Abstract
Thermal modification can improve the dimensional stability of wood without the use of biocides, but the process also changes the colour. Changes in relative amounts of chromophores and auxochromes of poplar extracts during thermal modification were investigated by pre-extraction of ground wood in increasingly polar solvents followed by thermal modification. Changes in wood colour and FTIR spectra were used to assess the effects of extraction/thermal modification, while selected extracts were analysed by GC–MS. Colour of non-modified poplar was mainly affected by ethanol extraction, while thermally modified poplar was most affected by 1,4-dioxane and acetone extraction. GC–MS analysis to characterize the main chromophores and auxochromes in the 1,4-dioxane and acetone extracts from non-modified or thermally modified poplar suggested that chromophores and/or auxochromes in extracts including carbonyl, vinyl, benzene ring and hydroxyl groups tended to be much more abundant in thermally modified poplar which accounts for the darkening of the wood.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood is an environmentally attractive renewable material, but it also has undesirable properties including hygroscopicity, dimensional instability and biodegradability that limit its application (de Oliveira Araújo et al. 2016; Deka and Saikia 2000). Traditional preservative treatments can effectively protect timber against biodeterioration, but some users are concerned about the risks associated with chemicals. Thermal modification was developed to enhance both dimensional stability and colour and has been increasingly used in some regions, especially Europe (Dubey et al. 2011). Thermal modification can be performed with simple production equipment to enhance dimensional stability (Priadi and Hiziroglu 2013), decay resistance (Tripathi et al. 2014; Li et al. 2018) and appearance (Li et al. 2018) of the wood.
Colour is an important attribute of wood, although there is no universal preference for a given timber (Wang et al. 2016; Chen et al. 2012). Colour depends on chromophores, which include a range of nitro, nitroso, azo, α-diketo and quinone compounds as well as chromophores that need a multi-group conjugate such as carbonyl, thiocarbonyl, benzene rings and carbon–carbon double and triples (Jeffries 1914). Chromophores contain unsaturated chemical bonds that readily transition into an excited state with a minimum amount of energy, and the wavelength of the absorbed light enters the visible-light region to develop colour. A compound containing a chromophore may not be coloured, and the introduction of auxochromes such as hydroxyls, amines and carboxyl groups may be necessary to produce colour (Sandoval-Torres et al. 2010). The introduction of an auxochrome into a coloured compound intensifies the colour.
Cellulose, hemicellulose, lignin and extractives degradation during thermal modification alters the chromophores and auxochromes, changing wood colour. Cellulose, hemicellulose and lignin degradation during thermal modification will also cause changes in the physical and chemical properties of wood. Physical and chemical properties of wood change at temperatures around 140–150 °C (Cademartori et al. 2012; Priadi and Hiziroglu 2013). Thermally modified wood flexural properties and other strength properties begin to decrease under the effects of temperature and time (Bal 2018; Gao et al. 2016; Cademartori et al. 2012). Researchers have unsuccessfully tried to establish a response model between thermally modified wood colour and mechanical strength, but the correlations were poor, possibly because of the role of extractives (Johansson and Moren 2006; Gonzalez-Pena and Hale 2009). Although extractives are present at relatively low levels and have little effect on the mechanical properties, they play an important role in wood colour, most typically by rendering heartwood a much darker colour than sapwood (Moya et al. 2012; Pandey 2005). Extractives tend to be rich in unsaturated and conjugated structures including tannins, phenols, stilbenes and quinones (Sandoval-Torres et al. 2010; Mayer et al. 2006; Burtin et al. 2000). These compounds tend to be relatively stable in existing heartwood, but some are less stable and prone to changes when heated. These processes are the premise for modifying wood colour using thermal modification (Chen et al. 2014).
The objective of this study was to explore the changes in the relative levels of chromophores and auxochromes in poplar extracts during thermal modification to analyze the contribution and mechanism of the extracts to colour changes of the thermally modified poplar. This study provides information useful for developing models using colour to predict changes in flexural properties of thermally modified wood.
Materials and methods
Materials
Wood preparation: Poplar (Populus tomentosa Carr.) trees were selected from plantations at Northwest A&F University in the Yangling District, Shaanxi Province, China, and the heartwood was selected to be cut into clear, defect-free lumber. The lumber was air-dried, and then, small shavings cut from the lumber were ground to pass a 40–60 mesh screen. The ground wood was oven-dried at 60 °C to 8% moisture content and weighed (nearest 0.01 g). Approximately 200 g of oven-dried wood was prepared.
Solvent extraction
The ground wood was successively extracted using petroleum ether (PE), ethyl acetate (EA), 1,4-dioxane (Diox), acetone (DMK), ethanol (EtOH), acetonitrile (ACN) and distilled water (DW) in a Soxhlet extractor. The ground wood samples with different polar range extracts removed were obtained, and then, an aliquot of each extracted ground wood was thermally modified (Fig. 1).
Twenty-five grams of un-extracted ground wood was thermally modified. Five grams of ground wood was wrapped in filter paper that had been pre-extracted in a 2:1 phenol/ethanol mixture. The filter paper packet was placed in a Soxhlet extractor, and 100 ml of petroleum ether (boiling range: 30–60 °C) was added to the round-bottom flask on the assembly. The solvent was heated to produce four reflux changes per hour, and the system was maintained for a minimum of 8 h or until the liquid in the siphon return tube was colourless. At the end of the extraction, the solvent was filtered to remove particulate and concentrated by rotary evaporation at 60 °C. The petroleum ether-extracted poplar wood flour was oven-dried at 60 °C to constant weight and weighed. This process was repeated on 35 samples (35 filter paper packets containing 5 g of ground wood) that were used in subsequent extractions.
Five extracted samples were set aside for further testing, and then, the remaining 30 samples were Soxhlet-extracted in ethyl acetate using the same extraction conditions. The resulting extract was collected and evaporated, while the wood was oven-dried and partitioned as described above. This process was successively repeated with Soxhlet extraction in 1,4-dioxane, acetone, ethanol, acetonitrile and, finally, distilled water to produce five ~ 25 g groups of extracted material plus one ~ 25 g non-extracted control for thermal modification.
Thermal modification
Extracted and non-extracted poplar wood flour samples were oven-dried at 60 °C and weighed (nearest 0.01 g) before being wrapped in tin foil and thermally treated for 4 h at 180 °C in a nitrogen medium at atmospheric pressure. The oven was turned off after 4 h and cooled to 30 °C, and then, the thermally modified wood was stored in a desiccator.
Colour measurement
The effect of extraction and thermal modification on wood colour was measured by the CIELab system with a CHNspec CS-802 Spectrophotometer (Hangzhou CHNspec Technology Co., Ltd., Hangzhou, Zhejiang, China) equipped with a D65 light source at a 10-degree observed angle with a 15-mm aperture at the point of measurement. Poplar wood flour from a given extraction was placed into three 40-mm large-surface light-transmitting quartz cuvettes. The colour values were measured at three locations on each cuvette, and the mean value was calculated. The colour parameters of the samples were expressed by the lightness L* (L* = 0 indicates pure black and L* = 100 indicates pure white), the red–green axis chromaticity index a* (red in plus values/green in minus values) and the yellow–blue axis chromaticity index b* (yellow in plus values/blue in minus values) values by CIELab colour system. The total colour difference (∆E*) was calculated according to the following equation:
where ∆L*, ∆a** and ∆b* are the differences between non-extracted and a given solvent extraction sequence of L*, a* and b*, respectively.
The results were analysed using a one-way ANOVA (P = 0.05) and a Tukey HSD post hoc test using the SPSS Statistics 22.0 software program.
FTIR spectroscopy analysis of extracts
The characteristics of 1,4-dioxane and acetone extracts from non-modified ground wood were examined by sequentially extracting additional ground wood with petroleum ether and ethyl acetate, followed by extraction with 1,4-dioxane and acetone. The extracted ground wood was thermally modified as described earlier, and then, these materials were extracted with 1,4-dioxane and acetone (Fig. 1). The dried 1,4-dioxane and acetone extracts were subjected to FTIR analysis using the KBr plate method on a Bruker Vertex 70 FTIR spectrometer (Bruker Optics Ltd., Coventry, UK) at 4 cm−1 resolution in the spectral region 4,000–400 cm−1 with 32 scans. The resulting spectra were used to examine characteristic peaks for chromophores and auxochromes.
GC–MS analysis of extracts
Five milligrams of a given evaporated 1,4-dioxane and acetone extract from non-modified or thermally modified poplar was derivatized by adding 25 µl of N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) and 7500 µl acetonitrile and heating at 60 °C in a water bath until the extract dissolved. The dissolved extract was filtered through a 0.22-µm organic nylon filter at least three times to remove any particulate.
The resulting filtrate was analysed by GC–MS on a TRACE1310-ISQLT GC–MS spectrometer (Thermo Fisher Scientific, Massachusetts, USA) with a TG-5MS fused silica column (30 m × 0.25 mm id., film thickness 0.25 µm). Ultra-high-purity helium (99.999%) was used as the carrier gas at a flow rate of 1 ml/min. The oven temperature was kept at 80 °C for 2 min, then programmed to 180 °C at a rate of 15 °C/min, kept constant at 180 °C for 2 min, then programmed to 280 °C at a rate of 10 °C/min and then kept constant at 280 °C for 10 min. ESI source parameters were: positive ionization modes and scan range from 45 to 450 m/z. Individual components representing > 0.5% of the total peak area were identified on the basis of comparison of their mass spectrum using the 2014 National Institute of Standards and Technology library database.
Results and discussion
Effects of extract with different polarities on the colour of poplar
Poplar tends to blacken, redden or become yellower following thermal modification (Salca et al. 2016). Extraction of poplar prior to thermal modification produced significantly different changes in colour parameters (P < 0.05), but there were no consistent trends in brightness difference (ΔL*), red–green axis chromaticity index difference (Δa*) or yellow–blue axis chromaticity index difference (Δb*) with solvent type (Table 1). Overall colour differences (ΔE*) from the non-extracted control were lowest after acetonitrile extraction, followed by distilled water, ethyl acetate, 1,4-dioxane, then acetone, petroleum ether and, finally, ethanol. The differences would suggest a pattern of altered colour changes with solvent type; however, the results did not consistently change with solvent polarity. Zanuncio et al. (2015) conducted similar studies and found that removing the cold water extract of Eucalyptus pellita and Pinus radiata wood before thermal modification changed the colour of the treated wood, while removing the dichloromethane extract did not affect the colour.
The ΔL* values, which represent brightness, increased the most in poplar extracted with ethanol or petroleum ether, while brightness declined in samples extracted in all the other solvents prior to thermal modification except 1,4-dioxane. The Δa* values, which reflect changes in the red-green region, increased significantly following both ethyl acetate and ethanol extraction, while they increased only slightly for the acetonitrile and distilled water extractions. Extraction in petroleum ether alone produced the greatest loss in Δa* following thermal modification, and the residual ground wood tended to be lighter coloured with more green. The Δb* values reflect changes along the yellow–blue axis and suggested that extraction in either ethyl acetate or ethanol produced the greatest increases, while extraction in acetone produced the greatest losses. The remaining solvents had relatively little effect on Δb*.
Extraction prior to thermal modification would be expected to remove existing chromophores that could be further modified during the heating process, but it also potentially exposes the cell wall polymers to modification.
Effects of extraction with different polarities on the colour of thermally modified poplar
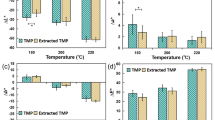
Thermal modification of non-extracted and extracted poplar resulted in darkening which was consistent with previous studies of thermal modification (Fig. 2). Samples that experienced smaller colour changes following solvent extraction tended to experience larger changes following thermal modification.
Example of ground poplar wood sequentially extracted in solvents prior to thermal modification and CIELab colour parameters associated with each treatment. Note: The smaller the colour difference before and after thermal modification of poplar after extraction, the greater the effect of this polar extract on the colour of thermally modified poplar. Values followed by the same letters do not differ significantly by Tukey HSD post hoc test (0.05)
The ΔL* of acetone and 1,4-dioxane extracted poplar samples tended to be lower than that of control. The Δa* values of thermally modified poplar were similar for all of the extracts except for those subjected to petroleum ether and acetone, while Δb* values declined for poplar extracted in the series with ethyl acetate, 1,4-dioxane, ethanol, acetonitrile or distilled water.
There were no consistent differences in ΔE* values of thermally modified poplar with extraction except for 1,4-dioxane and acetone, both of which had significantly smaller changes than the control. These results are consistent with previous reports (Gao et al. 2009, 2015). The differences noted in ΔE* with these two extraction sequences led us to further explore potential differences using FTIR and GC–MS.
FTIR analysis of the Diox and DMK extracts
FTIR analysis of the 1,4-dioxane and acetone extracts of non-modified and thermally modified wood (Fig. 3) suggested that the relative intensities of the O–H stretching vibrations (3394 and 3370 cm−1), conjugated aldehydes and esters C=O stretching vibrations (1723 cm−1), C=C or C=O stretching vibrations (1639 cm−1) were all weaker for the acetone extracts and the 1,4-dioxane sample, suggesting that the 1,4-dioxane extracts contained more hydroxyls, vinyls and carbonyls (Esteves et al. 2013).
The relative intensities of conjugated aldehydes and esters C=O stretching vibrations (1723 cm−1) and =C–H stretching vibrations of alkenes (878 cm−1) of the 1,4-dioxane extracted and thermally modified poplar (Diox-TMP) all increased relative to the non-thermally treated samples (Diox-UMP), while the C=C or C=O stretching vibrations (1639 cm−1) of the 1,4-dioxane extracted thermally modified samples decreased (Rajiv et al. 2016). These results suggest an increase in the presence of vinyl and conjugated carbonyl groups of aldehydes and esters in the thermally modified 1,4-dioxane extract and a corresponding decrease in conjugated carbonyl groups of aromatic structures.
The relative intensities of the O–H stretching vibrations (3370 cm−1), conjugated aldehydes and esters C=O stretching vibrations (1719 cm−1), C=C or C=O stretching vibrations (1639 cm−1) and benzene ring stretching vibrations (1515 cm−1) of thermally treated acetone extracted poplar (DMK-TMP) all increased compared to the non-thermally modified materials (DMK-UMP). These differences suggested increased levels of hydroxyl, vinyl, benzene ring and carbonyl groups with thermal modification.
GC–MS analysis of 1,4-bioxane and acetone extracts of thermally modified wood
The characteristics of acetone and 1,4-dioxane extracts of non-modified and thermally modified poplar were assessed because these two extracts tended to produce the greatest effects on colour changes following thermal modification.
Analysis of 1,4-dioxane extracts
The primary components in the resulting GC–MS chromatograms were characterized on the basis of peak area as well as the presence of either chromophores or auxochromes in the identified compounds (Tables 2 and 3).
1,4-Dioxane extraction of non-modified poplar resulted in a wide range of possible compounds. Nine compounds represented 37.26% of the total peak area, with 1-ethoxypropan-2-yl acetate or similar compounds representing over 19% of the total peak area. All nine of the peaks contained chromophores or auxochromes with six containing both structures. The results indicate that native poplar already contained a sizable number of potentially coloured compounds (Table 2).
Ten compounds were common in 1,4-dioxane extracts of thermally modified poplar, representing 50.69% of the peak area (Table 3). Seven of these compounds contained both chromophores and auxochromes. Interestingly, three compounds (4-hydroxybenzoic acid, glycerol and 2-hydroxyethyl palmitate) were found in both extracts. The remaining compounds were unique to each material illustrating the changes associated with thermal treatment. More compounds in 1,4-dioxane extracts of thermally modified poplar contained longer conjugate systems than those in non-thermally modified poplar, which may help explain the darkening associated with thermal modification (Yu et al. 2015).
The chromophores and auxochromes were mainly carbonyl, hydroxyl and benzene ring groups, and levels tended to be much higher in the thermally modified timber.
GC–MS analysis of acetone extracts
Acetone extracts of non-thermally modified poplar contained 12 compounds that contained either chromophores or auxochromes representing 59.32% of the peak area (Table 4).
Eight of these compounds contained both structures, while two only contained chromophores and two auxochromes. Glycerol was, by far, the most common compound in the extract. By contrast, only nine compounds were found in acetone extract of the thermally modified poplar, representing 55.54% of the total peak area (Table 5).
Eight of these compounds contained both chromophores and auxochromes, while one contained only auxochrome structures. Once again, glycerol was the most abundant compound, representing 40.41% of the peak area.
The chromophores and auxochromes in acetone extracts of both non-modified and thermally modified poplar were mainly carbonyl, hydroxyl and benzene ring groups. The glycerol content in the acetone extract of poplar before and after thermal modification did not change, and it contained a large amount of hydroxyls. Compounds with chromophores and auxochromes tended to be much higher in thermally modified poplar. These increases are consistent with the FTIR results showing increased benzene and carbonyl groups in thermally modified materials.
Four compounds were common to acetone extracts from both thermally modified and non-modified materials (glycerol, 4-hydroxylbenzanoic acid, 2-hydroxyethyl palmitate and 9, 12-octadecadienoic acid). Interestingly, 4-hydroxybenzoic acid was found in all four of the extracts, although it was only present at low levels. The compound composition of the thermally modified Populus tomentosa Carr. acetone extract (Table 5) was the result of further screening of chromophores and auxochromes. The presence of 4-hydroxylbenzanoic acid and 9,12-octadecadienoic acid was consistent with studies of thermally modified Populus nigra (Mecca et al. 2019).
Conclusion
The effect of extract changes during the thermal modification on the colour of thermally modified poplar was studied. The results suggest the colour of poplar without thermal modification was mainly affected by the petroleum ether and ethanol extracts, and the total colour difference (ΔE*) of poplar before and after extraction was 6.88 and 8.17, respectively. The colour of thermally modified poplar was mainly affected by DMK and Diox extracts; ΔE* of poplar with medium polar solvents (EA, Diox and DMK) extracts removed before thermal modification (13.3, 12.2 and 11.4) was lower than that of control group (14.9). The FTIR and GC–MS results suggest that one of the reasons for the darkening of thermally modified poplar was that the carbonyl, vinyl, benzene ring and hydroxyl groups which were chromophores and (or) auxochromes in extracts tended to be much higher in thermally modified poplar.
References
Bal BC (2018) A comparative study of some of the mechanical properties of pine wood heat treated in vacuum, nitrogen and air atmospheres. BioResources 13(3):5504–5511. https://doi.org/10.15376/biores.13.3.5504-5511
Burtin P, Jay-Allemand C, Charpentier JP, Janin G (2000) Modifications of hybrid walnut (Juglans nigra 23 × Juglans regia) wood color and phenolic composition under various steaming conditions. Holzforschung 54:333–338. https://doi.org/10.1515/HF.2000.006
Chen Y, Fan YM, Gao JM, Li HK (2012) Coloring characteristics of in situ lignin during heat treatment. Wood Sci Technol 46(1–3):33–40. https://doi.org/10.1007/s00226-010-0388-5
Chen Y, Tshabalala MA, Gao JM, Stark NM, Fan YM (2014) Color and surface chemistry changes of extracted wood flour after heating at 120℃. Wood Sci Technol 48(1):137–150. https://doi.org/10.1007/s00226-013-0582-3
de Cademartori PHG, Schneid E, Gatto DA, Beltrame R, Stangerlin DM (2012) Modification of static bending strength properties of Eucalyptus grandis heat-treated wood. Mat Res 15(6):922–927. https://doi.org/10.1590/S1516-14392012005000136
de Oliveira AS, Rocha Vital B, Oliveira B, Carneiro ADO, Lourenco A, Pereira H (2016) Physical and mechanical properties of heat-treated wood from Aspidosperma populifolium, Dipteryx odorata and Mimosa scabrella. Maderas-Cienc Tecnol 18(1):143–156. https://doi.org/10.4067/S0718-221X2016005000015
Deka M, Saikia CN (2000) Chemical modification of wood with thermosetting resin: effect on dimensional stability and strength property. Bioresource Technol 73(2):179–181. https://doi.org/10.1016/S0960-8524(99)00167-4
Dubey MK, Pang SS, Walker J (2011) Effect of oil heating age on colour and dimensional stability of heat-treated Pinus radiata. Eur J Wood Prod 69(2):255–262. https://doi.org/10.1007/s00107-010-0431-0
Esteves B, Marques AV, Domingos I, Pereira H (2013) Chemical changes of heat-treated pine and Eucalypt wood monitored by FTIR. Maderas-Cienc Tecnol 15(2):245–258. https://repositorio.ipv.pt/bitstream/10400.19/4301/1/MCT2012-0008_363%20last%20version_15102012_R1_27102012_7112012.pdf
Gao JM, Yi XS, Mu J, Meng LS (2009) Effects of extractives on induced discoloration of Betula costata trautv. treated by saturated steaming. J Beijing for Univ 31(S1):77–80. https://doi.org/10.13332/j.1000-1522.2009.s1.040
Gao S, Chen Y, Gao JM (2015) Effect of extractives on color changes of Eucalyptus urophylla×Eucalyptus grandis and its spectrum analysis. J Northeast for Univ 43(4):73–76. https://doi.org/10.4067/S0718-221X2013005000020
Gao H, Sun M, Cheng HY, Gao WL, Ding XL (2016) Effects of heat treatment under vacuum on properties of poplar. BioResources 11(1):1031–1043. https://doi.org/10.15376/biores.11.1.1031-1043
Gonzalez-Pena MM, Hale M (2009) Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 2: property predictions from colour changes. Holzforschung 63(4):394–401. https://doi.org/10.1515/HF.2009.077
Jeffries WL (1914) Color and structure in organic compounds. J Mitchell Soc 81–88. https://dc.lib.unc.edu/cdm/singleitem/collection/jncas/id/1171/rec/2
Johansson D, Morén T (2006) The potential of colour measurement for strength prediction of thermally treated wood. Holz Roh- Werkst 64(2):104–110. https://doi.org/10.1007/s00107-005-0082-8
Li RR, Xu W, Wang XD, Wang CG (2018) Modeling and predicting of the color changes of wood surface during CO2 laser modification. Jour Clean Prod 183:818–823. https://doi.org/10.1016/j.jclepro.2018.02.194
Mayer I, Koch G, Puls J (2006) Topochemical investigations on wood extractives and their influence on color changes in American black cherry (Prunus serotina Borkh.). Holzforschung 60:589–594. https://doi.org/10.1515/HF.2006.100
Mecca M, D’Auria M, Todaro L (2019) Effect of heat treatment on wood chemical composition, extraction yield and quality of the extractives of some wood species by the use of molybdenum catalysts. Wood Sci Technol 53:119–133. https://doi.org/10.1007/s00226-018-1057-3
Moya R, Fallas RS, Bonilla PJ, Tenorio C (2012) Relationship between wood color parameters measured by the CIELab system and extractive and phenol content in Acacia mangium and Vochysia guatemalensis from fast-growth plantations. Molecules 17:3639–3652. https://doi.org/10.3390/molecules17043639
Pandey K (2005) A note on the influence of extractives on the photo-discoloration and photo-degradation of wood. Polym Degard Stabil 87(2):375–379. https://doi.org/10.1016/j.polymdegradstab.2004.09.007
Priadi T, Hiziroglu S (2013) Characterization of heat-treated wood species. Mater Des Mater Des 49:575–582. https://doi.org/10.1016/j.matdes.2012.12.067
Rajiv P, Deepa A, Vanathi P, Vidhya D (2016) Screening for phytochemicals and FTIR analysis of Myristica dactyloides fruit extracts. Int J Pharm Pharm Sci 9(1):315–318. https://doi.org/10.22159/ijpps.2017v9i1.11053
Salca EA, Kobori H, Inagaki T, Kojima Y, Suzuki S (2016) Effect of heat treatment on colour changes of black alder and beech veneers. J Wood Sci 62(4):297–304. https://doi.org/10.1007/s10086-016-1558-3
Sandoval-Torres S, Jomaa W, Marc F, Puiggali JR (2010) Causes of color changes in wood during drying. For Stud China 12(4):167–175. https://doi.org/10.1007/s11632-010-0404-8
Tripathi S, Pant H, Kashyap AK (2014) Decay resistance against basidiomycetes fungi of heat-treated Pinus roxburghii and Mangifera indica wood. J Trop For Sci 26(2):203–207. https://www.frim.gov.my/v1/JTFSOnline/jtfs/v26n2/203-207.pdf
Wang Z, Sun BL, Liu JL (2016) Effect of thermo-vacuum treatment on the color and chemistry of larch wood. BioResources 11(1):2346–2360. https://doi.org/10.15376/biores.11.1.2349-2360
Yu SF, Liu Y, Li XJ, Luo WS (2015) Relations among different structure of reactive blue dyes and poplar dyeing properties. J Cent South Univ For T 35(2): 96–99, 108. https://doi.org/10.14067/j.cnki.1673-923x.2015.02.018
Zanuncio AJV, Carvalho AG, de Souza MT, Jardim CM, Carneiro ADO, Colodette JL (2015) Effect of extractives on wood color of heat-treated Pinus radiata and Eucalyptus pellita. Maderas-Cienc Tecnol 17(4):857–864. https://doi.org/10.4067/S0718-221X2015005000074
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31971590) and the Fundamental Research Funds for the Central Universities (2452019057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in the submission of this manuscript, and manuscript has been approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bi, Z., Yuan, J., Morrell, J.J. et al. Effects of extracts on the colour of thermally modified Populus tomentosa Carr.. Wood Sci Technol 55, 1075–1090 (2021). https://doi.org/10.1007/s00226-021-01304-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-021-01304-7






