Abstract
Antagonist muscle co-activation is thought to be partially regulated by cortical influences, but direct motor cortex involvement is not fully understood. Corticomuscular coherence (CMC) measures direct functional coupling of the motor cortex and muscles. As antagonist co-activation differs according to training status, comparison of CMC in agonist and antagonist muscles and in strength-trained and endurance-trained individuals may provide in-depth knowledge of cortical implication in antagonist muscle co-activation. Electroencephalographic and electromyographic signals were recorded, while 10 strength-trained and 11 endurance-trained participants performed isometric knee contractions in flexion and extension at various torque levels. CMC magnitude in 13–21 and 21–31 Hz frequency bands was quantified by CMC analysis between Cz electroencephalographic electrode activity and all electromyographic signals. CMC was significant in both 13–21 and 21–31 Hz frequency bands in flexor and extensor muscles regardless of participant group, torque level, and direction of contraction. CMC magnitude decreased more in antagonist than in agonist muscles as torque level increased. Finally, CMC magnitude was higher in strength-trained than in endurance-trained participants. These findings provide experimental evidence that the motor cortex directly regulates both agonist and antagonist muscles. Nevertheless, the mechanisms underlying muscle activation may be specific to their function. Between-group modulation of corticomuscular coherence may result from training-induced adaptation, re-emphasizing that corticomuscular coherence analysis may be efficient in characterizing corticospinal adaptations after long-term muscle specialization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antagonist co-activation is defined as the “unintentional concurrent activation of antagonist muscles during the activation of agonist muscles” (Duchateau and Baudry 2014). Antagonist muscles produce significant torque around the joint in the direction opposite to net torque, which increases joint stiffness to improve movement accuracy (Miller et al. 2000; Gribble et al. 2003; Stokes and Gardner-Morse 2003) and protect joints [for review, see Remaud et al. (2007)]. Despite the crucial functional role of antagonist muscles during both isometric and dynamic actions, the cortical mechanisms underlying their regulation are not fully understood (Duchateau and Baudry 2014).
Based on early investigations that revealed common firing rate fluctuations between agonist and antagonist motor unit pairs (De Luca and Mambrito 1987; De Luca and Erim 2002), a large body of evidence suggests that cortical level regulates the excitation of agonist–antagonist muscle pairs as a single pool via a common descending oscillatory drive (Boonstra et al. 2009; Mohr et al. 2015). This hypothesis is supported by other electromyography (EMG) studies showing similar fatigue-related changes in agonist and antagonist muscles during a fatigue protocol (Psek and Cafarelli 1993; Levenez et al. 2005; Levenez et al. 2008) and significant intermuscular oscillatory coupling of agonist and antagonist muscles (Wang et al. 2015). The possible role of the cortex in the regulation of antagonist muscle co-activation has been inferred from analysis of motor cortex oscillations recorded by electroencephalography (EEG) (Dal Maso et al. 2012). Consequently, measurement of functional coupling between activities of the motor cortex and antagonist muscles is required to confirm direct motor cortex involvement in the regulation of antagonist co-activation.
Corticomuscular coherence (CMC) analysis directly assesses frequency coupling (Rosenberg et al. 1989) between brain and muscle electrophysiological oscillatory activities recorded by EEG and EMG, respectively. Since the past 2 decades, CMC between the primary motor cortex and agonist muscles has been well characterized (Conway et al. 1995; Kristeva et al. 2007; Gwin and Ferris 2012; Muthuraman et al. 2012; Ushiyama et al. 2012; Campfens et al. 2013; Enders and Nigg 2015; Poortvliet et al. 2015; Lai et al. 2016). Indeed, during isometric contractions, motor cortex oscillations, which are carried down to the alpha-motoneurons via the corticospinal tract (Baker et al. 2003; Lemon 2008; Negro and Farina 2011), are significantly coupled with muscle activity in the 13–31 Hz, ‘beta’ band (β-band) (Muthuraman et al. 2012; Campfens et al. 2013; Poortvliet et al. 2015). Although the underlying mechanisms are still being debated (Witham et al. 2011), CMC reveals large-scale communication between remote populations of neuronal networks (Buzsaki and Draguhn 2004; Schnitzler and Gross 2005; Joundi et al. 2012) and represents suitable analysis for characterizing the cortical regulation of muscle activation (Ushiyama et al. 2010; Boonstra 2013). Consequently, comparison of CMC magnitude between EEG from the motor cortex and EMG from muscles acting as agonists and antagonists may help to further elucidate cortical mechanisms controlling antagonist muscle co-activation.
Interestingly, training status alters antagonist co-activation. Indeed, studies have reported lower antagonist co-activation in strength-trained (ST) than in endurance-trained (ET) or untrained participants during isometric contractions (Tillin et al. 2011; Dal Maso et al. 2012; Amarantini and Bru 2015). Significant changes also occur at the cortical and corticospinal tract levels between ST and ET participants (Carroll et al. 2002; Griffin and Cafarelli 2005; Falvo et al. 2010; Dal Maso et al. 2012). For instance, event-related desynchronization of 13–21 Hz cortical oscillations was higher in ST than ET during isometric contractions. Furthermore, CMC magnitude is sensitive to neural adaptations—induced by extended motor practice (Ushiyama et al. 2010; Perez et al. 2012; Ushiyama et al. 2012; Larsen et al. 2016). Therefore, CMC comparison between individuals with different training status may provide an in-depth understanding of the mechanisms that participate in the control of antagonist muscles.
The aim of the present study was to compare CMC between the motor cortex and agonist muscles versus CMC between the motor cortex and antagonist muscles to provide further insights regarding cortical mechanisms underlying antagonist co-activation. The current work is based on the same sample as in Dal Maso et al. (2012) where the effects of training status on event-related desynchronization of motor cortex oscillations were investigated during isometric voluntary contractions. Following the method developed by Bigot et al. (2011) for accurate assessment of CMC, the first general hypothesis was that CMC would be significant in both agonist and antagonist muscles, but of different magnitudes according to muscle function. Moreover, based on previous results showing higher event-related desynchronization in ST than in ET in the lower β-band (13–21 Hz), it was hypothesized that CMC magnitude would differ between ST and ET in this frequency band. In the upper β-band (21–31 Hz), event-related desynchronization was found to be modulated according to torque level and training status during flexion exertions only. These results led to further postulation that CMC magnitude differs with torque level with specific modulation according to training status in the upper β-band during flexion exertion only.
Methods
Ethics approval
The study protocol was in accordance with the Declaration of Helsinki for research on human subjects and followed local ethics guidelines of the Université de Toulouse (France). Study participants received explicit information about the experimental design and gave signed informed consent before the experimental procedures began.
Participants
Twenty-one right-footed men (assessed by inventory of foot preference (Chapman et al. 1987)) volunteered to participate in this study. They were assigned to 1 of the 2 following groups:
-
The ST group included ten participants engaged in regular lower limb strength training at least three times per week since at least 3 years (age 24.10 ± 4.31 years; height 1.77 ± 0.07 m; mass 79.60 ± 7.73 kg; mean ± SD). None of these participants was significantly involved in another physical activity.
-
The ET group included 11 participants engaged in endurance disciplines involving the lower limbs at least three times per week since at least 3 years (age 22.09 ± 2.30 years; height 1.80 ± 0.07 m; mass 77.45 ± 7.49 kg; mean ± SD). None of these participants was involved in an ST program.
The two groups were age-, height-, and mass-matched (p > 0.05). All participants had no lower limb injuries or neurological disorders.
Instrumentation
Net joint torque around the knee was recorded at 1000 Hz by calibrated dynamometer (System 4 Pro, Biodex Medical Systems, Shirley, NY, USA). Surface EMG signals were recorded at 1000 Hz by a Bagnoli-8 system and DE-2.1 single differential electrodes (Delsys, Boston, MA, USA). After suitable skin preparation (Hermens et al. 2000), EMG electrodes were placed over the bellies of representative right knee extensors, namely, the vastus medialis (VM) and rectus femoris (RF), and representative right knee flexors, namely, the biceps femoris (BF) and gastrocnemius (Ga) (Olney and Winter 1985; Amarantini et al. 2010). This electrode placement permitted to record EMG with no crosstalk contamination (Supplementary Figure 1). The reference electrode was placed on the left radial styloid. EEG signals were recorded at 1024 Hz by a 64-channel ActiveTwo system (BioSemi, Amsterdam, The Netherlands). The BioSemi system works in a “zero-Ref” set-up with ground and reference electrodes replaced by a so-called CMS/DRL circuit (for further information, refer to http://www.biosemi.com/faq/cms&drl.htm). EEG electrode locations followed the 10–20 international system. EMG and EEG electrode impedance was kept below 5 kΩ. Torque, EMG, and EEG data were time-synchronized offline with TTL pulse.
Experimental procedures
The experimental procedures have been described in detail elsewhere (Dal Maso et al. 2012). Briefly, participants were seated and secured to the dynamometer with the right knee flexed at 60°. They were then asked to perform:
-
1.
Three 4-s knee isometric maximum voluntary contractions (MVC), followed by 1-min rest in flexion and extension.
-
2.
Three 6-s so-called “relative MVC” (rMVC) in both directions of contraction separated by 1-min rest. During rMVC, participants were asked to achieve the greatest isometric knee torque while relaxing the upper body (for details, see Dal Maso et al. 2012 and Cremoux et al. 2013) to minimize contamination of EEG signals by contractions of muscles surrounding the head during MVC.
-
3.
Knee flexion and extension isometric contractions at 20, 40, 60, and 80% of rMVC are in random order. Participants were not required to stiffen their joints, so that the co-activation recorded over antagonist muscles was of unintentional origin. Participants received custom-made visual feedback of the target torque level (for details, see Dal Maso et al. 2012 and Cremoux et al. 2013). Twenty contractions, each lasting 6 s with 6-s rest in between, were recorded in each condition. A 3-min rest period was allowed after every 16 contractions.
Data analysis
All filters mentioned hereafter are zero-lag, fourth-order Butterworth filters.
Net joint torque was low-pass filtered at 10 Hz. A period of interest was defined for each contraction as a 3-s consecutive window where the sum of absolute error and the variability between required and exerted torque was minimal. Mean squared error of torque production was computed according to the method of Divekar and John (2013). No between-group difference was seen in flexion and extension (p > 0.05).
Figure 1 illustrates the processing steps for computing CMC. Raw EEG and EMG signals were 3–100 Hz band-pass filtered and 45–55 Hz notch filtered (Fig. 1a, b). EEG signals were average referenced. The Cz EEG electrode was selected as the electrode of interest, since (1) event-related desynchronization of the β-band was maximum at this electrode during pre-testing with 50 right knee extension movements (Supplementary Figure 1) and (2) its location on the EEG cap matched the location of the primary motor cortex of the lower limb. Previous studies on lower limb muscle contractions also selected the Cz electrode as the electrode of interest (Perez et al. 2007; Masakado and Nielsen 2008; Dal Maso et al. 2012). Continuous EEG and EMG data were then reduced to concatenated trials from −0.5 to +8 s relative to the onset of visual feedback and zero-aligned. CMC was computed in the time–frequency domain as electrophysiological signals are non-stationary processes (Zhan et al. 2006; Allen and MacKinnon 2010). Time–frequency CMC between Cz EEG signal and each unrectified EMG signal was calculated with the WaveCrossSpec Matlab toolbox for wavelet coherence analysis (Bigot et al. 2011: http://www.math.u-bordeaux1.fr/~jbigot/Site/Software_files/WavCrossSpec.zip). The parameters ‘nvoice’ (scale resolution of wavelets), ‘J1’ (number of scales), and ‘wavenumber’ (Morlet mother wavelet parameter) were, respectively, set to 0.125, 864, and 7 to yield accurate identification of oscillatory activity in the [0.0021:0.9967:104.6565] Hz frequency range. Magnitude-squared coherence was computed as follows:
where \( S_{\text{EEG/EMG}} \left( {\omega , u} \right) \) is the wavelet cross-spectrum between EEG and EMG time series at frequency \( \omega \) and time \( u \) (Fig. 1e); \( S_{\text{EEG}} \left( {\omega , u} \right) \) and \( S_{\text{EMG}} \left( {\omega , u} \right) \) are wavelet auto-spectra of EEG (Fig. 1c) and EMG (Fig. 1d) time series, respectively, at frequency \( \omega \) and time \( u \). Refer to Bigot et al. (2011) for detailed equations.
Steps taken to compute corticomuscular coherence with data from Cz EEG and VM EMG electrodes in an ST participant during knee extension performed at 20% of rMVC. Signals of a Cz EEG and b VM EMG electrodes. Wavelet auto-spectra of c Cz EEG and d VM EMG signals. e Wavelet cross-spectrum between Cz EEG and VM EMG signals: red contours identify areas in the time–frequency plane where correlation between the two signals is significant. f Representation of significant areas of wavelet magnitude-squared coherence between Cz EEG and VM EMG signals. For each participant, experimental condition, and each muscle, the corticomuscular coherence magnitude was quantified as the volume under magnitude-squared coherence values in the low-β (13–21 Hz) and high-β (21–31 Hz) bands where a significant correlation between EEG and EMG time series was detected on the wavelet cross-spectrum
There is an ongoing debate challenging the conventional EMG rectification for CMC analysis (Neto and Christou 2010; McClelland et al. 2012; Negro et al. 2015). Rectification of a zero-mean oscillatory signal, as EMG in our case, is a non-linear process that distorts its power spectrum properties (Neto and Christou 2010; McClelland et al. 2012). Especially for steady-state force tasks, the previous studies highlighted that there is no significant difference in CMC magnitude computed with rectified or unrectified EMG signals (Yao et al. 2007; Bayraktaroglu et al. 2011; Yang et al. 2016). Consequently, to meet both theoretical support and practical justification for the computation of CMC (Bigot et al. 2011; McClelland et al. 2012), CMC was computed from zero-mean centered EEG signals and unrectified EMG signals. As cautioned by Yang et al. (2016), computing CMC with unrectified EMG is suitable provided that EMG signals are motion artefact-free, which was the case of our data (Supplementary Figure 2).
CMC magnitude was quantified as volume under the time–frequency plane where CMC was significant, as detected on cross-spectrum, using the statistical test introduced by Bigot et al. (2011). This magnitude quantification was previously introduced in CMC (Yoshida et al. 2017; Cremoux et al. 2017) and intermuscular coherence (Charissou et al. 2016) studies. The significant threshold of corticomuscular coherence was Bonferroni-corrected to 0.05/4 as CMC was computed with four muscles (Winslow et al. 2016). Finally, for each participant and experimental condition, CMC magnitude in knee extensor and flexor muscle groups was obtained by averaging CMC magnitude in Cz EEG–VM EMG and Cz EEG–RF EMG and in Cz EEG–BF EMG and Cz EEG–Ga EMG, respectively.
Statistics
All dependent variables were tested for normality (Shapiro–Wilk test, α = 0.05), and all data met the assumption of homogeneity of variance (Levene’s test, all p > 0.05).
Three-factor group (ST vs. ET) × torque level (20 vs. 40 vs. 60 vs. 80%) × muscle function (agonist vs. antagonist) analysis of variance (ANOVA) was conducted on CMC magnitude separately in flexion and extension with repeated measures on torque level and muscle function. CMC magnitude was compared statistically in both 13–21 Hz and 21–31 Hz frequency bands.
Results
CMC magnitude in the 13–21 Hz frequency band
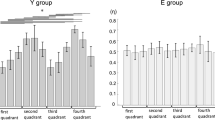
In both flexion and extension, ANOVA revealed a torque level effect (F 3,17 = 8.34; p = 0.001 and F 3,17 = 10.34; p < 0.001, respectively), a muscle function effect (F 1,19 = 30.46; p < 0.001 and F 1,19 = 86.70; p < 0.001, respectively), and torque level × muscle function interaction (F 3,17 = 7.59; p = 0.002 and F 3,17 = 9.72; p = 0.001, respectively) on CMC magnitude in the 13–21 Hz frequency band. CMC magnitude was higher in antagonist than in agonist muscles. Low-β CMC magnitude in antagonist muscles decreased with torque level, while it tended to remain constant across torque levels in agonist muscles (Fig. 2a, c). Low-β CMC magnitude was significantly higher in ST than in ET participants in flexion and extension (F 1,19 = 5.64; p = 0.028; Fig. 2b and F 1,19 = 4.73; p = 0.042; Fig. 2d, respectively).
Mean (+SE) of corticomuscular coherence magnitude in 13–21 Hz (left part) and 21–31 Hz (right part) frequency bands in agonist and antagonist muscles during flexion (upper part) and extension (lower part) contractions at 20, 40, 60, and 80% of rMVC. a, c, e and g represent significant torque level and muscle function effects and torque level × muscle function interaction, respectively, indicated by arrow, hash symbol, and triangle when applicable. b, d, f, and h represent the group effect indicated by asterisk when applicable
CMC magnitude in the 21–31 Hz frequency band
In both flexion and extension, ANOVA revealed a torque level effect (F 3,17 = 3.98; p = 0.026 and F 3,17 = 7.46; p = 0.002, respectively). CMC magnitude with agonist and antagonist muscles decreased with torque level (Fig. 2e, g). In extension only, high-β CMC was significantly higher than in ET participants (F 1,19 = 7.26; p = 0.014, Fig. 2h). In flexion only, high-β CMC was significantly higher with antagonist than with agonist muscles (F 1,19 = 80.95; p < 0.001, Fig. 2e).
Discussion
To provide further insights into cortical involvement underlying antagonist co-activation, the present study compared CMC in agonist and antagonist muscles and in ST and ET participants during knee isometric contractions performed at different torque levels. First, these findings revealed that CMC was significant with antagonist muscles in the broad β-band, providing clear experimental evidence that the cortex participates directly in the regulation of antagonist co-activation. Second, CMC magnitude in antagonist muscles decreased more than CMC magnitude in agonist muscles as torque level increased, which suggests specific regulation of muscle activation according to their function. Finally, higher CMC magnitude in the lower β-band for ST in comparison to ET participants may result from training-induced adaptation.
The cortex regulates antagonist muscle co-activation
Coherence analysis measures the strength of the coupling between two oscillatory signals (Rosenberg et al. 1989). While the previous studies repeatedly investigated CMC in agonist muscles (Kristeva et al. 2007; Gwin and Ferris 2012; Muthuraman et al. 2012; Ushiyama et al. 2012; Campfens et al. 2013; Enders and Nigg 2015; Poortvliet et al. 2015; Lai et al. 2016), the present work focused on CMC in antagonist muscles, considering their major functional role in human voluntary muscular contraction. Our findings revealed a significant coupling between the motor cortex and antagonist muscles in the broad β-band, regardless of the group of participants, direction of contraction, and torque level. Considering that CMC is thought to reflect communication between distant neural networks (Buzsaki and Draguhn 2004; Schnitzler and Gross 2005; Joundi et al. 2012) via pyramidal pathways (Baker et al. 2003; Lemon 2008; Negro and Farina 2011), the present study provides direct experimental evidence of the functional coupling between primary motor cortex and antagonist muscles during isometric knee contractions. This result agrees with the “common drive” theory (De Luca and Mambrito 1987; De Luca and Erim 2002), confirming the role of cortical control in antagonist co-activation.
Regulation of muscle activation is function-specific
CMC magnitude in the broad β-band was higher with antagonist than with agonist muscles in both groups of participants. Moreover, CMC magnitude in the low-β-band decreased more in antagonist than in agonist muscles as torque level increased. These results suggest that the regulation of muscle activation is function-specific, with strongest direct functional oscillatory communication between the brain and antagonist muscles. The following potential mechanisms may explain the observed modulation of CMC magnitude with muscle function.
First, CMC may not derive from direct and simple motor cortex-to-muscle descending oscillation propagation. Indeed, intrinsic spinal mechanisms occurring during voluntary contractions (Nielsen 2016) can alter CMC magnitude, as suggested by modeling-based investigation (Williams and Baker 2009; Watanabe and Kohn 2015). Ascending muscle-to-motor cortex drives are also essential in the establishment of corticomuscular coupling. Indeed, CMC is altered after ischemia or arm cooling (Pohja and Salenius 2003; Riddle and Baker 2005; Witham et al. 2011) and peripheral electrical stimulation (Lai et al. 2016), which are known to modulate peripheral nerve conduction time and afferent information, respectively. The contribution of spinal mechanisms and afferent information may, therefore, be specific to muscle function and torque production, causing torque level x muscle function β-band CMC magnitude interaction.
Second, proportions of direct corticospinal projections innervating muscles may have altered broad β-band CMC magnitude in agonist and antagonist muscles. Indeed, distal muscles, which have more direct corticospinal projections than proximal muscles, have higher CMC magnitude (Ushiyama et al. 2010). The latter finding indicates that the proportions of corticomotoneurons directly innervating muscles may be greater when they act as antagonists rather than as agonists.
Finally, the modulation of CMC magnitude may also be interpreted in light of the status quo theory (Engel and Fries 2010). Indeed, high CMC magnitude may reflect the maintenance of a stable state that efficiently processes feedback to recalibrate the sensorimotor system with minimum computational effort (Brown 2000). According to the present CMC results, the muscle function effect may indicate greater computational effort to regulate agonist muscle than antagonist muscle activation.
CMC analysis as a marker of training-induced adaptation
According to the previous analysis of the current database, both groups had comparable force capabilities, but ST participants had lower antagonist co-activation than ET participants in flexion (Dal Maso et al. 2012). In light of numerous investigations that have reported decreased antagonist co-activation in ST athletes (Carolan and Cafarelli 1992; Hakkinen et al. 2000; Griffin and Cafarelli 2005; Amarantini and Bru 2015), this result was interpreted as an ST-induced adaptation mechanism known to occur at cortical, corticospinal, and motor unit levels (Carroll et al. 2002; Griffin and Cafarelli 2005; Folland and Williams 2007; Falvo et al. 2010; Vila-Chã and Falla 2016). The present data indicate increased CMC in the ST group in the lower β-band during flexion and extension exertions and in the upper β-band during extension exertions only. This result agrees with the previous studies showing alteration of CMC with functional abilities of participants (Ushiyama et al. 2010; Larsen et al. 2016). Interestingly, the previous findings highlighted modulation of task-related spectral power of motor cortex oscillations in the lower β-band between ET and ST participants in both directions of contraction (Dal Maso et al. 2012). CMC magnitude, being sensitive to cortico-motoneuronal plasticity occurring after practice (Baker and Baker 2003; Hansen and Nielsen 2004; Perez et al. 2006; Mendez-Balbuena et al. 2012; Larsen et al. 2016), the observed increase of CMC in ST participants may be interpreted as a training adaptation effect. Different mechanisms may, therefore, participate in the control of muscle activation between groups, suggesting that CMC magnitude is a reliable marker of corticomotor adaptations.
Conclusion
The present study revealed a significant CMC between the motor cortex and both agonist and antagonist muscle activities. Its findings provide experimental evidence that the cortex is directly involved in the regulation of both agonist and antagonist muscle co-activation, and suggest that the mechanisms underlying muscle activation are specific to their function. The present observations also revealed that the strength of corticomuscular coupling is sensitive to practice-induced adaptation, emphasizing that CMC may be a relevant measure in investigating the effect of long-term corticomotor adaptation.
References
Allen DP, MacKinnon CD (2010) Time-frequency analysis of movement-related spectral power in EEG during repetitive movements: a comparison of methods. J Neurosci Methods 186:107–115. doi:10.1016/j.jneumeth.2009.10.022
Amarantini D, Bru B (2015) Training-related changes in the EMG-moment relationship during isometric contractions: further evidence of improved control of muscle activation in strength-trained men? J Electromyogr Kinesiol 25:697–702. doi:10.1016/j.jelekin.2015.04.002
Amarantini D, Rao G, Berton E (2010) A two-step EMG-and-optimization process to estimate muscle force during dynamic movement. J Biomech 43:1827–1830. doi:10.1016/j.jbiomech.2010.02.025
Baker MR, Baker SN (2003) The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol 546:931–942
Baker SN, Pinches EM, Lemon RN (2003) Synchronization in monkey motor cortex during a precision grip task. II. effect of oscillatory activity on corticospinal output. J Neurophysiol 89:1941–1953. doi:10.1152/jn.00832.2002
Bayraktaroglu Z, von Carlowitz-Ghori K, Losch F, Nolte G, Curio G, Nikulin VV (2011) Optimal imaging of cortico-muscular coherence through a novel regression technique based on multi-channel EEG and un-rectified EMG. Neuroimage 57:1059–1067. doi:10.1016/j.neuroimage.2011.04.071
Bigot J, Longcamp M, Dal Maso F, Amarantini D (2011) A new statistical test based on the wavelet cross-spectrum to detect time-frequency dependence between non-stationary signals: application to the analysis of cortico-muscular interactions. Neuroimage 55:1504–1518. doi:10.1016/j.neuroimage.2011.01.033
Boonstra TW (2013) The potential of corticomuscular and intermuscular coherence for research on human motor control. Front Hum Neurosci 7:855. doi:10.3389/fnhum.2013.00855
Boonstra TW, Daffertshofer A, Roerdink M, Flipse I, Groenewoud K, Beek PJ (2009) Bilateral motor unit synchronization of leg muscles during a simple dynamic balance task. Eur J Neurosci 29:613–622. doi:10.1111/j.1460-9568.2008.06584.x
Brown P (2000) Cortical drives to human muscle: the Piper and related rhythms. Prog Neurobiol 60:97–108
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304:1926–1929. doi:10.1126/science.1099745
Campfens SF, Schouten AC, van Putten MJ, van der Kooij H (2013) Quantifying connectivity via efferent and afferent pathways in motor control using coherence measures and joint position perturbations. Exp Brain Res 228:141–153. doi:10.1007/s00221-013-3545-x
Carolan B, Cafarelli E (1992) Adaptations in coactivation after isometric resistance training. J Appl Physiol 73(1985):911–917
Carroll TJ, Riek S, Carson RG (2002) The sites of neural adaptation induced by resistance training in humans. J Physiol 544:641–652
Chapman JP, Chapman LJ, Allen JJ (1987) The measurement of foot preference. Neuropsychologia 25:579–584
Charissou C, Vigouroux L, Berton E, Amarantini D (2016) Fatigue- and training-related changes in ‘beta’ intermuscular interactions between agonist muscles. J Electromyogr Kinesiol. doi:10.1016/j.jelekin.2016.02.002
Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR (1995) Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol 489(Pt 3):917–924
Cremoux S, Tallet J, Berton E, Dal Maso F, Amarantini D (2013) Does the force level modulate the cortical activity during isometric contractions after a cervical spinal cord injury? Clin Neurophysiol 124:1005–1012. doi:10.1016/j.clinph.2012.11.007
Dal Maso F, Longcamp M, Amarantini D (2012) Training-related decrease in antagonist muscles activation is associated with increased motor cortex activation: evidence of central mechanisms for control of antagonist muscles. Exp Brain Res 220:287–295. doi:10.1007/s00221-012-3137-1
De Luca CJ, Erim Z (2002) Common drive in motor units of a synergistic muscle pair. J Neurophysiol 87:2200–2204. doi:10.1152/jn.00793.2001
De Luca CJ, Mambrito B (1987) Voluntary control of motor units in human antagonist muscles: coactivation and reciprocal activation. J Neurophysiol 58:525–542
Divekar NV, John LR (2013) Neurophysiological, behavioural and perceptual differences between wrist flexion and extension related to sensorimotor monitoring as shown by corticomuscular coherence. Clin Neurophysiol 124:136–147. doi:10.1016/j.clinph.2012.07.019
Duchateau J, Baudry S (2014) The neural control of coactivation during fatiguing contractions revisited. J Electromyogr Kinesiol 24:780–788. doi:10.1016/j.jelekin.2014.08.006
Enders H, Nigg BM (2015) Measuring human locomotor control using EMG and EEG: current knowledge, limitations and future considerations. Eur J Sport Sci pp 1–11 doi: 10.1080/17461391.2015.1068869
Engel AK, Fries P (2010) Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol 20:156–165. doi:10.1016/j.conb.2010.02.015
Falvo MJ, Sirevaag EJ, Rohrbaugh JW, Earhart GM (2010) Resistance training induces supraspinal adaptations: evidence from movement-related cortical potentials. Eur J Appl Physiol 109:923–933. doi:10.1007/s00421-010-1432-8
Folland JP, Williams AG (2007) The adaptations to strength training: morphological and neurological contributions to increased strength. Sports Med 37:145–168
Gribble PL, Mullin LI, Cothros N, Mattar A (2003) Role of cocontraction in arm movement accuracy. J Neurophysiol 89:2396–2405. doi:10.1152/jn.01020.2002
Griffin L, Cafarelli E (2005) Resistance training: cortical, spinal, and motor unit adaptations. Can J Appl Physiol 30:328–340
Gwin JT, Ferris DP (2012) Beta- and gamma-range human lower limb corticomuscular coherence. Front Hum Neurosci 6:258. doi:10.3389/fnhum.2012.00258
Hakkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ (2000) Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol 83:51–62. doi:10.1007/s004210000248
Hansen NL, Nielsen JB (2004) The effect of transcranial magnetic stimulation and peripheral nerve stimulation on corticomuscular coherence in humans. J Physiol 561:295–306. doi:10.1113/jphysiol.2004.071910
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G (2000) Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10:361–374. doi:10.1016/S1050-6411(00)00027-4
Joundi RA, Jenkinson N, Brittain JS, Aziz TZ, Brown P (2012) Driving oscillatory activity in the human cortex enhances motor performance. Curr Biol 22:403–407. doi:10.1016/j.cub.2012.01.024
Kristeva R, Patino L, Omlor W (2007) Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. Neuroimage 36:785–792. doi:10.1016/j.neuroimage.2007.03.025
Lai MI, Pan LL, Tsai MW, Shih YF, Wei SH, Chou LW (2016) Investigating the effects of peripheral electrical stimulation on corticomuscular functional connectivity stroke survivors. Top Stroke Rehabil 23:154–162. doi:10.1080/10749357.2015.1122264
Larsen LH, Jensen T, Christensen MS, Lundbye-Jensen J, Langberg H, Nielsen JB (2016) Changes in corticospinal drive to spinal motoneurones following tablet-based practice of manual dexterity. Physiol Rep 4:e12684. doi:10.14814/phy2.12684
Lemon RN (2008) Descending pathways in motor control. Annu Rev Neurosci 31:195–218. doi:10.1146/annurev.neuro.31.060407.125547
Levenez M, Kotzamanidis C, Carpentier A, Duchateau J (2005) Spinal reflexes and coactivation of ankle muscles during a submaximal fatiguing contraction. J Appl Physiol 99(1985):1182–1188. doi:10.1152/japplphysiol.00284.2005
Levenez M, Garland SJ, Klass M, Duchateau J (2008) Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol 99:554–563. doi:10.1152/jn.00963.2007
Masakado Y, Nielsen JB (2008) Task-and phase-related changes in cortico-muscular coherence. Keio J Med 57:50–56
McClelland VM, Cvetkovic Z, Mills KR (2012) Rectification of the EMG is an unnecessary and inappropriate step in the calculation of Corticomuscular coherence. J Neurosci Methods 205:190–201. doi:10.1016/j.jneumeth.2011.11.001
Mendez-Balbuena I, Huethe F, Schulte-Monting J, Leonhart R, Manjarrez E, Kristeva R (2012) Corticomuscular coherence reflects interindividual differences in the state of the corticomuscular network during low-level static and dynamic forces. Cereb Cortex 22:628–638. doi:10.1093/cercor/bhr147
Miller JP, Croce RV, Hutchins R (2000) Reciprocal coactivation patterns of the medial and lateral quadriceps and hamstrings during slow, medium and high speed isokinetic movements. J Electromyogr Kinesiol 10:233–239
Mohr M, Nann M, von Tscharner V, Eskofier B, Nigg BM (2015) Task-dependent intermuscular motor unit synchronization between medial and lateral vastii muscles during dynamic and isometric squats. PLoS One 10:e0142048. doi:10.1371/journal.pone.0142048
Muthuraman M, Arning K, Govindan RB, Heute U, Deuschl G, Raethjen J (2012) Cortical representation of different motor rhythms during bimanual movements. Exp Brain Res 223:489–504. doi:10.1007/s00221-012-3276-4
Negro F, Farina D (2011) Linear transmission of cortical oscillations to the neural drive to muscles is mediated by common projections to populations of motoneurons in humans. J Physiol 589:629–637. doi:10.1113/jphysiol.2010.202473
Negro F, Keenan K, Farina D (2015) Power spectrum of the rectified EMG: when and why is rectification beneficial for identifying neural connectivity? J Neural Eng 12:036008. doi:10.1088/1741-2560/12/3/036008
Neto OP, Christou EA (2010) Rectification of the EMG signal impairs the identification of oscillatory input to the muscle. J Neurophysiol 103:1093–1103. doi:10.1152/jn.00792.2009
Nielsen JB (2016) Human spinal motor control. Annu Rev Neurosci 39:81–101. doi:10.1146/annurev-neuro-070815-013913
Olney SJ, Winter DA (1985) Predictions of knee and ankle moments of force in walking from EMG and kinematic data. J Biomech 18:9–20
Perez MA, Lundbye-Jensen J, Nielsen JB (2006) Changes in corticospinal drive to spinal motoneurones following visuo-motor skill learning in humans. J Physiol 573:843–855. doi:10.1113/jphysiol.2006.105361
Perez MA, Lundbye-Jensen J, Nielsen JB (2007) Task-specific depression of the soleus H-reflex after cocontraction training of antagonistic ankle muscles. J Neurophysiol 98:3677–3687. doi:10.1152/jn.00988.2007
Perez MA, Soteropoulos DS, Baker SN (2012) Corticomuscular coherence during bilateral isometric arm voluntary activity in healthy humans. J Neurophysiol 107:2154–2162. doi:10.1152/jn.00722.2011
Pohja M, Salenius S (2003) Modulation of cortex-muscle oscillatory interaction by ischaemia-induced deafferentation. NeuroReport 14:321–324. doi:10.1097/01.wnr.0000058518.74643.96
Poortvliet PC, Tucker KJ, Finnigan S, Scott D, Sowman P, Hodges PW (2015) Cortical activity differs between position- and force-control knee extension tasks. Exp Brain Res 233:3447–3457. doi:10.1007/s00221-015-4404-8
Psek JA, Cafarelli E (1993) Behavior of coactive muscles during fatigue. J Appl Physiol 74(1985):170–175
Remaud A, Guevel A, Cornu C (2007) Antagonist muscle coactivation and muscle inhibition: effects on external torque regulation and resistance training-induced adaptations. Neurophysiol Clin 37:1–14. doi:10.1016/j.neucli.2007.01.002
Riddle CN, Baker SN (2005) Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol 566:625–639. doi:10.1113/jphysiol.2005.089607
Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM (1989) The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53:1–31
Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6:285–296. doi:10.1038/nrn1650
Stokes IA, Gardner-Morse M (2003) Spinal stiffness increases with axial load: another stabilizing consequence of muscle action. J Electromyogr Kinesiol 13:397–402
Tillin NA, Pain MT, Folland JP (2011) Short-term unilateral resistance training affects the agonist-antagonist but not the force-agonist activation relationship. Muscle Nerve 43:375–384. doi:10.1002/mus.21885
Ushiyama J, Takahashi Y, Ushiba J (2010) Muscle dependency of corticomuscular coherence in upper and lower limb muscles and training-related alterations in ballet dancers and weightlifters. J Appl Physiol 109(1985):1086–1095. doi:10.1152/japplphysiol.00869.2009
Ushiyama J, Masakado Y, Fujiwara T et al (2012) Contraction level-related modulation of corticomuscular coherence differs between the tibialis anterior and soleus muscles in humans. J Appl Physiol 112(1985):1258–1267. doi:10.1152/japplphysiol.01291.2011
Vila-Chã C, Falla D (2016) Strength training, but not endurance training, reduces motor unit discharge rate variability. J Electromyogr Kinesiol 26:88–93. doi:10.1016/j.jelekin.2015.10.016
Wang L, Lu A, Zhang S, Niu W, Zheng F, Gong M (2015) Fatigue-related electromyographic coherence and phase synchronization analysis between antagonistic elbow muscles. Exp Brain Res 233:971–982. doi:10.1007/s00221-014-4172-x
Watanabe RN, Kohn AF (2015) Fast oscillatory commands from the motor cortex can be decoded by the spinal cord for force control. J Neurosci 35:13687–13697. doi:10.1523/jneurosci.1950-15.2015
Williams ER, Baker SN (2009) Renshaw cell recurrent inhibition improves physiological tremor by reducing corticomuscular coupling at 10 Hz. J Neurosci 29:6616–6624. doi:10.1523/jneurosci.0272-09.2009
Winslow AT, Brantley J, Zhu F, Contreras Vidal JL, Huang H (2016) Corticomuscular coherence variation throughout the gait cycle during overground walking and ramp ascent: a preliminary investigation. Eng Med Biol Soc 2016:4634–4637. doi:10.1109/embc.2016.7591760
Witham CL, Riddle CN, Baker MR, Baker SN (2011) Contributions of descending and ascending pathways to corticomuscular coherence in humans. J Physiol 589:3789–3800. doi:10.1113/jphysiol.2011.211045
Cremoux S, Tallet J, Dal Maso F, Berton E, Amarantini D (2017) Impaired corticomuscular coherence during isometric elbow flexion contractions in human with cervical spinal cord injury. Eur J Neurosci. doi:10.1111/ejn.13641
Yang Y, Solis-Escalante T, van de Ruit M, van der Helm FC, Schouten AC (2016) Nonlinear coupling between cortical oscillations and muscle activity during isotonic wrist flexion. Front Comput Neurosci 10:126. doi:10.3389/fncom.2016.00126
Yao B, Salenius S, Yue GH, Brown RW, Liu JZ (2007) Effects of surface EMG rectification on power and coherence analyses: an EEG and MEG study. J Neurosci Methods 159:215–223. doi:10.1016/j.jneumeth.2006.07.008
Yoshida T, Masani K, Zabjek K, Chen R, Popovic MR (2017) Dynamic cortical participation during bilateral, cyclical ankle movements: effects of aging. Sci Rep 7:44658. doi:10.1038/srep44658
Zhan Y, Halliday D, Jiang P, Liu X, Feng J (2006) Detecting time-dependent coherence between non-stationary electrophysiological signals–a combined statistical and time-frequency approach. J Neurosci Methods 156:322–332. doi:10.1016/j.jneumeth.2006.02.013
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dal Maso, F., Longcamp, M., Cremoux, S. et al. Effect of training status on beta-range corticomuscular coherence in agonist vs. antagonist muscles during isometric knee contractions. Exp Brain Res 235, 3023–3031 (2017). https://doi.org/10.1007/s00221-017-5035-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-5035-z