Abstract
The restoration of dead/degraded oyster reefs is increasingly pursued worldwide to reestablish harvestable populations or renew ecosystem services. Evidence suggests that oysters can improve water quality, but less is known about the role of associated benthic sediments in promoting biogeochemical processes, such as nutrient cycling and burial. There is also limited understanding of if, or how long postrestoration, a site functions like a natural reef. This study investigated key biogeochemical properties (e.g., physiochemical properties, nutrient pools, microbial community size and activity) in the sediments of dead reefs; 1-, 4-, and 7-year-old restored reefs; and natural reference reefs of the eastern oyster, Crassostrea virginica, in Mosquito Lagoon (FL, USA). Results indicated that most of the measured biogeochemical properties (dissolved organic carbon (C), NH4 +, total C, total nitrogen (N), and the activity of major extracellular enzymes involved in C, N, and phosphorus (P) cycling) increased significantly by 1-year postrestoration, relative to dead reefs, and then remained fairly constant as the reefs continued to age. Few differences were observed in biogeochemical properties between restored reefs of any age (1 to 7 years) and natural reference reefs. Variability among reefs of the same treatment category was often correlated with differences in the number of live oysters, reef thickness, and/or the availability of C and N in the sediments. Overall, this study demonstrates the role of live intertidal oyster reefs as biogeochemical hot spots for nutrient cycling and burial and the rapidity (within 1 year) with which biogeochemical properties can be reestablished following successful restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The magnitude of ecosystem services provided by oyster reefs is well established, with the monetary value placed at up to $99,000 ha−1 year−1, even when harvesting for human consumption is excluded (Grabowski et al. 2012). These services include the creation of benthic habitat for other invertebrates and fish, shoreline protection, wave attenuation, the removal of phytoplankton and suspended solids, and the enhancement of denitrification (e.g., Dame et al. 1984; Meyer et al. 1997; Newell et al. 2002; Peterson et al. 2003; Coen et al. 2007; Kellogg et al. 2013). Yet, despite their critical role in the environment, oyster populations have been decimated globally over the last century due to overharvesting, pollution, and disease. For example, it is estimated that globally, 85% of shellfish reefs have been lost (Beck et al. 2011); in Chesapeake Bay, the loss estimate of the eastern oyster, Crassostrea virginica, since 1980 is over 99% (Wilberg et al. 2011).
The growing recognition of the ecological importance of oysters, coupled with their rapid rate of population decline, has fueled a substantial effort to restore dead and degraded oyster reefs in several regions of the world. Although some restoration efforts focus on oyster restoration for aquaculture and harvest, a growing number of projects emphasize the potential role of oysters in improving water clarity and quality through filter feeding and enhanced nitrogen (N) removal (Leffler and Hayes 2004; Laing et al. 2006; Plutchak et al. 2010; Garvis et al. 2015; Mortazavi et al. 2015). Oyster reefs function to couple the benthic and pelagic food webs by sequestering suspended biomass and detritus from the water column, including large volumes of phytoplankton (Dame et al. 1984, 1991; Cressman et al. 2003). This material is converted temporarily into benthic biomass and then deposited/excreted onto the surrounding sediments in an altered chemical form (i.e., as dissolved inorganic nutrients), or at least altered particle size (i.e., aggregated into larger particles with mucus; Dame 1999). The biodeposits (i.e., feces and pseudofeces) from oysters typically have a significantly higher organic carbon (C), N, and phosphorus (P) concentration than the surrounding sediments (Newell et al. 2005), creating a chemical environment that is ideal for the development of a robust sediment microbial community, which may otherwise be limited by one or more of these key macronutrients (McClain et al. 2003; Reddy and DeLaune 2008).
Numerous studies have investigated the potential for this biodeposit-enriched environment to enhance denitrification, an important ecosystem service considering the role excess N often plays in algal blooms (e.g., Dame et al. 1991; Newell et al. 2002; Higgins et al. 2013; Kellogg et al. 2013; Pollack et al. 2013; Smyth et al. 2013; Hoellein et al. 2015; Dalrymple and Carmichael 2015; Mortazavi et al. 2015; Lindemann et al. 2016). However, there has yet to be a more comprehensive review of the potential role of benthic sediments associated with oyster reefs to serve as a generalized biogeochemical hot spot in the coastal environment, one which enhances the transformation and burial of a variety of biologically important elements via microbial metabolism. This study seeks to evaluate the role of intertidal oyster reefs in changing the (1) physiochemical properties of the sediment, through an investigation of differences in bulk density and pH; (2) the chemical properties of the sediment, via a quantification of extractable/bioavailable nutrients in the porewater and total nutrient pools; and (3) the microbial properties of the sediment, by determining differences in total microbial biomass, denitrification enzyme activity, and the activity of major extracellular enzymes involved in C, N, and P cycling. In addition to comparing these biogeochemical properties of live vs. dead oyster reef sediments, this research also strives to determine how long it takes a restored oyster reef to establish biogeochemical indicators and functions that are quantitatively equivalent to those found in a natural oyster reef. To achieve these objectives, 8 years of oyster reef restoration efforts in a coastal lagoon located in central Florida (USA) was leveraged in a space-for-time substitution experiment, along with dead reef and reference reef control treatments. It was hypothesized that it would take approximately 3 to 4 years for a restored oyster reef to exhibit biogeochemical properties comparable to a natural reef; a hypothesis based on monitoring data suggesting oyster recruitment surpassed a critical tipping point around this timeframe (Birch and Walters 2012). Furthermore, total nutrient pools and associated microbial activity were expected to increase linearly with time since restoration.
Methods
Site Description and Restoration History
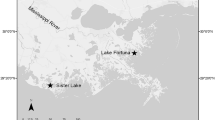
This study was conducted in the Indian River Lagoon (IRL), a shallow (average depth 1 m) estuary with a naturally restricted tidal exchange that extends along 251 km of the Atlantic coast of central Florida (Fig. 1). The greater IRL spans the biogeographical transition between temperate and subtropical climates, making it a hot spot for biodiversity and a backbone of the regional economy (Dybas 2002). However, the IRL is also characterized by degraded water quality, an increasing number of algal blooms and fish kills, and a dramatic decline in the amount of native oyster reef habitat (Garvis et al. 2015; St. Johns River Water Management District 2016).
Specifically, sampling took place within the boundaries of Canaveral National Seashore, located in the northernmost portion of the IRL, a region referred to as Mosquito Lagoon. The aerial coverage of intertidal shellfish reefs (predominately eastern oyster, C. virginica) has declined by 24% within Mosquito Lagoon as a whole, and 40% within Canaveral National Seashore, specifically, since 1943 (Garvis et al. 2015). The primary cause of reef degradation in this area is believed to be recreational boat wakes, which dislodge live oyster clusters and deposit them above the mean high water line, causing oyster death, and thus creating piles of loose, dead shells that are too elevated within the tidal frame for natural recolonization (Grizzle et al. 2002; Wall et al. 2005; Campbell 2015). Since 2007, a community-based oyster reef restoration effort has been undertaken within Canaveral National Seashore, resulting in the successful restoration of more than 77 patch reefs (totaling > 2.8 acres) as of 2016, with efforts ongoing (L.J. Walters, pers. comm.). The restoration process begins with locating dead reefs and raking the loose shell down to a low intertidal height. Mesh mats with attached, stabilized, disarticulated shells are then placed over this dead shell surface and weighted down with cement weights to establish a stable foundation for new oyster larvae to settle upon. Over time, oyster larvae from the surrounding water colonize this foundation and grow, forming a restored reef (Garvis et al. 2015).
Experimental Design
A total of 20 reefs were chosen for study in a space-for-time substitution experimental design. Four (4) representative reefs were chosen from each of 5 treatment categories: dead reefs (unrestored control), 1-year-old restored reefs (2015), 4-year-old restored reefs (2012), 7-year-old restored reefs (2009), and natural reefs (reference control), henceforth referred to as dead, 1-year restored, 4-year restored, 7-year restored, and reference, respectively (Fig. 1). The specific reefs chosen for this study were identified based on annual monitoring data that indicated these particular reefs had live oyster densities and reef thicknesses that were prototypical of all reefs within the condition/age class of interest, while also avoiding reefs that are the subject of other experiments that might have caused an additional disturbance or stress. A nested experimental design was employed in which each individual reef (subgroup, 20 total) was nested in combination with only one treatment (group, 5 total). Additionally, randomly selected field replicates (either 5 or 3 replicates depending on June or July sampling, respectively) were collected on each reef.
Treatment Characterization
Annual monitoring data on oyster success for each reef was analyzed to further characterize the biophysical differences between treatment conditions. Monitoring data included the mean number of live oysters per quadrat/per reef and mean reef thickness. The number of live oysters were counted on 30 randomly selected 0.25 m2 quadrats and averaged for each reef. Mean reef thickness was measured by using 10 unique quadrats of the same dimensions. Within these quadrats, the highest point of the oysters above the benthos was recorded. Two of the sampled reefs did not have annual monitoring data for 2016, so data from the next closest reef (< 20 m) of the same age was used.
A variety of physical and geographic properties were also measured for each reef to understand if there are inherent within-group or between-group differences that may serve as confounding variables during the study. These variables included reef area, spatial spread, distance from the nearest ocean inlet, latitude, distance from the nearest shore, channel width, and aerial coverage of neighboring reefs within a 50- and 100-m buffer area of the reef of interest. Reef area for restored reefs was calculated based on the total number of 0.25 m2 mesh restoration mats deployed at the site; these areas were digitized using geographic information systems from ESRI’s high-resolution World Imagery (GIS; ArcGIS 10.4.1, ERSI, Redlands, CA). For dead and reference reefs, areas were digitized using aerial photography provided by St. Johns River Water Management District, after Garvis et al. (2015), and the area was determined in ArcMap. The spatial spread/variability between reefs of the same treatment was calculated by creating a polygon around the outermost sampled reefs, defining the center point of the area, and then calculating straight line distances between that center point and each reef. Because the IRL is a tidally restricted lagoon, the distance between each reef and the nearest inlet for ocean water exchange was also of interest and was measured based on the shortest water flow path distance in ArcMap. For all reefs, Ponce de Leon inlet (29° 4′ 35.29″ N, 80°55′ 0.68″ W), located to the north of the study area, was the nearest inlet. Latitude was determined using a handheld GPS during field visits. Distance to shore was measured as the shortest linear distance from the edge of the reef to the nearest lagoonal shoreline, and channel width was measured as the span of open water from the edge of each reef to the adjacent shore; both measurements were performed in ArcMap using ESRI’s World Imagery. In the backwater areas where reefs are not on main channels and flow is restricted, the width of the nearest opening into the enclosed reef area was used as channel width because it dictates the flow of water past the reef. Finally, neighboring reef area was determined using two buffer widths, 50 and 100 m, around the reef of interest, using ArcMap. All reef area from naturally occurring and restored reefs in each of these buffers was summed to achieve the total neighboring reef area.
Field Sampling
Field sample collection took place over two separate sampling campaigns, one from June 6 to 15, 2016, and one from July 17 to 20, 2016. The same 20 reefs were visited once during each sampling campaign, making sure to visit at least 1 reef in each treatment during any individual sampling day in a stratified random sampling design. The reason for dividing the sampling efforts between two different time periods was the logistical constraints of doing several time-intensive analyses on each sample that were also highly time-sensitive (e.g., enzymes assays, denitrification enzyme activity (DEA), and microbial biomass carbon (MBC) are all recommended to be completed within 48 h of collection; Stenberg et al. 1998; DeForest 2009). Therefore, samples had to be collected in manageable quantities to ensure each analysis was completed using fresh sediment. During the June sampling, 5 reefs were visited and 5 randomly selected locations on each reef were sampled for sediment during 4 field trips (25 samples/trip = 100 samples total). Two days of laboratory sample processing took place between each field trip to ensure all analyses could be completed within the recommend hold time of samples. During the July sampling, 10 reefs were visited and 3 randomly selected locations on each reef were sampled for sediment during 2 field trips (30 samples/trip = 60 samples total). In June, each sediment sample was collected using a 7-cm diameter, 50-cm-long polycarbonate core tube beveled on the bottom and pounded into the substrate using a board and a rubber mallet. Due to the mineral nature of the substrate, compaction was deemed to be negligible. On restored reefs, the mesh restoration mats could be pulled up temporarily to sample the sediment directly below the reef (a mucky mixture of mineral matter and biodeposits). On reference reefs, cores were collected adjacent to and/or between shells, to the degree possible; on dead reefs, cores were collected of intertidal sediment directly adjacent to the dead shell mounds. All sediment cores did contain some shells within the sample, with large fragments (> 2 cm diameter) removed by hand before processing. Once a core was obtained, it was extruded from the core tube in the field, and the top 0–10 cm was collected, placed in an air-tight plastic bag, and put on ice for transport back to the laboratory. During the July sampling, the procedure was similar except that only the top 0–3 cm of sediment was collected due to the differing nature of the analysis performed on these sediments (i.e., extracellular enzyme assays, which tend to be more time-intensive and the activity restricted to the most surficial sediments). At the time of sediment sampling, a surface water grab sample was also collected above each reef in a 500-mL acid-washed Nalgene bottle and placed on ice for further processing at the laboratory. During the July sampling, surface water dissolved oxygen (DO), pH, and salinity were also recorded with a handheld sonde (ProDSS, YSI Inc., Yellow Springs, OH, USA).
Surface Water Analysis
Upon return to the laboratory, the grab surface water samples were immediately vacuum-filtered through a 0.45-μm membrane filter, acidified to a pH < 2 with double-distilled H2SO4, and stored at 4 °C. Water samples were analyzed within 28 days for total dissolved organic carbon (DOC), nitrate + nitrite (NO3 −), ammonium (NH4 +), and ortho-phosphate (PO4 3−). A Shimadzu TOC-L Analyzer (Shimadzu Scientific Instruments, Kyoto, Japan) was used to determine the concentration of nonpurgeable DOC in the water samples. Nutrient concentrations were determined colorimetrically on a SEAL AQ2 Automated Discrete Analyzer (Seal Analytical, Mequon, WI) using EPA Methods 353.2 Rev. 2.0, 350.1 Rev. 2.0, and 365.1 Rev. 2.0, respectively, for NO3 −, NH4 +, and SRP (USEPA 1993).
Sediment Physiochemical Properties
All sediment samples were immediately weighed upon return to the lab and homogenized by hand. A subsample of ~100 g was weighed in an aluminum tin and dried at 70 °C for 3 days in a gravity drying oven. Large shells (> 2 cm diameter) were excluded from the subsample. Once dried to a constant weight, sediment bulk density was calculated gravimetrically as the mass of solids divided by the volume of the sampling core tube. Soil pH was determined on field moist soils by creating a 1:5 sediment to DI water slurry, allowing it to equilibrate for 30 min, and then measuring pH of the solution with an Accumet benchtop pH probe (Accumet XL200, Thermo Fisher Scientific, Waltham, MA, USA).
Sediment Nutrient Pools
Sediment nutrient pools were divided into extractable pools (i.e., nutrients within the sediment porewater and extracted from the sediment surface following the addition of salts) and total pools (i.e., nutrients complexed within the sediment’s mineral and organic matter). Extractable sediment nutrient pools were determined for DOC, NO3 −, NH4 +, and PO4 3−. Extractable DOC was quantified following sediment extraction with 0.5 M K2SO4 as described below for MBC nonfumigate control samples. Extractable NO3 −, NH4 +, and PO4 3− were determined by adding 3 g wet sediment to a 40-mL centrifuge tube along with 25 mL of 2 M KCl. Centrifuge tubes were shaken vigorously by hand and then placed on an orbital shaker at 150 rpm and 25 °C for 1 h. Samples were then centrifuged at 4000 rpm and 10 °C for 10 min, and the supernatant vacuum-filtered through a 0.45-μm membrane filter, acidified with H2SO4 to a pH < 2, and stored at 4 °C. Samples were analyzed colorimetrically on a SEAL AQ2 Automated Discrete Analyzer (Seal Analytical, Mequon, WI) using EPA Methods 353.2 Rev. 2.0, 350.1 Rev. 2.0, and 365.1 Rev. 2.0, respectively, for NO3 −, NH4 +, and PO4 3− (USEPA 1993).
Total sediment nutrient pools consisted of organic matter content, total C, total N, and total P. All analyses were performed on dried sediment following 3 days in the drying oven at 70 °C. Samples were then mechanically ground down to individual particles with a Spex 8000 mixer/mill (Spex Sample Prep, Metuchen, NJ, USA). Organic matter content was determined by loss-on-ignition where dried soils were combusted at 550 °C for 5 h, and final weight was subtracted from initial weight. Total C and N content was quantified on an Elementar vario MICRO select (Elementar Analytical, Langenselbold, Germany). Total P was determined following acid digestion with 1 M HCl in accordance with Andersen (1976). Digestant was then analyzed colorimetrically on a SEAL AQ2 Automated Discrete Analyzer (Seal Analytical, Mequon, WI) using EPA method 365.1 Rev. 2.0 (USEPA 1993).
Sediment Microbial Properties
DEA, extracellular enzymes, and MBC analyses were all completed within 48 h of sample collection. Microbial biomass C was determined by the fumigation-extraction method after Vance et al. (1987). Briefly, two replicate 3 g samples of the sediment from the homogenized 0–10-cm depth increment were placed in 40-mL centrifuge tubes. One set was fumigated with 0.5 mL of pure chloroform for 24 h, while the other set served as a nonfumigated control. Both sets were extracted with 25 mL 0.5 M K2SO4, placed on an orbital shaker at 150 rpm and 25 °C for 1 h, and centrifuged for 10 min at 4000 rpm and 10 °C. The supernatant was vacuum-filtered through a 0.45-μm membrane filter, acidified with H2SO4 to a pH < 2, and stored at 4 °C. Samples were analyzed for DOC on a Shimadzu TOC-L Analyzer (Shimadzu Scientific Instruments, Kyoto, Japan). MBC was calculated as the difference in DOC between the fumigated samples and the nonfumigated control, divided by the mass of dry soil used. The DOC for the nonfumigated samples was considered extractable DOC.
Sediment DEA was determined on the top 0–10 cm of sediment collected in June using an acetylene inhibition method in accordance with Tiedje (1982) and modified by White and Reddy (1999). Five grams of sediment was placed in a 70-mL glass serum bottle using care to avoid large shell fragments. Bottles were evacuated to − 75 kPa and purged with O2-free N2 gas for 2 min to create anaerobic conditions. Eight milliliters of acetylene gas (C2H2) was added to each bottle while maintaining atmospheric pressure (Yoshinari and Knowles 1976), and bottles were placed on an orbital shaker at 150 rpm and 25 °C for 30 min to distribute the C2H2 gas. After 30 min, 8 mL of DEA solution (56 mg KNO3-N L−1, 288 mg dextrose C L−1, and 2 mg chloramphenicol L−1) was added to create a slight overpressure (Gardner and White 2010). Bottles were continuously shaken at 150 rpm 25 °C, and 1 mL headspace samples extracted at 30, 60, 90, and 120 min for analysis on a Shimadzu 2014 Gas Chromatograph equipped with an electron capture detector (ECD; Shimadzu Scientific Instruments, Kyoto, Japan). Headspace pressure was recorded at each sampling and N2O-N production rate was calculated over time, per kilogram of sediment, with consideration for the portion of gas in the aqueous phase using the Bunsen absorption coefficient (Tiedje 1982).
Extracellular enzyme activity assays were performed on a homogenized 0–3-cm sediment subsample collected in July. Assays utilized fluorescent model substrate 4-methylumbelliferone (MUF) for standardization, and fluorescently labeled (MUF) substrates specific to β-1-4-glucosidase (BG), β-N-acetylglucosaminidase (NAG), alkaline phosphatase (AP) enzymes, as indicators of C, N, and P cycling, respectively (Chrost and Krambeck 1986; Hoppe 1993). Incubation times, substrate concentrations, and analysis gain settings were optimized using enzyme kinetic studies of three different site sediments after German et al. (2011). Maximum potential was determined to be reached in 24 h for all three substrates, as was thus used as the incubation time. All assays were performed and incubated in the dark at 25 °C. Fluorescence was measured at excitation/emission wavelengths 360/460 on a BioTek Synergy HTX (BioTek Instruments, Inc., Winooski, VT, USA) and converted from absolute fluorescence units to moles of active enzymes per gram of dry soil after Bell et al. (2013). Enzyme activity (mol g−1 h−1) was determined as the difference in fluorescent tag liberation between the beginning (time = 0) and the end (time = 24 h) of assay.
Data Analysis
Statistical analysis was performed in SAS 9.4 (SAS Institute, Inc., Cary, NC, USA). All data sets were tested to determine if the assumptions of homogeneity of variance and normality were met using the Brown and Forsythe and Shapiro-Wilk tests, respectively. Where these assumptions were not met, data was transformed and further statistical analysis was conducted using the data that fulfilled the assumptions. Outliers were identified using a modified Thompson Tau. Differences in treatment characteristics and surface water variables among treatments were tested with a one-way ANOVA model. To test for significant differences between treatments among the various biogeochemical data sets (sediment physiochemical properties, nutrient pools, and microbial properties), a two-level nested random effects ANOVA was performed in which reefs (4 random) were nested within treatments (5 fixed). These predictor variables provided the best fit for the data, which was determined via a model selection process based on Akaike information criterion values (AIC). In SAS, both the PROC NESTED and PROC GLM commands were utilized—PROC NESTED was used to partition the variance, while PROC GLM was used to calculate p values if unequal sample sizes resulted from the removal of outliers. A least squares means post hoc test was used to identify significant differences in each pairwise comparison. Pearson’s product correlations were performed to determine correlations between reef characteristics and biogeochemical data and among biogeochemical variables themselves. For all tests, α = 0.05.
Results
Treatment Characteristics
Two key biophysical reef characteristics varied significantly among treatments: the average number of live oysters within a 0.25-m2 plot and the mean thickness of the reef (Fig. 2). In particular, 7-year restored, 4-year restored, and reference reefs had higher live oyster density than 1-year restored, and dead reefs had no live oysters. A similar pattern was observed for reef thickness, with 7-year restored having the highest average thickness, followed by 4-year and reference reefs, all of which were significantly greater than dead reefs.
Differences in the mean number of live oysters (a) and reef thickness (b) among the five treatment categories according to a one-way ANOVA. Error bars indicate standard error; different letters represent significantly different means (p < 0.05) according to a post hoc least squares means pairwise comparison
Eight other physical/geographical characteristics were also evaluated to assess the potential for confounding variables between treatments beyond reef age/condition; only one physical characteristic differed significantly between treatments (i.e., neighboring reef area within 100 m2; Table 1). The aerial coverage of all the reefs studied (n = 20) ranged in size from 44 to 4652 m2 (mean ± standard deviation, 564 ± 1020 m2). Reef area did not vary significantly with treatment but was generally greater for reference reefs, compared to all other reefs (Table 1). The spatial spread of reefs within the dead and 1-year restored treatment categories were generally higher than in the other treatment categories, but the effect was not significant. The mean distance between a reef and the nearest ocean inlet was 18 ± 2 km and did not differ by treatment. Distance from the inlet was negatively correlated with the latitude of the reef (r = − 0.55, p = 0.01) because the nearest inlet for each reef was located to the north of the study area. Latitude also did not vary significantly with treatment, but the variability in latitude was greatest among the 1-year restored and dead reefs, similar to the findings of spatial spread. In contrast, 4-year, 7-year, and reference reefs tended to be located nearer to one another (Fig. 1). The majority of the reefs studied (85%) were patch reefs, with an average of 35 ± 22 m between the edge of the reef and the nearest shoreline. Three (3) of the reefs studied were fringe reefs (located directly adjacent to the shore), and each fringe reef was in a different treatment category. Subsequently, there was not a significant difference in distance from the shore between treatments (Table 1). The width of the channel where the reefs were located varied from 45 to 381 m wide, but did not differ by treatment and was most variable for the 1-year restored reefs. The aerial coverage of other reefs in proximity to the reef of interest (i.e., neighboring reef area) was estimated for a 50- and 100-m radius buffer, indicating that reference reefs were generally located in an area of high reef density, followed by the 7-year restored reefs. Differences among treatments were significant within the 100-m buffer, with reference reefs having a higher density of surrounding reefs than dead reefs (Table 1). Additionally, neighboring reef area was positively correlated with reef area (r = 0.69, p < 0.001 for 50 m buffer; r = 0.80, p < 0.001 for 100 m buffer), indicating that larger reefs (often reference reefs) tended to be located in clusters near other reefs. Furthermore, the clusters of reefs tended to be located on smaller channels, indicated by a negative correlation with channel width (r = − 0.44, p = 0.05).
In terms of surface water chemistry, none of the variables measured showed significant differences among treatments, but some variables did vary slightly between the June and July sampling campaigns. Surface water NO3 − concentrations were below detection (BD) during both the June and July sampling (detection limit = 0.003 mg NO3 − L−1). Ammonium was also BD in June (detection limit = 0.07 mg NH4 + L−1) but averaged 0.11 ± 0.07 mg L−1 in July. Ortho-phosphate ranged from 0.02 ± 0.01 mg L−1 in June to 0.05 ± 0.01 mg L−1 in July, and DOC from 12.9 ± 2.7 to 19.7 ± 1.1 mg L−1 in June and July, respectively. In July, the average DO was 61.4 ± 11.1%, pH was 8.27 ± 0.04, and salinity was 30.3 ± 0.5 ppt (this data was not available for the June sampling).
Effect of Treatment on Biogeochemical Properties
Dead reefs had significantly higher sediment bulk density (1.37 ± 0.2 g cm−3) compared to all other treatment categories (1.09 ± 0.17 g cm−3; Table 1). Mean sediment pH was 8.40 ± 0.14, and although the effect of treatment was not quite significant (p = 0.07), there was a general trend of decreasing sediment pH with increasing reef age. The extractable nutrients of DOC, NH4 +, and PO4 3− all showed significant differences among treatments (Fig. 3; Table 2), with the exception of NO3 −, which was consistently BD (data not shown). In particular, both DOC and NH4 + peaked in concentration in the 1-year restored reefs. The DOC concentrations then decreased slowly as the reef age increased, whereas NH4 + concentrations remained fairly constant from 1-year restored through reference reefs. In contrast, PO4 3− concentrations were highest in 4-year restored reefs and dead reefs. Two of the four total nutrient pools measured (total C and total N) showed a significant treatment effect (Fig. 4; Table 2). Total C content was highest in the 1-year restored reefs and significantly lower in the dead reef sediments than all other treatments (Fig. 4b), while total N content generally increased with reef age, being highest in the 7-year restored and reference reefs and lowest in the dead reefs (Fig. 4c). Total P and organic matter content generally increased incrementally with reef age, but the treatment effect was not significant (Fig. 4a, d). The total biomass of the sediment microbial community, represented by MBC, did not differ significantly with treatment, nor did DEA. Denitrification enzyme activity was extremely low in all sediments (ranging from BD to 15.2 μg N2O-N kg−1) and highly variable between reefs of the same treatment and within a single reef (Table 2). All of the enzyme activity assays (BG, NAG, and AP) showed a significant treatment effect (Table 2). The general patterns were fairly consistent among the three enzymes assayed: the dead reefs generally had the lowest enzyme activity (significantly lower than all other treatments for AP) and the 1-year restored and reference reefs had the highest activity (with 7-year restored equally high for AP).
Changes in mean concentrations of extractable dissolved organic carbon (DOC; black line, primary axis), extractable ammonium (NH4 +; gray line, secondary axis), and extractable phosphate (PO4 3−; dashed line, secondary axis) according to treatment. Error bars indicate standard error; different letters represent significantly different means (p < 0.05) according to a post hoc least squares means pairwise comparison
Differences in mean concentration of sediment organic matter (a), total carbon (b), total nitrogen (c), and total phosphorus (d) according to treatment. Error bars indicate standard error; different letters represent significantly different means (p < 0.05) according to a post hoc least squares means pairwise comparison. Treatment and reef effects were calculated using a two-level nested random effects ANOVA in which reef was nested within treatment
Effect of Reef on Biogeochemical Properties
Several biogeochemical properties were found to vary significantly based on which individual reef the sample was obtained from within the treatment (i.e., a ‘reef effect,’ Table 2). Recognizing that individual reefs within the same treatment were not perfect replicates (Table 1), a posteriori correlational analysis was performed to investigate the relationship between biogeochemical properties on individual reefs and (1) the physical, geographical, and biophysical characteristics of each reef (Table 3) and (2) other biogeochemical properties (Table 4). A significant difference in bulk density among reefs of the same treatment (Table 2) may have been mediated by a negative relationship between reef thickness and bulk density (Table 3). Sediment pH was inversely correlated with reef area, the density of surrounding reefs (100 m), number of live oysters, and reef thickness, as well as all indicators of N and P availability (Table 4). Several biogeochemical variables were positively correlated with reef thickness (i.e., NH4 +, organic matter, total C, N, P, BG, and AP) and/or number of live oysters (i.e., organic matter, total N, P, and AP). Meanwhile, the density of surrounding reefs was correlated to NH4 +, PO4 3−, and total P. Otherwise, none of the other physical or geographical variables quantified for individual reefs were significantly correlated with the observed biogeochemical properties (Table 3).
Extractable DOC and NH4 + concentrations were positively correlated with other indicators of organic matter content in the sediment, such as total organic matter, C, N, and enzymes involved in C and N cycling (for DOC only; Table 4). Meanwhile, PO4 3− showed a negative correlation with AP and a positive correlation with sediment pH. All measured variables for sediment nutrients and microbial properties demonstrated either a significant (p < 0.001 to p ≤ 0.05), or weak (p > 0.05 to p < 0.1), positive correlation with sediment organic matter content, signifying the comprehensive importance of this variable. There was also a negative correlation between pH and organic matter, total N, and P (Table 4). None of the physical reef characteristics measured could explain the variability in DEA, but there was a significant positive correlation with C variables (DOC, total C, BG), organic matter content, and total N (Table 4). All enzyme activities were strongly correlated with one another and with N availability (both extractable NH4 + and total N). Furthermore, BG was positively correlated with DOC, and AP negatively correlated with PO4 3− (Table 4).
Discussion
Interest and involvement in oyster reef restoration is currently at an all-time high, with a growing number of projects focused on the reclamation of ecosystem services provided by these reefs, beyond just harvestable populations for human consumption (Coen et al. 2007; La Peyre et al. 2014; Guo et al. 2016). Despite these ecologically focused goals, few studies have attempted to quantify the role of intertidal oyster reefs in supporting biogeochemical indicators and functions within the coastal zone, nor have the monitoring efforts of past restorations adequately measured ecological impact from the standpoint of coastal sediment biogeochemistry. By leveraging sites from one of the longest running (10 years), highly successful, community-based oyster reef restoration efforts in the USA with annual monitoring, this study demonstrates that intertidal oyster reefs (both restored and reference) do support a variety of biogeochemical properties not seen on dead/degraded reefs and that several biogeochemical properties increase rapidly (within 1 year) following successful restoration efforts.
Sediment Physiochemical Properties
Sediment bulk density results indicate that the physical properties of dead reef sediments are significantly different than live reefs (restored and reference). Generally, bulk density is inversely correlated with organic matter content (e.g., Chambers et al. 2013), but the lack of a strong correlation between bulk density and organic matter in this study (p = 0.10) indicates that it is not the only contributing factor. Particle size analysis was not performed on these sediments, but observations indicated a higher percentage of sands at dead reefs and silts/clays beneath oyster reefs, which is consistent with the concept that the vertical structure of live reefs increases surface roughness, eddy formation, and turbulence, enhancing the trapping of fine sediments, relative to areas devoid of live reefs (Wildish and Kristmanson 1997; Styles 2015). The negative correlation between sediment bulk density and reef thickness provides further evidence of the importance of reef structure in altering local hydrodynamics and sedimentation patterns (Dame et al. 1984; Lenihan 1999).
Sediment pH showed a general trend of decreasing with reef age, being highest in dead reef sediments. The process of calcification for shell formation can result in a localized decrease in alkalinity as CO2 is produced (Frankignoulle et al. 1995; Waldbusser et al. 2013), a plausible explanation for the observed pattern when also considering the negative relationship between pH and reef area, surrounding reef density, and reef thickness. Furthermore, the negative relationship between sediment pH, organic matter content, and indicators of microbial activity (e.g., NAG and AP) suggests that CO2 and organic acids produced during microbial decomposition and respiration may also play a role in reducing sediment pH at older reefs. Meanwhile, the dissolution of shells at dead reefs appears to raise the sediment pH above that of the surrounding surface water (Waldbusser et al. 2011).
Sediment Nutrient Pools
Extractable nutrient data represents the DOC, NH4 +, and PO4 3− within the sediment porewater and that which is adsorbed to the cation exchange complex. These nutrient pools are considered to be readily available for uptake and assimilation (Reddy and DeLaune 2008) and may contribute to the flux, or regeneration of the nutrients sequestered during filter feeding, back into the water column. In this study, both extractable DOC and NH4 + increased significantly under live oyster reefs of all ages, relative to dead reefs (Fig. 3). Previous studies have found small fluxes of DOC from oyster beds, which are presumed to be a by-product of oyster metabolism or created during the degradation of sediment organic matter (Dame et al. 1991). Extractable DOC concentrations were strongly correlated with sediment organic matter content, total C and N, and the activity of BG and NAG, indicating the role of extracellular enzymes in breaking down organic substrates to produce available DOC. The positive flux of NH4 + from oyster reefs is well established in the literature, is often noted to be highest in the summer, and occurs independent of tidal cycle or water velocity (Dame et al. 1984, 1985, 1992; Kellogg et al. 2013). Studies agree that the vast majority of regenerated N from oysters is in the form of NH4 + due to the anoxic nature of marine sediments (Newell et al. 2005). Some suggest that the significant release of benthic NH4 + may even accelerate primary production, particularly in shellfish aquaculture systems, potentially offsetting some of the water quality benefits of phytoplankton uptake by shellfish beds (Asmus and Asmus 1991; Nizzoli et al. 2005; Murphy et al. 2015). Although previous studies typically focused on quantifying the mass balance of N-species within the water column as it flows over a reef or fluxes into the water, it makes sense that extractable NH4 + is also high in the sediment, creating a diffusion gradient that drives the flux across the sediment-water interface into more oxygenated surface water (Newell et al. 2005). The source of NH4 + is considered to be both metabolic by-products and a product of enhanced N cycling in the sediment (Kellogg et al. 2013). Extractable PO4 3− also demonstrated a significant treatment effect but did not follow the same dead vs. live reef pattern observed for extractable DOC and NH4 +. Instead, extractable PO4 3− concentrations peaked in 4-year restored and dead reefs and were lowest in older reefs (7-year restored and reference reefs). Few studies have investigated P-cycling dynamics in oyster reefs and the findings have been inconsistent. For example, an in situ mass balance study on a natural reef found no change in surface water PO4 3− concentrations as water flowed over the reef (Dame et al. 1984), while a flux study on a restored reef found a release of PO4 3− from the benthos that varied seasonally (Kellogg et al. 2013). In this study, the pattern of extractable PO4 3− may be explained by a significant positive relationship with pH and a negative relationship with AP. The correlation with pH could indicate that P availability is tied to iron (Fe) reduction in this system, as higher pH can promote the release of P from Fe compounds (Krom and Berner 1981; Huang et al. 2005). However, redox condition (oxygen availability) is also a key factor in determining P release, which was not measured in this study. Moreover, additional AP is synthesized when there is a strong demand for limited PO4 3− (e.g., Chrost 1991), thus mediating the relationship between PO4 3− availability and other potential environmental drivers, such as the treatment effects. Extractable NO3 − data was not presented because concentrations were consistently BD. This is expected considering NO3 − is the oxidized form of inorganic N and most marine sediments are anoxic below the top few centimeters (Newell et al. 2005). Additionally, a brown tide bloom (Aureombra lagunensis) persisted from February through October 2016 within the study region; our sampling occurred during this time (June and July 2016; E. Phlips, pers. comm.). The bloom may have assimilated most of the dissolved inorganic N within biomass, also explaining the low surface water NO3 − and NH4 + concentrations observed during sampling.
Total nutrient burial represents a longer-term storage reservoir and can occur in the sediments beneath oyster reefs when the excretion of biodeposits and the accumulation of organic and inorganic materials through sedimentation exceed the loss of nutrients through resuspension or assimilation by mobile organisms. Several ecologically important total nutrient pools showed a significant increase in the sediments when comparing dead reefs to 1-year restored reefs: organic matter content increased 164% after 1 year of restoration, total C increased by 236%, and total N increased by 260%. In other aquatic systems, such as wetland soils, total nutrient pools generally accumulate linearly over time as new deposits bury older deposits and the substrate accretes vertically (Reddy and DeLaune 2008). This does not appear to be the case in oyster reef sediments, as demonstrated by the lack of a linear increase in total nutrient pools over time (Fig. 4). Rather, the presence of a live reef results in a dramatic and rapid increase in sedimentary nutrient pools, followed by a dynamic equilibrium in which concentrations remain fairly constant as the reef continues to age. Therefore, although continuous oyster metabolism does consolidate biodeposits and the bed roughness created by the physical structure of the reef enhances particulate trapping (Newell et al. 2005; Styles 2015), there must also be a substantial degree of erosion and resuspension under high velocity conditions, coupled with organic matter mineralization, that prevents successive sedimentary nutrient accumulation. Although the treatment effect on total P was not significant, P did demonstrate the closest approximation of the linear accumulation in the sediment over time, presumably due to the high affinity for P to occlude with other minerals and the lack of a gaseous phase to promote the permanent removal of P from the system (Reddy and DeLaune 2008).
Kellogg et al. (2013) also found an order of magnitude increase in the concentration of sediment total C, N, and P under a restored subtidal reef (~ 6 years old) relative to an unrestored site, but did not investigate accumulation over time/reef age. Otherwise, previous studies investigating the role of oyster reefs on nutrients have focused on denitrification and flux rates in/out of the sediment (e.g., Kellogg et al. 2013, 2014; Smyth et al. 2013; Lindemann et al. 2016), biodeposit chemistry (e.g., Dalrymple and Carmichael 2015; Hoellein et al. 2015), or harvesting as a mechanism to remove the C, N, and P assimilated in oyster tissues and shells (e.g., Higgins et al. 2011); very little work has focused on total nutrient pools in the benthic sediments associated with oyster reefs.
Sediment Microbial Properties
Heterotrophic sediment microbial communities process organic substrates and associated nutrients for metabolism and subsequently promote nutrient cycling and organic matter mineralization, as well as serve as a potential food source for benthic organisms. This study investigated both the size of the microbial community (MBC) and their activity (DEA and extracellular enzyme assays). According to the results, the size of the microbial community (MBC) in the sediments was not affected by treatment, but did tend to be greater on individual reefs with greater substrate availability (organic matter and total C content). Overall, the concentrations of MBC observed in the benthic oyster reef sediments (775 ± 490 g kg−1) are only slightly below those found in nearby coastal wetland soils (e.g., 1298 ± 869 g kg−1; Chambers et al. 2013), despite the fact that these coastal wetland soils averaged 40% organic matter, compared to only ~ 10% organic matter in the benthic oyster reef sediments. The lack of a significant treatment effect is not overly surprising considering that microbial community size can be a poor indicator of microbial function due to functional redundancy and the ability of some microbes to remain present in a dormant state for extended periods of time (e.g., Nannipieri et al. 2003). However, it is still a useful indicator of substrate quality and turnover rate (e.g., DeBusk and Reddy 1998; White and Reddy 2000).
Denitrification enzyme activity was extremely low in all samples, which was likely attributable to low NO3 − availability during our sampling. Surface water samples collected in conjunction with sediments used for DEA analysis had NO3 − concentrations below detection. The low NO3 − availability may have been associated with the presence of brown tide during that time, as the NO3 − in the water column could have already been sequestered and assimilated by bloom concentrations of phytoplankton. As DEA is an indicator of the amount of NO3 − loading to the system, low NO3 − concentrations will dramatically limit denitrifying enzyme synthesis (White and Reddy 1999; Gardner and White 2010). Although DEA is not a direct measurement of in situ denitrification, under anaerobic, C-rich conditions, DEA has been shown to reflect the availability of NO3 − for soil microbes and the potential rate at which NO3 − is reduced at the time of sampling (Groffman and Tiedje 1989; Gardner and White 2010). In this study, no difference in DEA was found between treatments, but reef sediments with high C and N (total and extractable) did tend to have higher DEA. For comparison, DEA rates in freshwater streams and wetlands are approximately two orders of magnitude higher than those found in this study (e.g., Hale and Groffman 2006; Gardner and White 2010). Previous studies focusing on denitrification on/near oyster reefs have used significantly different quantification methods, including direct measurements of in situ denitrification (e.g., the production of N2 gas using membrane inlet mass spectrometry and/or labeled 15N) and gene expression, and are therefore difficult to compare to the findings for DEA in this study. Most oyster reef studies do indicate that denitrification is enhanced by the presence of live oysters, but the effect can vary significantly both spatially and temporally (Newell et al. 2002; Piehler and Smyth 2011; Kellogg et al. 2013, 2014; Pollack et al. 2013; Smyth et al. 2013; Lindemann et al. 2016). Additionally, the influence of reef age, density of live oysters, reef thickness, or natural vs. restored reefs on DEA had not been investigated prior to this study. Based on these previous findings, we anticipate a treatment effect would be evident (at least when comparing live vs. dead reefs) if using direct denitrification measurement methods, and/or if the DEA measurements were repeated over several seasons, including when NO3 − availability was greater.
The presence of live oysters strongly influenced the production of extracellular enzymes in benthic sediments, with activities on dead reefs being significantly lower than all (AP) or most (BG and NAG) other treatments (Table 4). Both BG and NAG are considered constitutive enzymes and are synthesized in proportion to the rate of C metabolism (BG) and N metabolism (NAG), whereas AP synthesis is induced by a limitation in extractable P (Chrost 1991; Leschine 1995; Makoi and Ndakidemi 2008). This concept is exemplified by the positive correlations between BG and extractable DOC, and NAG and extractable NH4+, and the negative correlation between AP and extractable PO4 3−. The higher enzyme activities under live reefs indicate more active C, N, and P cycling in these sediments.
Experimental Design Considerations
The 20 intertidal reefs chosen for this study were considered prototypical examples of the age class or condition they represented based on annual monitoring data (Walters 2016). They also represent a very long-running, well-monitored, and extensive intertidal oyster reef restoration effort in the USA, all of which were implemented by the same group using the same methods. Although only one physical reef characteristic varied significantly with treatment (i.e., density of neighboring reefs within 100 m), the amount of variance among reefs of the same treatment differed substantially by treatment for several variables. As with many restoration projects, efforts began with a focus on the sites in the most degraded state. Therefore, early restored sites (e.g., 7-year restored) were clustered within a core area in close proximity to natural reference reefs where natural recruitment was observed (L.J. Walters, pers. comm.). Over the years, as more and more reefs were restored, the footprint of the effort expanded outward to the north and south, encompassing a greater variety of sites over a larger geographic area. Despite these intrinsic differences among treatment categories, the variables of greatest significance when assessing restoration success (the number of live oysters and the thickness of the reef) showed a clear linear increase with years since restoration (Fig. 2), indicating the appropriateness of these reefs in assessing the impacts of reef age on biogeochemical properties. Interestingly, reference reefs averaged fewer live oysters and reduced reef thickness relative to the oldest restored sites. This is likely explained by the fact that both commercial and recreational oyster harvesting activities are permitted on natural reefs in the region, but are prohibited on the restoration sites, as they are designated by the National Park Service as protected research sites. Harvesters not only reduce live biomass but also typically target the tallest oysters or clusters protruding above the benthos, reducing the overall thickness of the reef. According to our results, both the number of live oysters and reef thickness were key variables for explaining the variance among several biogeochemical variables that could not be directly attributed to a treatment effect (i.e., age class). Higher densities of oysters mean greater production of biodeposits that can aggregate and settle on reef sediments (Newell et al. 2005), while increasing reef thickness results in greater bed roughness to enhance and preserve sediment deposition (Wildish and Kristmanson 1997; Styles 2015). Therefore, the dramatic increases in biogeochemical properties observed in this study after only 1-year postrestoration are strongly related to the year 1 density of live oysters (53.0 ± 30.6 per 0.25 m2) and reef thickness (109.2 ± 19.5 mm) observed in the warm, shallow waters of Mosquito Lagoon, FL. A similar oyster density and reef thickness will make these results most transferable to other oyster reef restoration projects.
Conclusions
This study represents the first attempt to provide a comprehensive quantification of a variety of biogeochemical properties on dead, restored, and natural intertidal oyster reefs (C. virginica) to better understand the biogeochemical function of benthic sediments beneath oyster reefs and assess the impact of reef age (i.e., years since restoration) on sediment biogeochemistry. Findings indicate that the physical, chemical, and microbial properties of benthic sediments differed significantly between dead and live reefs, regardless of the age of the reef, or whether it is a restored or natural reef. Specifically, the presence of live oyster reefs significantly decreased sediment bulk density and increased the concentrations of extractable DOC and NH4 +, total C and N, and the activity of major extracellular enzymes involved in C, N, and P cycling in the sediments, relative to dead reef sediments. Interestingly, time since restoration/reef age did not appear to be a significant factor in determining the magnitude of biogeochemical properties for any variables of interest. Rather, a rapid increase in sediment nutrient pools and microbial activity was observed 1 year after restoration (coinciding with a live oyster density and reef thickness of 53.0 ± 30.6 per 0.25 m2 and 109.2 ± 19.5 mm, respectively) and typically did not change significantly after that first year, nor did it differ significantly from natural reference reefs. In addition to finding several biogeochemical variables that changed with reef treatment, there was also significant variability observed between reefs of the same treatment. Correlational analysis suggests that this reef-to-reef variability may be related to slight differences in the number of live oysters, the thickness of the reef, and/or the availability of key nutrients in the sediment (e.g., extractable and total pools of C and N). Additional experimental research is warranted to better understand the specific factors influencing biogeochemical properties beyond reef age.
Overall, the findings of this study support the idea that live reefs may be considered ‘biogeochemical hot spots’—areas of sediment with disproportionately higher biogeochemical reaction rates than the surrounding sediments (McClain et al. 2003). Furthermore, there is strong evidence that the successful restoration of dead oyster reefs can rapidly (within 1 year) restore biogeochemical properties within the associated benthic sediments, resulting in significant increases in sedimentary nutrient availability, nutrient burial, and microbially mediated nutrient cycling. This data can help oyster reef restoration practitioners better evaluate the ecosystem services provided by their projects and the time scale on which ecological impacts are realized.
References
Andersen, J.M. 1976. An ignition method for determination of total phosphorus in lake sediments. Water Research 10: 329–331.
Asmus, R.M., and H. Asmus. 1991. Mussel beds: Limiting or promoting phytoplankton? Journal of Experimental Marine Biology and Ecology 148: 215–232.
Beck, Michael W., Robert D. Brumbaugh, Laura Airoldi, Alvar Carranza, Loren D. Coen, Christine Crawford, Omar Defeo, et al. 2011. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61: 107–116. https://doi.org/10.1525/bio.2011.61.2.5.
Bell, Colin W., Barbara E. Fricks, Jennifer D. Rocca, Jessica M. Steinweg, Shawna K. McMahon, and Matthew D. Wallenstein. 2013. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. Journal of Visualized Experiments. https://doi.org/10.3791/50961.
Birch, A., and L. Walters. 2012. Restoring intertidal oyster reefs in Mosquito Lagoon: the evolution of a successful model. TNC/NOAA Community-Based Restoration Partnership Program. 70 pp.
Campbell, D. 2015. Quantifying the effects of boat wakes on intertidal oyster reefs in a shallow estuary. Orlando: University of Central Florida.
Chambers, L.G., T.Z. Osborne, and K.R. Reddy. 2013. Effect of salinity pulsing events on soil organic carbon loss across an intertidal wetland gradient: A laboratory experiment. Biogeochemistry 115: 363–383. https://doi.org/10.1007/s10533-013-9841-5.
Chrost, R.J. 1991. Environmental control of the synthesis and activity of aquatic microbial ectoenzymes. In Microbial enzymes in aquatic environments, ed. R.J. Chrost, 29–59. New York: Springer.
Chrost, R.J., and H.J. Krambeck. 1986. Fluorescence correction for measurements of enzyme-activity in natural-waters using methylumbelliferyl substrates. Archiv Fur Hydrobiologie 106: 79–90.
Coen, Loren D., Robert D. Brumbaugh, David Bushek, Ray Grizzle, Mark W. Luckenbach, Martin H. Posey, Sean P. Powers, and S. Gregory Tolley. 2007. Ecosystem services related to oyster restoration. Marine Ecology Progress Series 341: 303–307. https://doi.org/10.3354/meps341299.
Cressman, K.A., M.H. Posey, M.A. Mallin, L.A. Leonard, and T.D. Alphin. 2003. Effects of oyster reefs on water quality in a tidal creek estuary. Journal of Shellfish Research 22: 753–762.
Dalrymple, D. Joseph, and Ruth H. Carmichael. 2015. Effects of age class on N removal capacity of oysters and implications for bioremediation. Marine Ecology Progress Series 528: 205–220. https://doi.org/10.3354/meps11252.
Dame, Richard F. 1999. Oyster reefs as components of estuarine nutrient cycling: inceidental or controlling? In Oyster reef habitat restoration: a synopsis and synthesis of approaches, ed. Mark W. Luckenbach, Roger Mann, and James A. Wesson, 267–280. Williamsburg: W&M Publish. https://doi.org/10.21220/V5NK51.
Dame, Richard F., Richard G. Zingmark, and Elizabeth Haskin. 1984. Oyster reefs as processors of estuarine materials. Journal of Experimental Marine Biology and Ecology 83: 239–247.
Dame, Richard F., T.G. Wolaver, and S.M. Libes. 1985. The summer uptake and release of nitrogen by an intertidal oyster reef. Netherlands Journal of Sea Research 19: 265–268. https://doi.org/10.1016/0077-7579(85)90032-8.
Dame, Richard F., Norbert Dankers, Theo Prins, Henk Jongsma, and Aad Smaal. 1991. The influence of mussel beds on nutrients in the western Wadden Sea and eastern Scheldt estuaries. Estuaries 14: 130–138. https://doi.org/10.1007/BF02689345.
Dame, Richard F., John D. Spurrier, and Richard G. Zingmark. 1992. In situ metabolism of an oyster reef. Journal of Experimental Marine Biology and Ecology 164: 147–159. https://doi.org/10.1016/0022-0981(92)90171-6.
DeBusk, W.F., and K.R. Reddy. 1998. Turnover of detrital organic carbon in a nutrient-impacted Everglades marsh. Soil Science Society of America Journal 62: 1460–1468. https://doi.org/10.2136/sssaj1998.03615995006200050045x.
DeForest, Jared L. 2009. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biology and Biochemistry 41: 1180–1186. https://doi.org/10.1016/j.soilbio.2009.02.029.
Dybas, C.L. 2002. Florida’s Indian River lagoon: An estuary in transition. Bioscience 52: 554–559.
Frankignoulle, M, M. Pichon, and J-P. Gattuso. 1995. Aquatic calcification as a source of carbon dioxide. In Carbon sequestration in the biosphere, ed. Max A. Beran, 265–271. Berlin Heidelberg: Springer-Verlag.
Gardner, L.M., and J.R. White. 2010. Denitrification enzyme activity as an indicator of nitrate movement through a diversion wetland. Soil Science Society of America Journal 74: 1037–1047. https://doi.org/10.2136/sssaj2008.0354.
Garvis, Stephanie K., Paul E. Sacks, and Linda J. Walters. 2015. Formation, movement, and restoration of dead intertidal oyster reefs in Canaveral National Seashore and Mosquito Lagoon, Florida. Journal of Shellfish Research 34: 251–258. https://doi.org/10.2983/035.034.0206.
German, Donovan P., Michael N. Weintraub, A. Stuart Grandy, Christian L. Lauber, Zachary L. Rinkes, and Steven D. Allison. 2011. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biology and Biochemistry 43: 1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017.
Grabowski, Jonathan H., Robert D. Brumbaugh, Robert F. Conrad, Andrew G. Keeler, J. Opaluch, Charles H. Peterson, Michael F. Piehler, Sean P. Powers, and Ashley R. Smyth. 2012. Economic valuation of ecosystem services provided by oyster reefs. Bioscience 62: 900–909. https://doi.org/10.1525/bio.2012.62.10.10.
Grizzle, R.E., J.R. Adams, and L.J. Walters. 2002. Historical changes in intertidal oyster (Crassostrea virginica) reefs in a Florida lagoon potentially related to boating activities. Journal of Shellfish Research 21: 749–756.
Groffman, Peter M., and James M. Tiedje. 1989. Denitrification in north temperate forest soils: Relationships between denitrification and environmental factors at the landscape scale. Soil Biology and Biochemistry 21: 621–626. https://doi.org/10.1016/0038-0717(89)90054-0.
Guo, Lin, Fei Xu, Zhigang Feng, and Guofan Zhang. 2016. A bibliometric analysis of oyster research from 1991 to 2014. Aquaculture International 24: 327–344. https://doi.org/10.1007/s10499-015-9928-1.
Hale, R.L., and P.M. Groffman. 2006. Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. Journal of Environmental Quality 35: 2425–2432. https://doi.org/10.2134/jeq2006.0164.
Higgins, Colleen B., Kurt Stephenson, and Bonnie L. Brown. 2011. Nutrient bioassimilation capacity of aquacultured oysters: Quantification of an ecosystem service. Journal of Environment Quality 40: 271. https://doi.org/10.2134/jeq2010.0203.
Higgins, Colleen B., Craig Tobias, Michael F. Piehler, Ashley R. Smyth, Richard F. Dame, Kurt Stephenson, and Bonnie L. Brown. 2013. Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Marine Ecology Progress Series 473: 7–27. https://doi.org/10.3354/meps10062.
Hoellein, Timothy J., Chester B. Zarnoch, and Raymond E. Grizzle. 2015. Eastern oyster (Crassostrea virginica) filtration, biodeposition, and sediment nitrogen cycling at two oyster reefs with contrasting water quality in Great Bay Estuary (New Hampshire, USA). Biogeochemistry 122: 113–129. https://doi.org/10.1007/s10533-014-0034-7.
Hoppe, Hans-Georg. 1993. Use of fluorogenic model substrates for extracellular enzyme activity (EEA) measurement of bacteria. In Handbook of methods in aquatic microbial ecology, eds. Paul F. Kemp, Barry F. Sherr, Evelyn B. Sherr, and Jonathan J. Cole, 423–431. Boca Raton: CRC Press LLC.
Huang, Qinghui, Zijian Wang, Chunxia Wang, Shengrui Wang, and Xiangcan Jin. 2005. Phosphorus release in response to pH variation in the lake sediments with different ratios of iron-bound P to calcium-bound P. Chemical Speciation and Bioavailability 17: 55–61. https://doi.org/10.3184/095422905782774937.
Kellogg, M. Lisa, Jeffrey C. Cornwell, Michael S. Owens, and Kennedy T. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series 480: 1–19. https://doi.org/10.3354/meps10331.
Kellogg, M. Lisa, Ashley R. Smyth, Mark W. Luckenbach, Ruth H. Carmichael, Bonnie L. Brown, Jeffrey C. Cornwell, Michael F. Piehler, Michael S. Owens, D. Joseph Dalrymple, and Colleen B. Higgins. 2014. Use of oysters to mitigate eutrophication in coastal waters. Estuarine, Coastal and Shelf Science 151: 156–168. https://doi.org/10.1016/j.ecss.2014.09.025.
Krom, M.D., and R.A. Berner. 1981. The diagenesis of phosphorus in a nearshore marine sediment. Geochimica et Cosmochimica Acta 45: 207–216.
Laing, I., P. Walker, and F. Areal. 2006. Return of the native - is European oyster (Ostrea edulis) stock restoration in the UK feasible? Aquatic Living Resources 19: 283–287. https://doi.org/10.1051/Alr:2006029.
Leffler, Merrill and Pauli Hayes. 2004. Oyster research and restoration in U.S. coastal waters: research priorities and strategies. www.mdsg.umd.edu/sites/default/files/files/store/oysterrestoration_summary.pdf. Accessed 23 Aug 2017.
Lenihan, Hunter S. 1999. Physical-biological coupling on oyster reefs: How habitat structure influences individual performance. Ecological Monographs 69: 251–275.
Leschine, S.B. 1995. Cellulose degradation in anaerobic environments. Annual Review of Microbiology 49: 399–426. https://doi.org/10.1146/annurev.micro.49.1.399.
Lindemann, Samantha, Chester B. Zarnoch, Domenic Castignetti, and Timothy J. Hoellein. 2016. Effect of eastern oysters (Crassostrea virginica) and seasonality on nitrite reductase gene abundance (nirS, nirK, nrfA) in an urban estuary. Estuaries and Coasts 39: 218–232. https://doi.org/10.1007/s12237-015-9989-4.
Makoi, Jhjr, and P.A. Ndakidemi. 2008. Selected soil enzymes: Examples of their potential roles in the ecosystem. African Journal of Biotechnology 7: 181–191.
McClain, Michael E., Elizabeth W. Boyer, C. Lisa Dent, Sarah E. Gergel, Nancy B. Grimm, Peter M. Groffman, Stephen C. Hart, et al. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6: 301–312. https://doi.org/10.1007/s10021-003-0161-9.
Meyer, David L., Edward C. Townsend, and Gordon W. Thayer. 1997. Stabilization and erosion control value of oyster cultch for intertidal marsh. Restoration Ecology 5: 93–99. https://doi.org/10.1046/j.1526-100X.1997.09710.x.
Mortazavi, Behzad, Alice C. Ortmann, Lei Wang, Rebecca J. Bernard, Christina L. Staudhammer, J. Donald Dalrymple, Ruth H. Carmichael, and Alice A. Kleinhuizen. 2015. Evaluating the impact of oyster (Crassostrea virginica) gardening on sediment nitrogen cycling in a subtropical estuary. Bulletin of Marine Science 91: 323–341. https://doi.org/10.5343/bms.2014.1060.
Murphy, Anna E., Iris C. Anderson, and Mark W. Luckenbach. 2015. Enhanced nutrient regeneration at commercial hard clam (Mercenaria mercenaria) beds and the role of macroalgae. Marine Ecology Progress Series 530: 135–151. https://doi.org/10.3354/meps11301.
Nannipieri, P., J. Ascher, M.T. Ceccherini, L. Landi, G. Pietramellara, and G. Renella. 2003. Microbial diversity and soil functions. European Journal of Soil Science 54: 655–670. https://doi.org/10.1046/j.1351-0754.2003.0556.x.
Newell, R.I.E., Jeffrey C. Cornwell, and Michael S. Owens. 2002. Influence of simulated bivalve biodeposition and microphytobenthos on sediment nitrogen dynamics: A laboratory study. Limnology and Oceanography 47: 1367–1379. https://doi.org/10.4319/lo.2002.47.5.1367.
Newell, R.I.E, T.R. Fisher, R.R. Holyoke, and J.C. Cornwell. 2005. Influence of eastern oysters on nitrogen and phosphorus regeneration in Chesapeake Bay, USA. In The comparative roles of suspension feeders in ecosystems, ed. R. Dame and S. Olenin, 93–120. Dordrecht: Springer pp. 93–120.
Nizzoli, Daniele, David T. Welsh, Marco Bartoli, and Pierluigi Viaroli. 2005. Impacts of mussel (Mytilus galloprovincialis) farming on oxygen consumption and nutrient recycling in a eutrophic coastal lagoon. Hydrobiologia 550: 183–198. https://doi.org/10.1007/s10750-005-4378-9.
Peterson, Charles H., Jonathan H. Grabowski, and Sean P. Powers. 2003. Estimated enhancement of fish production resulting from restoring oyster reef habitat: Quantitative valuation. Marine Ecology Progress Series 264: 249–264. https://doi.org/10.3354/meps264249.
La Peyre, Megan, Jessica Furlong, Laura A. Brown, Bryan P. Piazza, and Ken Brown. 2014. Oyster reef restoration in the northern Gulf of Mexico: Extent, methods and outcomes. Ocean and Coastal Management 89: 20–28. https://doi.org/10.1016/j.ocecoaman.2013.12.002.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere. https://doi.org/10.1890/ES10-00082.1.
Plutchak, Rochelle, Kelly Major, Cebrian Just, C. Drew Foster, Mary Elizabeth C. Miller, Andrea Anton, Kate L. Sheehan, Kenneth L. Heck, and Sean P. Powers. 2010. Impacts of oyster reef restoration on primary productivity and nutrient dynamics in tidal creeks of the north Central Gulf of Mexico. Estuaries and Coasts 33: 1355–1364. https://doi.org/10.1007/s12237-010-9327-9.
Pollack, Jennifer, David Yoskowitz, Hae Cheol Kim, and Paul A. Montagna. 2013. Role and value of nitrogen regulation provided by oysters (Crassostrea virginica) in the Mission-Aransas Estuary, Texas, USA. PloS One 8: 6–13. https://doi.org/10.1371/journal.pone.0065314.
Reddy, K.R., and R. DeLaune. 2008. Biogeochemistry of wetlands: Science and applications. New York: CRC.
Smyth, Ashley R., Suzanne P. Thompson, Kaylyn N. Siporin, Wayne S. Gardner, Mark J. McCarthy, and Michael F. Piehler. 2013. Assessing nitrogen dynamics throughout the estuarine landscape. Estuaries and Coasts 36: 44–55. https://doi.org/10.1007/s12237-012-9554-3.
St. Johns River Water Management District. 2016. The Indian River Lagoon: An estuary of national significance. http://www.sjrwmd.com/indianriverlagoon/. Accessed 26 Oct 2016.
Stenberg, B., M. Johansson, M. Pell, K. Sjodahl-Svensson, J. Stenstrom, and L. Torstensson. 1998. Microbial biomass and activities in soil as affected by frozen and cold storage. Soil Biology & Biochemistry 30: 393–402. https://doi.org/10.1016/s0038-0717(97)00125-9.
Styles, Richard. 2015. Flow and turbulence over an oyster reef. Journal of Coastal Research 31: 978–985. https://doi.org/10.2112/JCOASTRES-D-14-00115.1.
Tiedje, J.M. 1982. Denitrification. In Methods of soil analysis. Part 2, ed. A.L. Page, 1011–1026. Madison: ASA-SSSA.
USEPA. 1993. Methods for the determination of inorganic substances in environmental samples, EPA/600/R-93/100. Washington: U.S. Environmental Protection Agency.
Vance, E.D.D., P.C.C. Brookes, and D.S.S. Jenkinson. 1987. An extraction method for measuring soil microbial biomass-C. Soil Biology & Biochemistry 19: 703–707. https://doi.org/10.1016/0038-0717(87)90052-6.
Waldbusser, George G., Ryan A. Steenson, and Mark A. Green. 2011. Oyster shell dissolution rates in estuarine waters: Effects of pH and shell legacy. Journal of Shellfish Research 30: 659–669. https://doi.org/10.2983/035.030.0308.
Waldbusser, George G., Eric N. Powell, and Roger Mann. 2013. Ecosystem effects of shell aggregations and cycling in coastal waters: An example of Chesapeake Bay oyster reefs. Ecology 94: 895–903. https://doi.org/10.1890/12-1179.1.
Wall, L.M., Linda J. Walters, R.E. Grizzle, and P.E. Sacks. 2005. Recreational boating activity and its impact on the recruitment and survival of the oyster Crassostrea virginica on intertidal reefs in Mosquito Lagoon, Florida. Journal of Shellfish Research 24: 965–973. https://doi.org/10.2983/0730-8000(2005)24.
Walters, Linda J. 2016. Oyster reef deployment and monitoring: Final technical report. Indian River Lagoon National Estuary Program, 25 pp.
White, J.R., and K.R. Reddy. 1999. Influence of nitrate and phosphorus loading on denitrifying enzyme activity in Everglades wetland soils. Soil Science Society of America Journal 63: 1945. https://doi.org/10.2136/sssaj1999.6361945x.
White, J.R., and K.R.R. Reddy. 2000. Influence of phosphorus loading on organic nitrogen mineralization of Everglades soils. Soil Science Society of America Journal 64: 1525. https://doi.org/10.2136/sssaj2000.6441525x.
Wilberg, Michael J., Maude E. Livings, Jennifer S. Barkman, Brian T. Morris, and Jason M. Robinson. 2011. Overfishing, disease, habitat loss, and potential extirpation of oysters in upper Chesapeake Bay. Marine Ecology Progress Series 436: 131–144. https://doi.org/10.3354/meps09161.
Wildish, D.J., and D.D. Kristmanson. 1997. Benthic suspension feeders and flow. Cambridge: Cambridge University Press.
Yoshinari, Tadashi, and Roger Knowles. 1976. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochemical and Biophysical Research Communications 69: 705–710. https://doi.org/10.1016/0006-291X(76)90932-3.
Acknowledgements
The authors would like to thank Lacie Anderson, Phyllis Klarmann, Meagan Mindalie, Jaice Metherall, John Heiland, and Janet Ho for assistance with field sampling, as well as the cooperation of Canaveral National Seashore and the St. Johns Water Management District in the completion of this study. This work was supported by the Indian River Lagoon National Estuarine Program and the National Science Foundation, under the Coupled Natural-Human Systems program, award #1617374.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marco Bartoli
Rights and permissions
About this article
Cite this article
Chambers, L.G., Gaspar, S.A., Pilato, C.J. et al. How Well Do Restored Intertidal Oyster Reefs Support Key Biogeochemical Properties in a Coastal Lagoon?. Estuaries and Coasts 41, 784–799 (2018). https://doi.org/10.1007/s12237-017-0311-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0311-5