Abstract
Balance disorders after stroke have a particularly detrimental influence on recovery of autonomy and walking. The present study is aimed at assessing the effect of proprioceptive stimulation by neck muscle vibration (NMV) on the balance of patients with right hemispheric lesion (RHL) and left hemispheric lesion (LHL). Thirty-one (31) patients (15 RHL and 16 LHL), mean age 61.5 years (±10.6), mean delay 3.1 (±1.6) months after one hemispheric stroke were included in this prospective study. The mean position in mediolateral and anteroposterior plane of the CoP (center of pressure) and the surface were evaluated using a force platform at rest and immediately after 10 min of vibration on the contralesional dorsal neck muscle. NMV decreases the lateral deviation balance induced by the stroke. Twenty patients (64.5 %) experienced a visual illusion of light spot moving toward the side opposite stimulus. These patients showed more improvement by vibration than those without visual illusion. There was an interaction between sensitivity and side of stroke on the effect of NMV. Proprioceptive stimulation by NMV reduces postural asymmetry after stroke. This short-term effect of the vibration is more effective in patients susceptible to visual illusion. This result was consistent with a central effect of NMV on the structures involved in the elaboration of perception of body in space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major part of a stroke patient’s rehabilitation is focused on balance training, because balance recovery crucially contributes to the end result. Balance is a prognostic factor for autonomy, transferring and walking recovery (Bohannon and Leary 1995; Fong et al. 2001; Nardone et al. 2009). Postural disorders can be explained by motor or sensory deficit (Geurts et al. 2005; Sackley 1990; Winstein et al. 1989), and spatial cognition deficit may add to balance disturbance (Rode 1998).
Compared to patients with left hemispheric lesion (LHL), those with RHL take longer to recover balance and independence (Rode 1998; Perennou 1999). And in left hemiplegics, slow balance recovery is likely to be related to spatial cognition disorders (Goto et al. 2009; Katz et al. 1999; Perennou 1999). Despite this overriding issue of balance disorder management, no treatment techniques specifically addressing this cognitive component of imbalance are currently being used. First tested for the correction of spatial bias in cases of neglect, vestibular, visual or proprioceptive are likely to be of interest in managing the postural deficiencies associated with cognitive disorders after stroke (Rode 1998; Perennou 1999). Several stimulations have been tested to improve body position shifts related to spatial cognition disorders (Bonan et al. 2015). But to our knowledge, neck vibrative stimulation has not yet been tested as a means of improving postural asymmetry after stroke.
The neck muscle proprioception system plays a particularly crucial role in the perception of the body in space and in postural orientation because of its direct links with the vestibular and oculomotor systems (Biguer et al. 1988; Kavounoudias et al. 1999; Marsden et al. 2005). Messages from the proprioceptive receptors of the neck and vestibular receptors in conjunction with eye direction information help to localize objects relative to the body. Vibration is a potent proprioceptive stimulus for the primary endings of the muscle spindles (Gilhodes et al. 1992). In addition to segmental response, neck muscle vibration (NMV) has been shown to produce global effects probably through activation of the brain function related to multisensory integration that helps to restore the body representation (Ivanenko et al. 1999; Karnath et al. 2002; Pettorossi and Schieppati 2014).
Our working hypothesis was that proprioceptive stimulation by neck muscle vibration could improve mental body representation in space and consequently reduce the postural disturbance associated with cognitive impairment after stroke.
The aim of our study was to assess the effects of proprioceptive stimulation by one NMV session on balance disturbances in stroke patients. We also evaluated the relative importance of side of lesion, susceptibility to visual illusion and sensitivity.
Materials and methods
This prospective study was conducted in the physical and rehabilitation medicine (PRM) department of the University Hospital of Rennes and in the neurological PRM unit of Kerpape Center. From April 2011 to April 2013, all patients with either right or left hemispheric stroke were included if they met the inclusion criteria.
Population
The group consisted of thirty-one patients: 25 men and 6 women with a mean age of 61.5 years (standard deviation (SD) 10.6), all of them right-handed and who were admitted to the study less than 6 months after their first hemispheric stroke. Patients were included provided they were able to stand up with closed eyes for 30 s and had a lateral ipsilesional deviation of the CoP. Patients were not included if they were over 80 years of age, had a neurological history before stroke or suffered from vertigo, vestibular dysfunction, amblyopia or reduced alertness. Aphasic patients were not included if they failed to understand the procedure. A complete examination was performed: functional independence using the Barthel Index (Mahoney and Barthel 1965), motor impairment using the motricity index (Collin and Wade 1990) and the functional ambulation classification modified (FAC, Holden et al. 1984). Postural performances were considered by the Postural Assessment Scale for Stroke (PASS). Neglect was evaluated by the Bells test, line bisection and figure copy and considered to be present if at least two tests were positive.
After having reviewed the research project, the local Ethics Committee of Rennes University Hospital has issued a favorable opinion (Number 11.12). Before testing, we collected informed written patient consent.
Experimental protocol
Neck muscle vibration
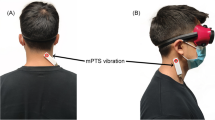
Subjects were made to sit in a dark room. A red light spot was projected on the wall in front of patient. Patients were classified as susceptible to vibration if a visual illusion of light spot deviation occurred toward the side opposite the stimulation. In this case, the vibrator was positioned on the neck so as to ensure that the vibration would cause the maximal illusion of light spot deviation. When no illusion was induced, the position of the vibrator was in the position mostly used in responsive patients: the para-occipital area on the contralesional side. In this position, vibration is presumably above the semispinalis and splenius (Fig. 1).
Representation of patient with right hemisphere lesion (represented by a cross) receiving a contralesional side vibration (represented by a cube). This patient is susceptible to visual illusion: In the dark, the patient feels an illusion of light spot movement toward the side opposite the vibration area (illusion of spot deviation represented by an arrow
After vibration positioning, the subjects received neck muscle vibration for 10 min while blindfolded (Fig. 2). Following previous studies about neck muscle vibration (Gilhodes et al. 1992; Biguer et al. 1988), we applied vibration on the side toward which we expect displacement. So the vibration is set up on the left side when the cerebral lesion is on the right.
The vibration was continuously delivered at 80 Hz by a plastic tube 7 cm long and 3 cm diameter (VB 115®, Techno Concept, France) held in place by a rubber band. Stimulation was given in the sitting position, which reduces the risk of falling that can be induced by a change of balance (Duclos et al. 2007; Wierzbicka et al. 1998).
Quantification of the postural responses to neck vibration
The first evaluation was carried out at rest. Patients stood on a force platform (SATEL®, France) for 25 s, and the displacement of the center of pressure (CoP) was recorded (Brun et al. 1993). The instruction given to the patient was to maintain a standing position, arms at the sides. The first evaluation consisted of 4 sessions: 2 sessions with open eyes (OE) and 2 sessions with closed eyes (CE). For the “closed eyes” situation, the patient was provided with a headband to reduce the risk of eyes opening. The second evaluation was carried out with the same protocol immediately after 10 min of dorsal neck vibrations in sitting position, beginning with the eyes-closed assessment (Fig. 3).
Pattern of evaluation and stimulation procedure with time: t1—two posturographies (25 s each) with eyes open; t2—two posturographies (25 s) with eyes closed; t3—vibration positioning in darkness; t4—10 min of neck muscle vibration (NMV); t14—two posturographies (25 s) with eyes closed after NMV; t15—two posturographies (25 s) with eyes open after NMV
For each session, the mean position of the CoP in the mediolateral (ML) plane and in the anteroposterior (AP) plane (in mm) and the surface (mm2) were calculated. The average position during each of the two closed-eyes sessions and the average position during each of the two open-eyes sessions were then calculated for the first evaluation (at rest) and the second evaluation (after vibration). The difference in the mean position of the CoP (mm) between the first (at rest) and the second (after vibration) evaluation was calculated first with closed eyes and then with open eyes. Expressed in terms of pre-post difference, the difference was positive if the position of the CoP had improved once it had been completed and negative if it was worse.
Statistics
We used SAS software. Mean ML and AP position of the CoP and the surface during the eyes-open and the eyes-closed situations were compared at rest and after vibration with Wilcoxon test. The alpha level was set at 5 %. We tested the effect of 3 factors on the variation using a mixed model with repeated measure subject adjusted to the rest position of the CoP: side of the lesion (RHL vs. LHL); susceptibility to visual illusion versus non-susceptibility to visual illusion; normal sensitivity versus low sensitivity. For multiple comparisons, we used a Bonferroni correction.
Results
Clinical data (Table 1)
The evaluation was conducted on average 3.1 months after stroke (SD 1.6). The cerebral lesion was right in 15 cases and left in 16 cases: 19 ischemic and 12 hemorrhagic.
Four patients had left spatial neglect. The motricity index was 49.4/100 (SD 24.6).
Epicritic sensitivity is tested twice compared to the healthy side by a foam point on thigh, leg and foot. The test is abnormal if the patient describes one zone of hypoesthesia or anesthesia on the contralesional side. Sensitivity was normal in 16 patients, abnormal in 15 patients (14 with hypoesthesia, one with anesthesia). The mean modified functional ambulation classification was 3.7/8 (SD 2.1). There was no difference between the RHL group and the LHL group (Table 1).
The posturography results in LHL and RHL groups are summarized in Table 2, and in absolute terms, no difference was noted between the two groups.
Effect of neck muscle vibration on all patients
The mean mediolateral CoP position of the patients was significantly displaced after vibration toward the hemiplegic side, i.e., to the left for the RHL and to the right for the LHL (the variation was positive) (ML deviation: 20.7 ± 11.5 mm before NMV to 18.5 ± 13.5 mm after NMV; mean variation 3.1; p < 0.005) (Tables 3, 4). An example of deviation is presented in Fig. 4 for a LHL patient. Average AP position and surface did not change after vibrations (Table 3).
Effect of susceptibility to visual illusion, side of the stroke, sensitivity and neglect
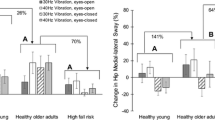
Twenty patients (64.5 %) (11 RHL and 9 LHL) were found to be susceptible to visual illusion as they described the illusion of light deviation toward side of the lesion. The effect of vibration was present in patients who felt visual illusion (mean displacement variation: 4.7 vs. −0.08 mm, p < 0.05) Table 5.
Patients with a sensitivity deficit were more improved than those without it (4.3 vs. 1.7; p < 0.05). There is no dependence between motor capacities.
Overall, there was an interaction effect between side of stroke and sensitivity (p < 0.05). When comparing the two sides using multiple-comparison Bonferroni correction, we noted a very wide variation in hyposensitive patients with LHL 8.51 versus with RHL 1.08 (p = 0.19) (Fig. 5). While the effect of neglect was not statistically studied because the size of the group was insufficient (4 persons), these patients seemed to be more deviated at rest and to have more deviation in an eyes-open situation after NMV.
Discussion
Balance maintenance requires multiple afferent information (from vestibular, visual and proprioceptive receptors) and efferent information (from motor effectors) in order to elaborate internal spatial representations of the body in space (Andersen et al. 1993; Merfeld et al. 1999). This information can be transformed into different and changing systems of coordinates, using allocentric referential (environmental information) or egocentric referential (related to the subject’s body), with regard to the position of the subject in space, and the task to be accomplished. This spatial cognitive processing can be altered by a stroke lesion, leading to a bias in body orientation (Perennou 1999; Rode 1998).
One session of NMV produces an immediate repositioning movement toward the stimulus. In addition, as previously described, in some patients it provokes the illusion of a lateral translation of the visual environment toward the contralateral side of the stimulus, which is typified by the illusory perception of movement to the right of a stationary light spot when the left neck muscle has received a vibration (Wierzbicka et al. 1998). As in other studies, we selected an area where the vibrations cause the greatest deviation of the light spot to the opposite side. Similarly to other authors, we found variable susceptibility to NMV visual illusion (Karnath et al. 2002; Schindler 2004). Nearly 2/3 of our patients were sensitive to visual illusion. Individual preference for proprioceptive input could explain this variability in behavior, a hypothesis supported by previous studies (Kluzic et al. 2007; Vibert et al. 2006). Interestingly, we found that the efficacy of NMV in correcting the postural bias was higher in patients who experience this visual illusion of an environmental displacement. This finding could be interpreted as no inhibition of descending signal in patients visually susceptible, and in this case, NMV might influence more the postural proprioceptive. NMV not only triggers a repositioning movement in the direction of the stimulation, but also provokes an illusory movement of the visual environment. This illusion was concomitant to a deviation of the subjective perception of the body (Karnath et al. 2002; Magnusson and Fransson 2006). NMV consequently seems to influence the relative position of the body with respect to space.
This hypothesis is also reinforced by the study of Bottini (2001), which carried out position emission tomography scan during neck muscle vibration and showed cerebral activity in areas of multisensory integration, i.e., the insula, parietal operculum and superior temporal gyrus. Finally, some authors have studied cerebral activation during the illusion of movement produced by muscle vibrations, using functional magnetic resonance imaging (Naito et al. 2007; Romaiguère et al. 2003). Romaiguère et al. compared the cortical activities arising in subjects, with or without an illusion of movement. The illusion of movement specifically activated not only the controlateral sensorimotor and premotor, but also the parietal cortices in which the representation of body in space is elaborated. Moreover, Weiller et al. (1996) showed that while activation in the sensorimotor cortex was almost identical during voluntary and passive movements, the inferior parietal lobule was more active during passive movements, which suggests that a sensation of movement occurring in the absence of any voluntary command could lead to ascertaining by the central nervous system of the body representation in the parietal cortex. Altogether, these arguments are consistent with a central effect of NMV. NMV could ameliorate postural imbalance after stroke due to spatial cognitive impairment through central activation of the cortical areas involved in the mental representations of body (Roll 2003).
It should be added that neck vibrations seem particularly effective as a means of modulating the egocentric frame of reference since they produce a bias in the subjective perception of the body median with “straight-ahead” perception deviated to the side of the vibration (Karnath et al. 1993, 2002). Moreover, neck vibrations have been shown to improve motor actions in a neglected space, i.e., tactile and visuospatial exploration and copying tasks in neglect patients (Johannsen et al. 2003; Schindler et al. 2002). Finally, another study has shown that unlike optokinetic manipulation, neck vibration does not seem to effectively contribute to judgments of object size, thereby suggesting that NMV may not act on the allocentric references (Schindler 2004).
A few studies have evaluated the effect of sensory stimulations on postural asymmetry in stroke patients. Prism adaptation using an artificial deviation of visual environment induced by 10° optical prisms was shown to effectively reduce postural asymmetry in right-lesioned patients (Tilikete et al. 2001). In 1998, Rode studied the effect of vestibular caloric stimulation which reduces the postural asymmetry. Finally, optokinetic and galvanic stimulations have been recently tested in stroke patients (Bonan et al. 2016). Both optokinetic and galvanic vestibular stimulations could modulate the position of the hemiparetic’s center of pressure (CoP), and the postural effect was nearly double for RHL patients compared to that for LHL. Our study is the first study testing the effectiveness of contralesional dorsal neck muscle vibration (NMV) as a means of reducing postural asymmetry in patients undergoing stroke rehabilitation. In our study, however, we found no significant difference between the RHL and the LHL groups in variation of the CoP after vibration. That said, our post-stroke delay was nearly 3 months, compared to 6 months in Rode’s study, and we assume that this difference explains the discrepancy. In our patients, postural asymmetry at rest in RHL and LHL was quite similar, which is probably due to the fact that over 3 months, spatial cognitive impairment is not limited to RHL. It would be useful to compare these findings to the symptoms of neglect that are known to be initially present in both RHL and LHL. At a later stage, the LHL patients recovered more rapidly from the visuospatial disturbances and differences in visuospatial behavior that arise only at a distance between the RHL and the LHL.
Perennou et al. tested 3 groups of stroke patients (RHL with neglect, RHL without neglect and LHL) by another proprioceptive stimulation: transcutaneous electrical stimulation on neck muscles during sitting position on a rocking platform (Perennou et al. 1996). The stimulation effectively reduced sitting balance instability in neglect patients only. In our study, stability in the standing posture was not improved since the surface did not change. However, it is quite difficult to compare our study with the latter study because stimulation was given in a sitting position on a rocking platform and evaluation was performed under stimulation. It should also be recalled that our neglect patients were too few for statistical analysis. The statistical analysis was performed excluding neglect patients but do not influence results.
An interesting result of our study was that NMV was more effective in patients with abnormal sensitivity, especially in LHL. These patients with low sensitivity are probably more disturbed in their sensory integration and could be more receptive to sensory stimulation because of a higher spatial distortion. Those results were viewed with caution because of reduced statistical power due to the small sample. The particular effect in LHL could be explained by a more potent action of the stimulation due to the fact that the central structures of the right hemisphere receiving multiple sensory information involved in the elaboration of the representation of body in space have been spared.
In our study, contrary to what was expected an anterior–posterior deviation was not found. It could be explained by results in standard norms in our patients before stimulation, and probably because the stimulation is unilateral. Furthermore, our primary aim is to research the effect on the mediolateral deviation, so we positioned the vibrator to have a maximum of light spot deviation toward the opposite side of stimulation. If there was an illusion of vertical spot movement, we changed the vibrator position.
The order of post-stimulation tests (open eyes or closed eyes) has not been randomized. This would have been interesting, but we chose to conduct the assessment eyes closed just after stimulation so as not to cancel the effect of vibration by eyes opening. Visual feedback has a stabilizing influence on postural control, providing additional information on position and orientation. Maintenance of eye closure during evaluation increased the efficiency of neck vibration in many patients, thereby suggesting that sensory recalibration may be less effective when visual afference is present. In previous studies it was equally important to suppress visual cues in order to obtain a better result in terms of sensorial stimulation (Karnath et al. 2002; Gomez et al. 2009). Our findings are also consistent with the notion that the effect of sensory stimulation is induced by an illusion. The presence of visual cues definitely prevents the illusion of body translation relative to the visual environment elicited by vibration. This could be especially crucial for stroke patients who have been shown to be visually dependent (Bonan et al. 2013; Yelnik 2005).
It is known that neck proprioception interacts with vestibular system. Vibration of the neck muscles modifies the perception of vestibular self-motion (Pettorossi et al. 2015). We cannot separate the interaction neck proprioception and the vestibular sense, but to our knowledge no study of the direct effect of neck vibration on vestibular system has been carried out. The stimulation is carried out some distance from the mastoid, and to limit the vestibular activation, we used a low vibration amplitude. Other study with vestibular evoked potential could answer this question.
We studied the immediate effect of neck muscle vibration after stimulation because fatigability of hemiplegic patients in a standing position did not allow for prolonged assessments. But in other studies on healthy subjects, correction of the bias, which was maintained throughout proprioceptive stimulation, did not stop once the vibration sensation had run its course, and even persisted after stimulation stopped (Karnath et al. 2002). This result suggests a long-lasting and significant change in the perception of the body in space. Pettorossi et al. (2015) found that high-frequency neck muscle vibration induces an effect during 4 h on self-motion perception. Filippi et al. in (2009) found 90 days, a decrease in the area of sway of CoP after quadriceps vibration. However, the vibration of neck muscles appears to be effective in the long term on neglect symptoms if they are repeated (Johannsen et al. 2003). The long-term effectiveness of vibration in the specific indication of the balance disorder due to poor representation of body in space has to be confirmed in further study. That said, its long-lasting effect over several sessions remains to be verified, even though it has already been tested for symptoms of visuospatial neglect (Schindler 2004; Johannsen et al. 2003). Shindler showed that an effect on neglect symptoms could last at least 2 months. Johannsen demonstrated that ten 20-min NMV sessions may have an effect on neglect symptoms lasting for more than 1 year (Johannsen et al. 2003). Improvement for neglect symptoms was even more pronounced when the vibration was associated with visual scanning (Schindler 2004) or occupational therapy (Kamada et al. 2011). As regards postural control, it would be of major interest to have it performed in association. As for neglect, it could be carried out as a supplement to traditional rehabilitation (with the advantage that it requires no additional time for the therapist) either simultaneously with balance exercises in a seated or standing position, or else during dynamic exercises. After our preliminary study, we have chosen to test in a new study the long-term effect of repetitive NMV sessions.
NMV is technically simple, easy to use in rehabilitation centers and very cheap. In our study, side effects were not described, as they had been in previous studies (Biguer et al. 1988). The technique will be easy to apply, especially in the immediate post-stroke period, when spatial disorders (body misorientation and neglect) are the most flagrant. It can conveniently be used before balance acquisition as the patient can remain in a seated position, and once the vibration is in place, his cooperation is not required.
Conclusion
One session of NMV could reduce postural asymmetry after stroke. The durability of its effects will require confirmation in future studies. Several findings underscore its therapeutic effects on the postural disturbances related to spatial cognition impairment, the effects being achieved through central action on the structures involved in the representation of the body in space. It could therefore be useful to include this technique in balance rehabilitation in order to specifically treat the cognitive component of postural disturbances.
References
Andersen RA, Snyder LH, Li CS, Stricanne B (1993) Coordinate transformations in the representation of spatial information. Curr Opin Neurobiol 3:171–176
Biguer B, Donaldson IML, Hein A, Jeannerod M (1988) Neck muscle vibration modifies the representation of visual motion and direction in man. Brain 111:1405–1424
Bohannon RW, Leary KM (1995) Standing balance and function over the course of acute rehabilitation. Arch Phys Med Rehabil 76:994–996
Bonan IV, Marquer A, Eskiizmirliler S, Yelnik AP, Vidal PP (2013) Sensory reweighting in controls and stroke patients. Clin Neurophysiol 124:713–722
Bonan I, Chochina L, Moulinet-Raillon A, Jamal K, Leblong E, Leplaideur S (2015) Effect of sensorial stimulations on postural disturbances related to spatial cognition disorders after stroke. Clin Neurophysiol 45:297–303
Bonan IV, Leblong E, Leplaideur S, Laviolle B, Tasseel Ponche S, Yelnik AP (2016) The effect of optokinetic and galvanic vestibular stimulations in reducing post-stoke postural asymmetry. Clin Neurophysiol 127(1):842–847
Bottini G (2001) Cerebral representations for egocentric space. Functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain 124:1182–1196
Brun V, Dhoms G, Henrion G (1993) Balance sway in hemiplegic patients: evaluation method and correlative study. Ann Réadapt Méd Phys 36:169–177
Collin C, Wade DT (1990) Assessing motor impairment after stroke: a pilot reliability study. J Neurol Psychiatry 53:576–579
Duclos C, Roll R, Kavounoudias A, Roll JP, Forget R (2007) Vibration-induced post effects: a means to improve postural asymmetry in lower leg amputees. Gait Posture 26:595–602
Filippi GM, Brunetti O, Botti FM, Panichi R, Camerota F, Cesari M, Pettorossi VE (2009) Improvement of stance control and muscle performance induced by focal muscle vibration in young-elderly women: a randomized controlled trial. Arch Phys Med Rehabil 90(12):2019–2025
Fong KN, Chan CC, Au DK (2001) Relationship of motor and cognitive abilities to functional performance in stroke rehabilitation. Brain Inj 15:443–453
Geurts ACH, De Haart M, Van Nes IJW, Duysens J (2005) A review of standing balance recovery from stroke. Gait Posture 22:267–281
Gilhodes JC, Gurfinkel VS, Roll JP (1992) Role of Ia muscle spindle afferents in post-contraction and post-vibration motor effect genesis. Neurosci Lett 135:247–251
Gomez S, Patel M, Magnusson M, Johansson L, Einarsson EJ, Fransson PA (2009) Differences between body movement adaptation to calf and neck muscle vibratory proprioceptive stimulation. Gait Posture 30:93–99
Goto A, Okuda S, Ito S, Matsuoka Y, Ito E, Takahashi A, Sobue G (2009) Locomotion outcome in hemiplegic patients with middle cerebral artery infarction: the difference between right and left-sided lesions. J Stroke Cerebrovasc Dis 18:60–67
Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl-Baker L (1984) Clinical gait assessment in the neurologically impaired. Phys Ther 64:35–40
Ivanenko YP, Talis VL, Kazennikov OV (1999) Support stability influences postural responses to muscle vibration in humans. Eur J Neurosci 11:647–654
Johannsen L, Ackermann H, Karnath HO (2003) Lasting amelioration of spatial neglect by treatment with neck muscle vibration. J Rehabil Med 35:249–253
Kamada K, Shimodozono M, Hamada H, Kawahira K (2011) Effects of 5 min of neck muscle vibration immediately before occupational therapy on unilateral spatial neglect. Disabil Rehabil 33:23–24
Karnath HO, Christ K, Hartje W (1993) Decrease of controlateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain 116:383–396
Karnath HO, Reich E, Rorden C, Fetter M, Driver J (2002) The perception of body orientation after neck-proprioceptive stimulation. Exp Brain Res 143:350–358
Katz N, Hartman-Maier A, Ring H, Soroker N (1999) Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Arch Phys Med Rehabil 80:379–384
Kavounoudias A, Gilhodes JC, Roll R, Roll JP (1999) From balance regulation to body orientation: two goals for muscle proprioceptive information processing. Exp Brain Res 124:80–88
Kluzik J, Peterka RJ, Horak FB (2007) Adaptation of postural orientation to changes in surface inclination. Exp Brain Res 178(1):1–17
Magnusson M, Fransson PA (2006) Cervical muscle afferent play a dominant role over vestibular afferent during bilateral vibration of neck muscles. J Vestib Res 16(3):127–136
Mahoney FI, Barthel D (1965) Functional evaluation: the Barthel index. Md State Med J 14:61–65
Marsden JF, Playford DE, Day BL (2005) The vestibular control of balance after stroke. J Neurol Neurosurg Psychiatry 76:670–678
Merfeld DM, Zupan L, Peterka RJ (1999) Humans use internal models to estimate gravity and linear acceleration. Nature 398:615–618
Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, Sadato N (2007) Human limb-specific and non-limb-specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci 25:3476–3487
Nardone A, Godi M, Grasso M, Guglielmetti S, Schieppati M (2009) Stabilometry is a predictor of gait performance in chronic hemiparetic stroke patients. Gait Posture 30:5–10
Perennou D (1999) Postural balance following stroke: toward a disadvantage of the right brain-damaged hemisphere. Rev Neurol 155:281–290
Perennou D, Pelissier C, Amblard B (1996) Posture and postural control following a cerebrovascular accident: a review. Ann Réadapt Méd Phys 39:497–513
Pettorossi VE, Schieppati M (2014) Neck proprioception shapes body orientation and perception of motion. Front Hum Neurosci. doi:10.3389/fnhum.2014.00895
Pettorossi VE, Panichi R, Massimo Botti F, Biscarini A, Filippi GM, Schieppati M (2015) Long lasting effects of neck muscle vibration and contraction on self motion perception of vestibular origin. Clin Neurophysiol 126:1886–1900
Rode G (1998) Postural asymmetry reduction by vestibular caloric stimulation in left hemiparetic patients. Scand J Rehabil Med 30:9–14
Roll JP (2003) Physiologie de la kinesthèse. Intellectica 36–37:49–66
Roll JP, Roll R (1988) From eye to foot: a proprioceptive chain involved in postural control. In: Amblard B, Berthoz A, Clarac F (eds) Posture and gait. Elsevier, Amsterdam, pp 155–164
Romaiguère P, Anton JL, Roth M, Casini L, Roll JP (2003) Motor and parietal cortical areas both underlie kinesthesia. Cogn Brain Res 16:74–82
Sackley CM (1990) The relationship between weight-bearing asymmetry after stroke, motor function and activities of daily living. Physiother Theory Pract 6:179–185
Schindler I (2004) Convergent and divergent effects of neck proprioceptive and visual motion stimulation on visual space processing in neglect. Neuropsychologia 42:1149–1155
Schindler I, Kerkhoff G, Karnath HO, Keller I, Goldenberg G (2002) Neck muscle vibration induces lasting recovery in spatial neglect. J Neurol Neurosurg Psychiatry 73:412–419
Tilikete C, Rode G, Rossetti Y, Pichon J, Li L, Boisson D (2001) Prism adaptation to rightward optical deviation improves postural imbalance in left-hemiparetic patients. Curr Biol 11:524–528
Vibert N, Hoang T, Gilchrist DP, MacDougall HG, Burgess AM, Roberts RD, Vidal PP, Curthoys IS (2006) Psychophysiological correlates of the inter-individual variability of head movement control in seated humans. Gait Posture 23(3):355–363
Weiller CJ, Jüptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, Müller S, Diener HC, Thilmann AF (1996) Brain representation of active and passive movements. Neuroimage 4:105–110
Wierzbicka MM, Gilhodes JC, Roll JP (1998) Vibration induced postural posteffects. J Neurophysiol 79:143–150
Winstein CJ, Gardner ER, McNeal DR, Barto PS, Nicholson DE (1989) Standing balance training: effect on balance and locomotion in hemiparetic adults. Arch Phys Med Rehabil 70:755–762
Yelnik A (2005) Evolution of the concepts concerning rehabilitation treatment for hemiplegic patients. Ann Read Med Phys 48:270–277
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leplaideur, S., Leblong, E., Jamal, K. et al. Short-term effect of neck muscle vibration on postural disturbances in stroke patients. Exp Brain Res 234, 2643–2651 (2016). https://doi.org/10.1007/s00221-016-4668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-016-4668-7