Abstract
The eastern oyster (Crassostrea virginica) and the reefs they create provide significant ecosystem services. This study measured their possible role in nutrient mitigation through bioassimilation, burial, and oyster-mediated sediment denitrification in near-shore shallow water (< 1 m water depth) and deep-water (> 1 m water depth) oyster reefs in Louisiana. Nitrogen (N) and carbon (C) in shell and tissue differed by oyster reproductive status, size, and habitat type. Changes in tissue percent N and C post-spawning combined with significant reductions in tissue dry weight from the release of gametes, resulted in 20 and 46% reductions in tissue N and C load (mg), respectively, for a 100-mm oyster. Oyster reefs did not enhance burial rates, with burial range rates estimated at 1.4–2.6 g N m−2 year−1, and 26.9–43.8 g C m−2 year−1. Closed-system ex situ incubations indicated net denitrification in all habitat types studied, with the highest rates exceeding 600 µmol N m−2 h−1 during the summer, but no enhancement attributable to oyster reefs specifically. Within the highly productive, organic-rich wetland complex systems of coastal Louisiana, oyster reefs were not associated with enhanced denitrification, likely due to the organic-rich setting, and redundant supplies of organic nitrogen and carbon from adjacent marshes. Context remains critical in determining ecosystem provision of habitats, and efforts to extrapolate and predict nitrogen removal across locations necessitates consideration of local conditions. Considering the large extent of reefs and oyster production across coastal Louisiana, oyster habitats may still contribute to N and C mitigation, but their unique contribution likely comes from bioassimilation, and removal of the oysters from the system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Excessive nutrient loading to coastal systems negatively impacts estuarine and marine communities (Valiela et al. 1992; Howarth et al. 2000). In otherwise nutrient-limited systems, nitrogen (N) and phosphorus from agricultural and urban runoffs stimulate primary productivity and alter biogeochemical cycling (Vitousek et al. 1997). Estuaries with high primary productivity may experience increased production of algae, and often become hypoxic as these primary producers die, decompose, and reduce available oxygen (Howarth et al. 2000). However, suspension feeders, such as oysters, have been shown to have significant impacts on estuarine water quality and may provide valuable services by potentially serving as nutrient sinks (e.g., Higgins et al. 2013; Kellogg et al. 2013; Smyth et al. 2013a, 2015; Mortazavi et al. 2015; Humphries et al. 2016).
Approximately 85% of oyster reefs across the globe have been degraded or lost over the past 130 years largely due to overharvesting, disease, and poor management practices (Beck et al. 2011). Concomitant with these reductions, many coastal estuaries have also experienced increases in anthropogenic nutrient inputs (Zu Ermgassen et al. 2013) leading to significant management and restoration efforts to address both issues. Enhancing populations of suspension feeding bivalves, such as the eastern oyster, Crassostrea virginica, through restoration of reefs has been proposed as a means of mitigating coastal eutrophication (Kellogg et al. 2014 and references therein). Despite numerous studies quantifying various ecosystem services, such as habitat provision and shoreline protection provided by oyster reefs across the Atlantic and Gulf coasts (Coen et al. 2007), our understanding of oyster-mediated eutrophication mitigation remains limited (e.g., Higgins et al. 2011; Kellogg et al. 2014; Humphries et al. 2016).
Oyster-mediated nutrient mitigation may occur through three possible mechanisms: (1) bioassimilation (Soogsanjinda et al. 2000; Higgins et al. 2011; Carmichael et al. 2012), (2) burial (Newell et al. 2005), and (3) denitrification (Newell et al. 2002). Bioassimilation, the uptake of nutrients into shell and tissue, provides one potential means of permanent N and C removal from an estuarine system if oysters are harvested, or through burial of shells. Several studies have quantified assimilation in oyster tissue and shell to calculate potential removal from a system (i.e., Higgins et al. 2011; Dalrymple and Carmichael 2015). Generally, studies identify a relatively narrow range of N and C assimilation, with strong positive relationships between oyster shell height, and N and C assimilation (i.e., Higgins et al. 2011). At the same time, comparisons across studies indicate N and C sequestration in oysters likely vary according to growing conditions (i.e., aquaculture versus reef), genetic history, oyster health, physiological (reproductive) state (Dalrymple and Carmichael 2015), and possibly estuarine nutrient status (Fertig et al. 2010; Dalrymple and Carmichael 2015). Ultimately, N removal only occurs if the oysters are removed from the system.
N and C sequestration may be associated with burial of N found in oyster biodeposits as well as N and C held in oyster shell, which can persist beneath reefs over geologic time scales (Kellogg et al. 2014). Burial rates are likely influenced by local deposition rates, sediment biogeochemistry, and hydrodynamics (Butzeck et al. 2015). While limited data exist for oyster-enhanced burial rates (Kellogg et al. 2014), the existence of remnant oysters, and “black” shells beneath reefs, combined with the potential of enhanced biodeposition on and adjacent to reefs suggests the potential for increased burial in these locations.
Sequestration or removal of N by oysters through denitrification may occur via several possible pathways. Broadly, oysters and their reefs may enhance denitrification through impacts on the availability of C or N. Oyster reefs are hypothesized to impact denitrification directly through increased deposition from either their biodeposits, their presence, or due to their structural complexity impacts on water movement (Hoellein and Zarnoch 2014). Oysters assimilate approximately 50% of filtered particulate organic matter and excrete the remainder as feces or pseudofeces to the sediment surface (Newell and Jordan 1983; Dalrymple and Carmichael 2015). Collectively termed as biodeposits, these ammonium-rich excretions may contribute to long-term burial and denitrification by stimulating benthic microbial processes (Newell et al. 2005; Hoellein et al. 2015).
Numerous studies focusing on whether oysters and their reefs enhance denitrification rates have yielded variable results. Some studies reported increased sediment denitrification rates associated with oysters, oyster reefs (e.g., Piehler and Smyth 2011; Kellogg et al. 2013; Smyth et al. 2013a, 2015), and aquaculture projects (Humphries et al. 2016), while others failed to find enhanced rates (Nizzoli et al. 2006; Higgins et al. 2013; Mortazavi et al. 2015). Several laboratory studies report higher denitrification associated with living oysters, or living oysters and sediments, suggesting that living oysters alone can mediate the environment to enhance denitrification (Caffrey et al. 2016). Differences reported from field studies, and between field and laboratory studies may partially reflect the different microhabitats sampled (i.e., living oysters, sediments adjacent to reef), but also the highly variable settings (i.e., temperature, nutrient status) between field studies. Specifically, parameters such as salinity, temperature, dissolved oxygen, hydrology, turbidity, bivalve density, sediment organic matter, and habitat context, among other factors (Carmichael et al. 2012; Smyth et al. 2013b, 2015; Kellogg et al. 2014) may be critical in determining whether oyster reefs enhance denitrification within a given location.
The concept of oyster reef-enhanced sediment denitrification is based on our assumption that through biodeposition, oysters provide needed organic N and C to the sediment microbial community that otherwise would have been limiting (Newell et al. 2002, 2005; Dame et al. 2002; Carmichael et al. 2012; Kellogg et al. 2014). In organic-rich systems, however, enhanced inputs of organic C and N may not alleviate limiting factors, and, may even contribute to increased sulfide accumulation and anoxia, which ultimately may reduce denitrification (Joye and Hollibaugh 1995). While general consensus exists that oyster reefs provide some nutrient reduction, there remains a need to more completely quantify relationships between nutrient sequestration and different environmental conditions and settings (Carmichael et al. 2012; Kellogg et al. 2014; Smyth et al. 2015).
Despite increased attention to the contributing role of natural, restored, and aquaculture oyster sites to nutrient mitigation along the east coast of the United States (Higgins et al. 2011; 2013; Piehler and Smyth 2011; Carmichael et al. 2012; Kellogg et al. 2013; Humphries et al. 2016), few data exist quantifying the contribution of oyster reefs to N and C mitigation within the northern Gulf of Mexico (but see Dalrymple and Carmichael 2015; Mortazavi et al. 2015). In Louisiana in particular, the potential contribution of oyster reefs to nutrient mitigation could be enormous given that Louisiana oyster populations include over 6.9 × 105 ha of public water bottoms that are managed for oyster reefs alone (LDWF 2013). This acreage of reefs fails to include an estimated 1.6 × 105 ha of private leased water bottoms (LDWF 2013), non-managed oyster reefs, and oyster reef restoration projects which remain unquantified, but are significant (La Peyre et al. 2014a). Reefs in this region tend to be characterized as a matrix of mud and reef material with minimal reef elevation and exist within shallow-water estuaries surrounded by productive marsh (Beck and La Peyre 2015). In addition, this region of the northern Gulf of Mexico remains one of the most highly eutrophic areas in the United States with the Mississippi River delivering an annual flux of nearly 1.5 × 106 mg N, a rate which has more than doubled since 1965 (Vitousek et al. 1997; Rivera-Monroy et al. 2013).
Given the highly eutrophic nature of Louisiana estuaries and unique reef characteristics, we hypothesized that the potential contributions of oyster reefs to N and C removal in this region may differ compared to previous reported studies. To examine this hypothesis, we quantified the three primary mechanisms of nutrient mitigation by which oyster reefs in Louisiana estuaries may contribute, and discuss the results in the context of Louisiana’s coast. This study sampled shallow-water, near-edge (< 1 m water depth) and deep-water (> 1 m water depth) oyster reefs to: (1) measure N and C assimilation in oyster tissue and shell of small (< 75 mm) and large (> 75 mm) oysters during pre- and post-spawning periods, (2) examine the role of oyster reefs and reference sediments to act as nutrient sinks by quantifying N and C buried in sediments, and (3) measure N2 fluxes to determine the influence of oysters in contributing to N removal via net denitrification. These data may be useful in further understanding the contributions of oysters to the ecological health and integrity of estuaries as new restoration projects are implemented and new strategies for the improvement of water quality are considered.
Methods
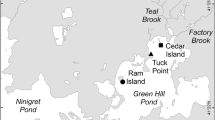
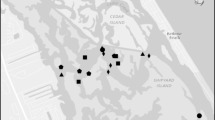
We quantified nutrient bioassimilation, burial, and sediment denitrification across three habitat types in two deltaic estuaries in coastal Louisiana: shallow-water reefs, deep-water reefs, and sub-tidal reference (bare sediment; Fig. 1). The two estuaries, Terrebonne (Sister (Caillou) Lake) and Breton Sound (Lake Fortuna) support extensive oyster reef complexes. Sister Lake is an estuarine lake surrounded by emergent marsh habitat, with a mean salinity of 11.8 ± 0.6 (mean ± standard error for 2003–2015; Continuous Data Recorder: USGS07381349). It is connected to the Gulf of Mexico through a pass at the southern end of the lake. Since 1940, Sister Lake has been a designated Public Oyster Seed Reservation, with approximately 30% of bottoms classified as oyster beds (LDWF 2013). Lake Fortuna, located in Breton Sound, Louisiana, is a mixture of microtidal bays, lakes, bayous, and canals surrounded by emergent marsh, with a mean salinity of 12.2 ± 0.7 (2003–2015; USGS07374526). Surveys indicate that approximately 10% of water bottoms in this area are oyster beds (LDWF 2013). Oyster reefs at both sites are actively managed and harvested and are characterized as a matrix of mud and reef material, with minimal reef elevation (Beck and La Peyre 2015). Oyster reefs persist in both shallow-water (< 1 m) and deep-water (> 1 m) environments, offering a unique opportunity to compare nutrient mitigation at reefs which vary in depth and proximity to shoreline.
For deep-water reefs, we selected a reef area in each estuary. Both reefs were distant (> 100 m) from the nearest shoreline, and in waters greater than 1 m depth. Surveys indicated adult oyster (> 75 mm shell height, SH) densities of 7.2 individuals (ind) m−2 (Sister Lake) and 4.8 ind m−2 (Lake Fortuna; LDWF 2013) in summer, typical of sub-tidal market-sized oyster reef densities in this region. For shallow-water reefs, we selected shoreline-adjacent reefs at both locations, with less than 1 m water depth. Adult oyster densities (> 75 mm SH) were estimated at 22 ind m−2 (Sister Lake, Casas et al. 2015) and 28.7 ind m−2 (Lake Fortuna, La Peyre, M.K., U.S. Geological Survey, unpubl. data). Sub-tidal reference sites were selected that were in close proximity to the deep-water and shallow-water oyster reef treatments, but at least 100 m away from either reef. Water depths at these treatments during sampling ranged from 1 to 2 m.
Bioassimilation
Oysters were collected in spring 2015 (February–March) using a hand-pulled oyster dredge (deep-water reefs) or by hand (shallow-water reefs). Furthermore, to compare nutrient content pre- (spring) and post (summer)-spawning (spawn comparison), oysters were also sampled from Sister Lake in August 2015. Twenty individuals from each of two size classes (< 75 mm (small) and ≥ 75 mm (large)) were harvested from each reef habitat type, placed in bags and returned to the laboratory at Louisiana State University Agricultural Center (spring (pre-spawn): 2 size classes × 2 locations × 2 habitat types × 20 oysters = 160 oysters; summer (post-spawn): 2 size classes × 2 habitat types × 20 oysters = 80 oysters).
In the laboratory, oysters were cleaned to remove any sediment and/or barnacles from the outer shell using a metal brush and water. For each oyster, the following morphometric measurements were recorded: wet weight (mg), shell length (mm), shell height (mm), and shell width (mm). Oysters were shucked to obtain shell wet weight (mg) and tissue wet weight (mg). Dry weight (mg) of shell and tissue separately was determined by placing shell and tissue in an oven at 60 °C to a constant weight (~ 7 days).
Tissue was then ground into a fine powder using mortar and pestle. Each shell half was cut with a table saw to remove a 2-cm segment from umbo to margin. The 2-cm segments were ground into a fine powder using a mortar and pestle. Tissue and shell subsamples were packaged and analyzed for total N and total C contents using an elemental analyzer (Costech 1040 CHNOS elemental combustion system; Louisiana State University Wetland Biogeochemistry Analytical Laboratory, Baton Rouge, LA). Shells from Sister Lake August 2015 sampling were not analyzed for N or C because it was assumed that nutrient concentrations in shell represented long-term assimilation and did not change within a 4-month period (Dalrymple and Carmichael 2015). Total nutrient content (mg) for oyster tissue and shell was calculated using the following formula:
Burial
In August 2015, triplicate sediment cores (14 cm inside diameter × 30 cm length) were collected at all sites and habitat types using a piston corer (2 sites × 3 habitats × 3 replicates). Adjustments were made at reefs where oyster densities were high to ensure that uniform sediment cores were collected. Upon collection, cores were separated into 3-cm increments, placed in labeled Ziploc® bags and brought back to the laboratory where they were dried in an oven at 60 °C to a constant weight (~ 7 days). After sediments dried, they were ground into a fine powder using mortar and pestle or a Wiley Mill and weighed for determination of bulk density. Subsamples of sediment from each 3-cm increment were packaged and analyzed for N and C contents using an elemental analyzer (Costech 1040 CHNOS elemental combustion system; Louisiana State University Wetland Biogeochemistry Analytical Laboratory, Baton Rouge, LA). Burial rates were conservatively estimated using reported accretion rates for Sister Lake of 2.0 mm year−1 (Freeman and Roberts 2013), and for Lake Fortuna, 3.5 mm year−1 (Smith 2009).
Denitrification
To quantify denitrification rates, we used the closed-system incubation method (Miller-Way and Twilley 1996). Triplicate sediment cores (10 cm inside diameter and 35 cm length) with overlying water were collected by hand at all of our sample locations (2 sites × 3 habitat types × 3 replicates) in August 2015 (summer) and January 2016 (winter). Each core consisted of 8–10 cm of sediment, with overlying water collected in the rest of the core, and initial conditions were reported including temperature (oC), dissolved oxygen (mg l−1), N2–N (µmol), NO3− (µmol) and NH4+ (µmol) (Table 1). At collection, cores were fitted with air-tight bottoms and lids, and transported to a dark environmental chamber located in the Oyster Wet Lab, Louisiana State University Agricultural Center, Baton Rouge, LA. Approximately, 100 L of surface water was also collected at each site in carboys and filtered using a series of filters down to 0.5 µm. Upon arrival at the environmental chambers, lids were removed and cores placed in a water bath at in situ conditions (28 °C for summer, 16.5–18 °C for winter). Air was gently bubbled into the surface water of each core overnight to prevent the depletion of dissolved oxygen in the water column prior to the beginning of the incubation (Miller-Way and Twilley 1996).
The next day, the overlying water was siphoned from each core and replaced with filtered water from the carboys. The cores were then fitted with air-tight lids that had inflow ports connected to the carboys and outflow ports connected to sampling valves. Lids also had magnetic stir bars that circulated water throughout the incubation. In addition to the nine treatment cores that were incubated during each experiment for each season, two control cores were also filled with filtered site water (no sediment), capped, and placed in the water bath. If necessary, corrections could be made for any changes that occurred in the control cores (Fulweiler and Nixon 2012). Incubations were conducted in the dark to minimize variability caused by benthic autotrophic processes (Miller-Way and Twilley 1996).
During sampling, dissolved oxygen and temperature were measured and recorded using a Hach LDO101-01 dissolved oxygen probe. Replicate water samples (12 mL) were collected for N2/Ar analyses (Bay Instruments, Pfeiffer 200 ME Mass Spectrometer) in Exetainer® glass vials using a gravity-fed system, preserved with 200 µL of 50% ZnCl2 solution, and capped with air-tight lids (Higgins et al. 2013). Additionally, a 60-mL water sample was collected for inorganic nutrient analysis. Samples were filtered through glass fiber filters (Whatman GF/F 25 mm, pre-combusted for 3 h at 450 °C) and processed for ammonium (NH4+) and combined nitrite + nitrate (NO2− + NO3−; hereafter referred to as nitrate). This sampling procedure was repeated so that each core could be sampled at five evenly spaced intervals before conditions inside the core became hypoxic (< 2 mg L−1 DO), which equated to approximately every hour during summer incubations and every 12 h during winter incubations. Thus, summer and winter incubations totaled approximately 5 and 48 h, respectively. Carboys were sampled three times during the course of the incubation (beginning, middle, and end) using the methods described above to obtain concentrations of dissolved inorganic nutrients entering the cores. The upper 4 cm of sediment was harvested to obtain percent organic matter via loss on ignition (Ball 1964).
Corrected concentrations of N2 and dissolved nutrients were calculated by subtracting the concentration of either N2 or dissolved nutrients entering the core from the concentrations estimated for that sample period. N2 and dissolved nutrient fluxes were then determined from the slope of a 5-point linear regression of concentration as a function of time. If the relationship was nonlinear (p > 0.05), the flux was considered zero. Fluxes for N2 and dissolved nutrients were then calculated using the following equation:
Since the N2:Ar ratio calculates the net N2 flux as gross denitrification minus gross nitrogen fixation, negative N2 fluxes were considered net N fixation and positive fluxes were considered net denitrification (Henry and Twilley 2014; Fulweiler et al. 2008). Dissolved nutrient fluxes generated in the control cores were subtracted from the fluxes in the experimental cores to correct for any changes observed in the reference (control) cores. We applied a dilution correction to each core to account for nutrients entering from the carboys during each sampling event (mean ± SE: 1.43 ± 0.03% dilution correction; Heffner 2013). Fluxes were then standardized by overlying water volume and sediment area.
Statistical analyses
Differences in tissue and shell N and C were examined by habitat type (shallow-water reef, deep-water reef, sub-tidal reference) and size class (small, large) using ANOVA while blocking on site. At one site only (Sister Lake), differences between pre- and post-spawn tissue N and C by size class and habitat type were also examined. Total N and C in sediment were examined by depth prior to combining layers for the analysis of bulk density, N, C, and organic matter concentrations using an ANOVA by habitat type, blocking on site. Last, differences in N2, NO3−, NH4+, and O2 fluxes were examined by habitat type, blocking on site for each season separately (summer, winter). Denitrification efficiency was calculated following Seitzinger (1988). Data were log transformed or arcsine transformed to meet normality and homogeneity of variance assumptions when necessary. Tukey’s HSD was used when significant models were indicated. A significance level of alpha < 0.05 was used.
Regression models using total nutrient mass (N, C) were used to examine the relationship between N and C in shell and tissue to shell height (mm). Furthermore, linear regression was used to examine the relationship of N2 fluxes to sediment oxygen demand in all treatments (shallow-water, deep-water, reference) during summer and winter incubations. All statistical analyses were done in R, version 3.1.3 (R Core Development Team, 2015). Unless otherwise indicated, mean ± one standard error was reported.
Results
Bioassimilation
Oysters used in the study covered a broad size range, with shell heights ranging from 36.4 to 129.3 mm, and dry weights for tissue and shell ranging from 8.5 to 52 mg and 3551 to 263,450 mg, respectively (Table 2). Across all samples, tissue N ranged from 10 to 400 mg (3.6–14.4%) and shell N ranged from 10 to 370 mg (0.11–0.40%). More than 53% of N was in oyster tissue, even though tissue dry weight represented just 2.2% of the total oyster dry weight. C ranged from 40 to 2420 mg (30.0–59.81%) in oyster tissue and from 480 to 32,890 mg (6.81–15.45%) in oyster shell. Less than 10% of C was in oyster tissue.
There were no significant differences between small and large oysters for percent N or C in pre- and post-spawning populations (results not shown), thus size classes were combined for further statistical analysis. For percent tissue N and C, there were significant interactions between habitat type and spawning status (Table 3). Post-spawning oysters had higher percent tissue N than pre-spawning oysters (p < 0.001), with a greater increase in deep-water compared to shallow-water reefs. Significant and highly predictive linear relationships were found between tissues N and C for pre-spawn oysters with shell height, but not for post-spawn oysters (Fig. 2).
Across estuaries for pre-spawning oysters, tissue percent N differed significantly by habitat type with individuals from deep-water reefs having higher values than those from shallow-water reefs (Table 4). There were no significant differences in tissue C. In shallow-water habitats, small oysters had significantly more N than large oysters, which in turn had significantly greater N in shells compared to both oyster size classes in deep-water habitats. Shell percent C varied significantly by size in both habitats, but not between habitats. Tissue and shell N and C had significant linear relationships with shell height, although there was a large amount of unexplained variation (Fig. 3).
Burial
There was no significant difference in sediment bulk density, percent N, or percent C by depth, so all depths within each core were combined for statistical analyses. Sediment bulk density was significantly greater at deep water reefs compared to sub-tidal sediments, which had significantly greater bulk density than shallow-water reefs (Table 5). In contrast, sediments N and C were significantly greater at shallow-water reefs compared to the other habitat types, which did not differ from one another. Calculated burial rates ranged from 1.4 to 2.6 g N m−2 year−1, and 26.9 to 43.8 g C m−2 year−1.
Denitrification
Net denitrification rates varied by season, with higher rates during summer incubations (> 500 µmol m2 h−1) compared to winter (< 100 µmol m2 h−1), but not by habitat type (Table 6; Fig. 4). Overall mean N2 fluxes and nitrate (NO3−) fluxes were positive, with highest fluxes during summer incubations (N2—shallow: 562.5 ± 208.4 µmol m2 h−1; deep: 649.2 ± 165.5 µmol m2 h−1; reference: 612.6 ± 12.3 µmol m2 h−1; NO3−—shallow: 39.7 ± 21.5 µmol m2 h−1; deep: 63.4 ± 50.6 µmol m2 h−1; reference: 0 µmol m2 h−1; Fig. 4a, 4b) and lower fluxes in winter (N2—shallow: 52.7 ± 9.9 µmol m2 h−1; deep: 23.9 ± 5.4 µmol m2 h−1; reference: 40.6 ± 1.6 µmol m2 h−1; NO3−—shallow: 26.3 ± 17.5 µmol m2 h−1; deep: 4.3 ± 3.0 µmol m2 h−1; reference: 0 µmol m2 h−1). Ammonium fluxes showed no clear patterns and ranged from − 360.7 to 232.5 μmol N m−2 h−1. Oxygen fluxes also did not differ significantly between habitat types (Fig. 4c). Denitrification efficiency was high (> 80%) across all habitat types and seasons tested (Fig. 4d). SOD and N2 flux relationships varied significantly by season, and within each habitat, although a general regression of increasing sediment oxygen demand (SOD) with increasing N2 flux occurred (Fig. 5).
Mean (± SE) summer and winter a N2 flux, b NO3− flux, c O2 flux and d denitrification efficiency (%) at deep-water reefs, shallow-water reefs, and sub-tidal reference sites (no oysters). Letters show the results of seasonal two-way ANOVA by habitat type, blocking on site. Different letters indicate significance among means. Note that fluxes of NO3− at reference sites were zero
Scatter plot of N2 fluxes to sediment oxygen demand across all habitat types and seasons. Points represent mean ± SE for each season (black circle: summer, gray circle: winter) by habitat type (deep-water reefs, shallow-water reefs, and sub-tidal reference) combination. Note that sediment oxygen demand is presented as a positive flux
Discussion
Oysters and the reefs they create contribute to nutrient mitigation through bioassimilation of N and C in tissue and shell, but failed to enhance N and C burial, or provide enhanced N removal through denitrification when compared to a sub-tidal reference site. For bioassimilation, reproductive status and oyster size (shell height) influenced oysters’ relative contribution and should be considered in developing estimates of potential N and C removal. In contrast, burial rates were not enhanced on oyster reefs, compared to reported burial rates in adjacent water bottoms and marshes. Similarly, denitrification rates were not enhanced on oyster reefs (shallow or deep), compared to reference (mud bottom) sites, despite some of the highest recorded rates of denitrification at oyster reefs documented during the summer. Within the organic-rich setting of the Louisiana delta system, denitrification rates at oyster reefs are likely not limited by lack of high-quality organic inputs. Considering the large extent of reefs and high production across Louisiana, oysters and their reefs contribute to nutrient mitigation across the landscape, although their unique contribution in this region likely only comes from bioassimilation, and their potential extraction following harvest.
Reproductive status may be an important factor to consider when predicting the role of oysters in mitigating nutrient loads through bioassimilation. Overall, tissue N (mg) and C (mg) decreased post-spawning. With the release of gametes during reproduction, tissue dry weight can be reduced from 50 to 90% (Ren et al. 2003) with a potentially equivalent reduction in N and C within the oyster tissue. In a 2-year study of N and C contents in mussels (Mytilus edulis L.), Rodhouse et al. (1984) found that 52% and 57% of the N and C budget, respectively, went to reproduction (gamete production), with approximately 30% to somatic maintenance, and the rest to shell, resulting in a significant drop in mussel N and C contents after reproduction. Similarly, N and C likely contribute to oyster somatic maintenance (tissue) and reproduction (gametes), explaining the drop in N and C post-spawn. Similar trends of decreasing N sequestration during late summer were found in Mobile, Alabama, which the authors attributed to either potential spawning or a temporary unidentified environmental stressor (Dalrymple and Carmichael 2015). In our study, spawning clearly had occurred, although it is also possible some unidentified stressor may explain the change.
Our data highlight a critical aspect of working with a shellfish species such as the oyster where an easily measurable indicator (i.e., shell height, mm) is often used as a surrogate for other measures. As shell height can only increase through time, decreases in condition from reproduction, or poor environmental conditions (i.e., starvation) are not reflected in changes in shell height. Specifically, the use of shell height has been suggested as a means to estimate potential nutrient removal through oyster harvest (i.e., Higgins et al. 2011). The results of this study indicate that if only tissue is being removed from the system, nutrient removal for a 100-mm oyster may be overestimated by as much as 20% (N) and 46% (C) if the oysters are collected post-spawn. Shell is often returned to the water to provide substrate for future populations; thus in many cases N and C removal estimates may only be relevant to oyster tissue. If, however, the entire organism (shell + tissue) is removed, N and C estimates for a 100-mm oyster could still vary by as much as 12% depending on whether the removal occurred pre- or post-spawning. Predictions based solely on shell height (mm) could greatly overestimate nutrient removal through bio-extraction.
Another goal of this project was to estimate long-term burial of N and C associated with Gulf Coast oyster reefs. As sediment accretion rates within coastal systems are highly variable and differ according to deposition, erosion, and compaction processes of organic and mineral particles at each site (Butzeck et al. 2015), generating accurate burial rates from which to extrapolate estimates of nutrient removal remains difficult and should be interpreted with caution. The estimated burial rates extrapolated were 5–10 times lower for N and C compared to marsh and open water estimates reported previously in this region (Smith et al. 1985). Overall, we found higher N and organic matter concentrations in shallow-water reef sediments compared to deep-water reefs and sub-tidal mud bottoms. The augmented concentrations were likely due to inputs from the adjacent and highly productive marsh, combined with the potentially greater edge reef complexity that can increase sediment trapping. This assumption is supported by recent work along the Atlantic U.S. coast that quantified C sequestration at oyster reefs and found that marsh edge reefs sequestered C at a rate similar to adjacent marsh habitats (Fodrie et al. 2017). Oyster reefs may enhance nutrient burial in some instances by increasing sediment accretion rates through biodeposition, but as with other ecosystem services provided by oyster reefs, other factors including adjacent habitats, hydrodynamics, and local management likely play a significant role as well (La Peyre et al. 2014b, 2015; Smyth et al. 2015; Fodrie et al. 2017).
Denitrification rates measured in the present study are at the upper end for rates measured at oyster reefs in the United States (Kellogg et al. 2014; Humphries et al. 2016); however, they fall within the range of measured values for coastal Louisiana systems, which were found to reach values > 2500 μmol m−2 h−1 (Rivera-Monroy et al. 2010). The structural complexity of the reef, or the density of oysters on the reef, may also play a critical role in determining the actual impact of reef in supplying organic N and C to the sediment microbial community (e.g., Dame et al. 2002; Piehler and Smyth 2011; Carmichael et al. 2012; Smyth et al. 2015). However, the similarities between reef and non-reef areas may be partially explained by the unique nature of Louisiana reef complexes and the history of bottom type and land use change in the region.
In Louisiana, the reefs are generally composed of an extensive complex of soft sediments and oysters, with minimal elevation due to the micro-tidal environment, and a long history of active harvest which tends to scatter the reefs, and may not provide significant structural complexity (LDWF 2013; Beck and La Peyre 2015). For example, the Sister Lake site in this study is an interior estuarine lake with over 30% of the water bottom classified as oyster reef, which is scattered throughout the entire lake area rather than concentrated into discrete, well-defined reef areas (LDWF 2013). Thus, denitrification associated with oyster reefs in these systems may be less localized, with organic and particulate matter in the water column influencing nutrient cycling at neighboring habitat types within each estuary. This is in direct comparison with oyster reefs in systems on the east coast, where reefs tend to have higher elevation than the surrounding mud bottom and have better-defined boundaries compared to reefs in Louisiana.
Alternatively, the high rates of denitrification across habitat types suggest that in this environment, the oyster presence failed to change local conditions to enhance denitrification. This finding is similar to another study conducted on the US Gulf coast, which concluded that site-specific biogeochemistry may determine the impact of oysters on N cycling (Mortazavi et al. 2015). Higgins et al. (2013) examined the effects of aquacultured oysters on N removal rates, and also failed to find an effect, which they had hypothesized could result from increased biodeposition. Biodeposition from oysters provides the most frequently cited explanation for enhanced denitrification at oyster reefs (i.e., Newell et al. 2002), but relies on the assumption that organic nutrients are limiting, and that biodeposits remain in sufficient concentration in the area that they are deposited. These Louisiana reef complexes and adjacent soft-bottom sediments form a part of an extensive complex of a highly productive marsh ecosystem, which contributes large amounts of organic N and C to the sediment microbial community. As a result, without other changes to the system, enhanced biodeposition may not translate to higher denitrification rates either directly through oyster filter feeding or reef complexity. Redundancy between ecosystem services has been suggested in other studies related to denitrification, when located adjacent to salt marshes and seagrass beds which provide organic matter (Smyth et al. 2015).
Similarly, as each oyster contributes to biodeposition through their individual filtering activities, oyster density or biomass may need to reach a critical threshold to enhance organic N and C measurably. While past work has suggested some nonlinear, and possible threshold relationship between oyster density and N assimilation (i.e., Dame et al. 2002; Smyth et al. 2015), differences in the range of oyster size classes reported, methods of estimation and extrapolations to common units, and the seasonal timing of density measurements make these relationships difficult to compare. For example, summer oyster densities often are elevated due to recent mass spawning events (Casas et al. 2015), with large numbers of small oysters which likely contribute little to biodeposition (Haven and Morales-Alamo 1966). In Louisiana, densities of market size oysters (> 75 mm) are the most commonly reported densities, as the inclusion of smaller oysters can vary 1000-fold throughout the year due to multiple spawning events, and low survival of smaller oysters (Casas et al. 2015). For the Sister Lake shallow-water reef used in this study, we reported a density of ~ 25 ind m−2; however, longer term data collection demonstrated densities over the last few years exceeding 100 ind m2 (> 75 mm), or 500 ind m2 (> 25 mm) (Casas et al. 2015). With high oyster growth rates in coastal Louisiana (Lowe et al. 2017), and highly variable densities, determining what density best captures potential reef contributions to biodeposition remains tricky. Furthermore, since estimated biodeposition rates for oysters provide ranges by grams of oyster tissue per day (Haven and Morales-Alamo 1966; Higgins et al. 2013), determining relationships or thresholds would likely be more successful if biomass was estimated and reported, rather than densities. Further research into threshold densities within the context of the local environment would be helpful in determining target restoration densities (Smyth et al. 2015). This line of inquiry is particularly important as a number of studies have demonstrated higher denitrification rates when comparing treatments with and without live oysters (Kellogg et al. 2013; Smyth et al. 2013b; Humphries et al. 2016). In this study, we only incubated cores with sediments, and found no enhancement of denitrification, similar to other studies which examined denitrification in reef-associated cores (sediments) (Higgins et al. 2013; Mortazavi et al. 2015). Further work which includes oysters within the incubations may be useful, although would require different techniques as our cores were too small to incorporate living oysters.
Across all habitat types, denitrification rates were higher during summer incubations (> 500 μmol m−2 h−1) than in winter (< 100 μmol m−2 h−1), which have been associated with higher metabolism among microbial communities in warmer temperatures (Smyth et al. 2013b). Summer and winter nitrate fluxes were positive at all sites, which may indicate coupled nitrification–denitrification (Fulweiler and Nixon 2012; Henriksen et al. 1983). Yet these positive fluxes may also indicate that the capacity for these sediments to maintain high denitrification efficiency is limited, with factors such as sediment composition, hydrology, microbial composition, and proximity to the sediment aerobic/anaerobic interface influencing rates in these systems (Carmichael et al. 2012; Mortazavi et al. 2015). Though we observed positive fluxes of nitrate to the overlying water, dissolved inorganic N fluxes were negative for all habitat types across both seasons, suggesting that these habitats are sinks for N.
Past studies have suggested the use of sediment oxygen demand (SOD) to predict denitrification in coastal systems, as N2 fluxes have been shown to increase linearly with an increase in sediment oxygen consumption (Piehler and Smyth 2011; Smyth et al. 2013b; Humphries et al. 2016). This relationship is common in coastal systems where coupled nitrification–denitrification is controlled by the availability of organic C (Henry 2012; Smyth et al. 2013a). However, in systems such as the Mississippi River Delta where organic matter is subject to high variation, other factors, including bivalve density and hydrodynamics, may also influence denitrification rates. This premise is supported in the present study, where sediment organic matter concentrations did not affect rates of denitrification, high variation between SOD, SOM and N2 flux relationships occurred between locations and findings similar to those reported in Alabama (Mortazavi et al. 2015). Interestingly, this increasing variation with increasing SOD and N2 flux is evident in data reported from other regions as well (i.e., Humphries et al. 2016).
While many studies on the U.S. east coast suggest that oyster habitat rivals or exceeds the denitrification capacity of other estuarine habitats (i.e., Piehler and Smyth 2011; Kellogg et al. 2013; Smyth et al. 2013b), results from these Louisiana reefs did not show this, similar to a past study in Alabama (Mortazavi et al. 2015). Oyster reefs did not enhance or exceed denitrification rates compared to surrounding estuarine habitat types. With significant high-quality inputs of new organic matter from the surrounding, extensive and highly productive marshes, oyster reefs in this setting may provide a redundant role, as enhancing organic N and C to the sediments would fail to alleviate any existing limitation on denitrification. Oyster reefs in this region contribute uniquely to nutrient removal, largely through assimilation in shell and tissue, and provide functional redundancy in removal through denitrification. Given the prolific annual oyster harvest and the extensive reef systems across coastal Louisiana (LDWF 2013), oyster-mediated nutrient mitigation across the coastal region of the state remains important, but contributions likely occur primarily through bioassimilation. Estimates of N and C removal through bioassimilation are based solely on extrapolations from oyster size (shell height), and numbers may vary by as much as 50% depending on oyster reproductive status at the time of removal; oyster condition, reflecting local water quality conditions, may similarly influence bioassimilation and removal.
References
Ball DF (1964) Loss on ignition as an estimate of organic matter and organic carbon in noncalcareous soils. J Soil Sci 15:84–92
Beck S, La Peyre MK (2015) Effects of oyster harvest activities on Louisiana reef habitat and resident nekton communities. Fish Bull 113:327–340
Beck MW, Brumbaugh RD, Airoldi L, Carranza A, Coen LD, Crawford C, Defeo O, Edgar GJ, Hancock B, Kay MC, Lenihan HS, Luckenbach MW, Toropova CL, Zhang G, Guo X (2011) Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61:107–116
Butzeck C, Eschenbach A, Grongroft A, Hansen K, Nolte S, Jensen K (2015) Sediment deposition and accretion rates in tidal marshes are highly variable along estuarine salinity and flooding gradients. Estuar Coasts 38:434–450
Caffrey JM, Hollibaugh JT, Mortazavi B (2016) Living oysters and their shells as sites of nitrification and denitrification. Mar Pollut Bull. https://doi.org/10.1016/j.marpolbul.2016.08.038
Carmichael RH, Walton W, Clark H (2012) Bivalve-enhanced nitrogen removal from coastal estuaries. Can J Fish Aquat Sci 69:1131–1149
Casas SM, La Peyre J, La Peyre MK (2015) Restoration of oyster reefs in an estuarine lake: population dynamics and shell accretion. Mar Ecol Prog Ser 524:171–184
Coen LD, Brumbaugh RD, Bushek D, Grizzle R, Luckenbach MW, Posey MH, Powers SP, Tolley A (2007) Ecosystem services related to oyster restoration. Mar Ecol Prog Ser 341:303–307. https://doi.org/10.3354/meps341303
Dalrymple DJ, Carmichael RH (2015) Effects of age class on N removal capacity of oysters and implications for bioremediation. Mar Ecol Prog Ser 528:205–220
Dame R, Bushek D, Allen D, Lewitus A, Edwards D (2002) Ecosystem response to bivalve density reduction management implications. Aquat Ecol 36:51–65
Fertig B, Carruthers TJB, Dennsion WC, Fertig EJ, Altabet MA (2010) Eastern oyster (Crassostrea virginica) δ15N as a bioindicator of nitrogen sources: observations and modeling. Mar Pollut Bull 60:1288–1298
Fodrie FJ, Rodriguez AB, Gitman RK, Grabowski JH, Lindquist NL, Peterson CH, Piehler MF, Ridge JT (2017) Oyster reefs as carbon sources and sinks. Proc R Soc B 284:20170891
Freeman AM, Roberts HH (2013) Storm layer deposition on a coastal Louisiana lake bed. J Coast Res 29:31–42
Fulweiler RW, Nixon SW (2012) Net sediment N2 fluxes in a southern New England estuary: variations in space and time. Biogeochemistry 111:111–124
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2008) Net sediment N2 fluxes in a coastal marine system: experiment manipulations and a conceptual model. Ecosystems 11:1168–1180
Haven D, Morales-Alamo R (1966) Aspects of biodeposition by oysters and other invertebrate filter feeders. Limnol Oceanogr 11:487–498
Heffner L (2013) Responses of nitrogen cycling to nutrient enrichment in New England salt marshes over an annual cycle. PhD, University of Rhode Island, USA
Henriksen K, Rasmussen MB, Jensen A (1983) Effect of bioturbation on microbial nitrogen transformations in the sediment and fluxes of ammonium and nitrate to the overlying water. Ecol Bull 35:193–205
Henry KM (2012) Linking nitrogen biogeochemistry to different stages of wetland soil development in the Mississippi River delta, Louisiana. MS thesis, Department of Oceanography and Coastal Sciences, Louisiana State University, Baton Rouge, LA
Henry KM, Twilley RR (2014) Nutrient biogeochemistry during the early stages of delta development in the Mississippi River deltaic plain. Ecosystems 17:327–343
Higgins CB, Stephenson K, Brown BL (2011) Nutrient bioassimilation capacity of aquacultured oysters: quantification of an ecosystem service. J Environ Qual 40:271–277
Higgins CB, Tobias C, Piehler MF, Smyth AR, Dame RF, Stephenson K, Brown BL (2013) Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Mar Ecol Prog Ser 473:7–27
Hoellein TJ, Zarnoch CB (2014) Effect of eastern oysters (Crassostrea virginica) on sediment carbon and nitrogen dynamics in an urban estuary. Ecol Appl 24:271–286
Hoellein TJ, Zarnoch CB, Grizzle RE (2015) Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hamshire, USA). Biogeochemistry 122:113–129
Howarth R, Anderson D, Cloern J, Elfring C, Hopkinson C, Lapointe B, Malone T, Marcus N, McGlathery K, Sharpley A, Walker D (2000) Nutrient pollution of coastal rivers, bays, and seas. Issues Ecol 7:1–16
Humphries AT, Ayvazian SG, Carey JC, Hancock BT, Grabbert S, Cobb D, Strobel CJ, Fulweiler CJ (2016) Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove nitrogen at comparable high rates. Front Mar Sci 3:1–10
Joye SB, Hollibaugh JT (1995) Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270:623–625
Kellogg ML, Cornwell JC, Owens MC, Paynter KT (2013) Denitrification and nutrient assimilation on a restored oyster reef. Mar Ecol Prog Ser 480:1–9
Kellogg ML, Smyth AR, Luckenbach MW, Carmichael RH, Brown BL, Cornwell JC, Piehler MF, Owens MS, Dalrymple DJ, Higgins CB (2014) Use of oysters to mitigate eutrophication in coastal waters. Estuar Coast Shelf Sci 151:156–168
La Peyre MK, Furlong J, Brown L, Brown KM, Piazza B (2014a) Oyster reef restoration in the northern Gulf of Mexico: extent, methods and outcomes. Ocean Coast Manag. https://doi.org/10.1016/j.ocecoman.2013.12.002
La Peyre MK, Humphries A, Casas S, La Peyre JF (2014b) Temporal variation in development of ecosystem services from oyster reef restoration. Ecol Eng 63:33–44
La Peyre MK, Serra K, Joyner TA, Humphries A (2015) Assessing shoreline exposure and oyster habitat suitability maximizes potential success for sustainable shoreline protection using restored oyster reefs. PeerJ 3:e1317. https://doi.org/10.7717/peerj.1317
LDWF. Louisiana Department of Wildlife and Fisheries. 2013. Oyster stock assessment report of the public oyster seed areas of Louisiana. Oyster data report series 19. Baton Rouge, LA
Lowe M, Sehlinger T, Soniat T, La Peyre MK (2017) Interactive effects of water temperature and salinity on growth and mortality of eastern oysters, Crassostrea virginica: a meta-analysis using 40 years of monitoring data. J Shellfish Res 36:1–15
Miller-Way T, Twilley R (1996) Theory and operation of continuous flow systems for the study of benthic-pelagic coupling. Mar Ecol Prog Ser 140:257–269
Mortazavi B, Ortmann AC, Wang L, Bernard RJ, Staudhammer CL, Dalrymple JD, Carmichael RH, Kleinhuizen AA (2015) Evaluating the impact of oyster (Crassostrea virginica) gardening on sediment nitrogen cycling in a subtropical estuary. Bull Mar Sci 91:323–341
Newell RIE, Jordan SJ (1983) Preferential ingestion of organic material by the American oyster Crassostrea virginica. Mar Ecol 13:47–53
Newell RIE, Cornwell JC, Owens MS (2002) Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: a laboratory study. Limnol Oceanogr 47:1367–1379
Newell RIE, Fisher TR, Holyoke RR, Cornwell JC (2005) Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In: RiF D, Olenin S (eds) The comparative roles of suspension feeders in ecosystems, vol 47. NATO science series IV—earth and environmental sciences. Springer, Dordrecht, The Netherlands, pp 93–120
Nizzoli D, Welsh DT, Fano EA, Viaroli P (2006) Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Mar Ecol Prog Ser 315:151–165
Piehler MF, Smyth AR (2011) Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2:1–16
R Core Development Team (2015) R: a language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna. https://www.R-project.org
Ren JS, Marsden ID, Ross AH, Schiel DR (2003) Seasonal variation in the reproductive activity and biochemical composition of the Pacific oyster (Crassostrea gigas) from the Marlborough Sounds, New Zealand. N Z J Mar Freshw Res 37:171–182
Rivera-Monroy VH, Lenaker P, Twilley RR, DeLaune RD, Lindau CW, Nuttle W, Habib E, Fulweiler RW, Castaneda-Moya E (2010) Denitrification in coastal Louisiana: a spatial assessment and research needs. J Sea Res 63:157–172
Rivera-Monroy VH, Branoff B, Meselhe E, McCorquodale A, Dortch Q, Steyer GD, Visser J, Wang H (2013) Landscape-level estimation of nitrogen removal in coastal Louisiana wetlands: potential sinks under different restoration scenarios. J Coast Res 67:75–87
Rodhouse PG, Roden CM, Hensey MP, Ryan TH (1984) Resource allocation in Mytilus edulis on the shore and in suspended culture. Mar Biol 84:27–34
Seitzinger SP (1988) Dentrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Smith RP (2009) Historic sediment accretion rates in a Louisiana coastal marsh and implications for sustainability. Department Environmental Sciences, University of Louisiana, Lafayette
Smith CJ, DeLaune RD, Patrick WH Jr (1985) Fate of riverine nitrate entering an estuary: I. Denitrification and nitrogen burial. Estuaries 8:15–21
Smyth AR, Geraldi NR, Piehler MF (2013a) Oyster-mediated benthic-pelagic coupling modifies nitrogen pools and processes. Mar Ecol Prog Ser 493:23–30
Smyth AR, Thompson SP, Siporin KN, Gardner WS, McCarthy MJ, Piehler MF (2013b) Assessing nitrogen dynamics throughout the estuarine landscape. Estuar Coast 36:44–55
Smyth AR, Piehler MF, Grabowski JH (2015) Habitat context influences nitrogen removal by restored oyster reefs. J Appl Ecol 52:716–725
Soogsanjinda P, Matsudea O, Uamamoto T, Rajendran N, Maeda H (2000) The role of suspended oyster culture on nitrogen cycle in Hiroshima Bay. J Oceanogr 56:223–231. https://doi.org/10.1023/A:1011143414897
Valiela I, Foreman K, LaMontagne M, Hersh D, Costa J, Peckol P, DeMeo-Anderson B, D’Avanzo C, Babione M, Sham CH, Brawley J, Lajtha K (1992) Couplings of watersheds and coastal waters: sources and consequences of nutrient enrichment in Waquoit Bay, Massachusetts. Estuaries 15:443–457
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilamn DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Zu Ermgassen PSE, Spalding MD, Grizzle RE, Brumbaugh RD (2013) Quantifying the loss of a marine ecosystem service: filtration by the eastern oyster in US estuaries. Estaur Coasts 36:36–43
Acknowledgements
This work was funded through the Coastal Science Assistantship Program, through Louisiana Sea Grant and the Coastal Protection and Restoration Authority of Louisiana, as well as the Louisiana Department of Wildlife and Fisheries through the U.S. Geological Survey Louisiana Fish and Wildlife Cooperative Research Unit. Thanks to Dr. Chris Swarzenski for comments on an earlier version of this manuscript. Early discussion with Drs. Bryan Piazza and Michael Piehler contributed to the development of this project. Input and discussion with Drs. Austin Humphries, S. Ayvazian and Boze Hancock informed this work. Many thanks to Robert Twilley, Edward Castañeda, Andy Nyman, Jerome La Peyre, John White, Mike Kaller, Brett Collier, Kristy Capelle, Romain Lavaud, Christian Flucke, Songjie He, Scott Harlamert, Steven Madere, Jay Curole, and Tommy Blanchard for their help and expertise. Data are available through MKL. Reviews from three anonymous reviewers significantly improved this manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed.
Additional information
Responsible Editor: S. W. A. Naqvi.
Reviewed by S. Ayvazian, R. H. Carmichael and N. E. Ray.
Rights and permissions
About this article
Cite this article
Westbrook, P., Heffner, L. & La Peyre, M.K. Measuring carbon and nitrogen bioassimilation, burial, and denitrification contributions of oyster reefs in Gulf coast estuaries. Mar Biol 166, 4 (2019). https://doi.org/10.1007/s00227-018-3449-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-018-3449-1