Abstract
We previously showed that standing on an inclined surface resulted in an after-effect of leaning in many healthy, blindfolded subjects when they returned to standing on a horizontal surface (Kluzik et al. in Exp Brain Res 162:474–489, 2005). The direction of leaning depended on the direction of prior surface inclination, always in a direction that preserved the relative alignment between the body and the support surface. For example, subjects leaned forward after they stood on a toes-up-inclined surface. In the present study, we investigated how the amplitude of surface inclination affected postural muscle activity, joint position, body segment orientation, and body center of mass (CoM) and foot center of pressure (CoP) locations before, during, and after subjects stood on an inclined surface. We asked whether the mechanism that underlies the lean after-effect involves regulation of local postural variables, such as the position of the ankle joint or the level of muscle activity, or whether instead, the mechanism involves regulation of global, whole-body postural variables that can only be determined by multisensory processing, such as orientation of the trunk or the body’s CoM. In one experiment, we found that varying the amplitude of a toes-up surface inclination between 2.5° and 10° had a systematic, linear, effect on the post-incline orientation of the trunk and head, but did not systematically affect the post-incline orientation of the legs, position of the ankle joint, the level of EMG activity, or the location of the CoP. In a second experiment, we found that preventing the legs from leaning in the post-incline period did not abolish leaning of the upper body. These findings suggest that (1) the body-to-support-surface relationship is an important reference for the CNS internal representation of postural orientation which is subject to adaptive modification and (2) the adaptive mechanism underlying the post-incline after-effect of leaning acts at the level of global, whole-body postural variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Healthy people have little difficulty controlling their posture or gait when transitioning from an inclined to a horizontal surface. However, even healthy people experience a momentary disorientation immediately after the surface conditions change, for example, when stepping off a boat, off an inclined treadmill, or out of inclined ski boots. Many patients with sensory or central nervous system disorders experience severe disorientation on altered surfaces and cannot easily adapt their posture to changing surface conditions (Gordon et al. 1995b; Nachum et al. 2004; Martin 1965; Nashner et al. 1982).
Evidence from a number of studies suggests that the sensorimotor systems involved in spatial orientation must be actively recalibrated, or must adapt, when conditions in the environment are altered (Lackner and DiZio 2000; Rieser et al. 1995; Weber et al. 1998). For example, after a period of walking on a rotating, circular treadmill, subjects with eyes closed walk in curved trajectories when attempting to walk straight ahead in a way that suggests learning of a new foot-to-trunk relative relationship, an effect called Podokinetic After-Rotation or PKAR (Gordon et al. 1995a; Weber et al. 1998). Likewise, after jogging for several minutes on a treadmill, subjects with eyes closed jogged forward, backward or sideways when attempting to jog-in-place on a stationary surface, and the direction of drift when attempting to jog-in-place depended on whether the subject had previously jogged forward, backward or sideways when on the treadmill (Anstis 1995). These adaptation after-effects maintained the same body-to-surface relationship as used in the prior surface conditions, suggesting that the experience with an altered surface condition caused a recalibration of how somatosensory information was interpreted to determine the relationship of the body to the surface. We recently showed that a similar adaptive process might be involved in the control of postural orientation when subjects adapt to a change in the inclination of the support surface (Kluzik et al. 2005).
After a period of standing on an inclined surface, we found that most healthy blindfolded subjects leaned when they returned to standing on a horizontal surface (Kluzik et al. 2005). The duration of the post-incline lean varied widely across subjects, ranging from a few seconds to as long as 4 min of leaning, but was highly consistent within subjects upon repeated testing, even when test sessions were months apart. Subjects leaned in the post-incline period, even though vestibular otolith information, foot-pressure somatosensory information, and proprioceptive information related to muscular effort should have allowed subjects to detect that they were leaned away from vertical with respect to gravity. The direction of leaning depended on the direction of surface inclination, such that leaning maintained a similar relative alignment between the body and the surface as when the subjects had stood on the inclined surface. Subjects leaned forward after standing on a toes-up inclined surface, backward after standing on a toes-down inclined surface, and rightward after standing on a right-side-up inclined surface. This result suggests that leaning may have occurred because of a sensorimotor adaptation involving the postural orientation system, although the exact nature of this underlying mechanism and which controlled postural variables underwent adaptation is not clear.
In the present study, we investigated how the amplitude of surface inclination affected postural variables during and after subjects stood on an inclined surface. By identifying which variables systematically changed or were held constant in response to altered surface orientation, we expected to gain a better understanding of the mechanism that underlies the leaning after-effect. One explanation for the post-incline lean could be that when someone stands an inclined surface, the nervous system adaptively recalibrates the set point for postural orientation, with the postural set point defined as a preferred, habitual postural alignment that the nervous system tries to maintain (Lestienne and Gurfinkel 1988; Lacquaniti et al. 1990). Upon return to a horizontal surface, the person might lean because of persistence of the adapted set point, which only gradually readapts toward the habitual set point for standing on a horizontal surface. If leaning were caused by an adaptive mechanism for adjusting the postural set point, then we would expect to find specific, systematic relationships between specific postural variables and the support surface as a function of the features of the surface inclination, such as the amplitude of inclination.
Another explanation for the post-incline lean could be an after-contraction effect in ankle muscles, which undergo large changes in length and postural demands when surface inclination changes. After-contraction effects are involuntary muscle contractions and movements that follow cessation of a period of sustained muscle activity and are believed to be caused by either peripheral (Hagbarth and Nordin 1998) or central (Duclos et al. 2004; Ghafouri et al. 1998; Gurfinkel et al. 1989) adaptive changes involving the muscle spindles. If leaning were caused by after-contraction effects, we would expect to find a pattern of tonic muscle activation in the post-incline period that systematically related to the pattern of tonic muscle activation observed while subjects stood on the inclined surface. For example, it is possible that shortened ankle dorsiflexors were tonically active when subjects stood on the inclined surface (Aniss et al. 1990), and that tonic activation of ankle dorsiflexors in the post-incline period pulled subjects into a leaned posture.
Identification of which postural variables undergo systematic adaptation to different amplitudes of surface inclination may provide insight into which variables are predominantly used by the central nervous system to regulate postural orientation. Postural orientation is a complex sensorimotor task that involves establishing a postural reference for body orientation with respect to the external world and the body segments to one another, while simultaneously regulating equilibrium (Gurfinkel et al. 1995; Horak and Macpherson 1996; Lestienne and Gurfinkel 1988; Massion 1994). Several lines of evidence suggest that the nervous system organizes postural orientation by controlling global, kinematic variables, such as leg length and orientation in space (Lacquaniti et al. 1990; Maioli and Poppele 1991) or how the trunk is oriented with respect to the support surface (Fung and Macpherson 1995; Gurfinkel et al. 1981, 1995; Horak and Macpherson 1996), variables which cannot be sensed directly and which require multisensory integration, rather than local joint angles or muscle activation, which can be sensed more directly. Force-related variables, such as contact forces against the ground, could also be used to estimate and control the location of the body’s center of mass (CoM) with respect to the base of support (Lacquaniti and Maioli 1994; Massion 1994). Information about the location of the center of pressure (CoP) from ground reaction forces, derived from the integration of somatosensory receptor systems in the foot, could provide information for orientation of the whole body CoM, as long as movement is slow and body configuration is not drastically altered (Kavounoudias et al. 1998).
In this study, we investigated how systematically varying the amplitude of surface inclination affected global kinematic, kinetic, and local joint variables during and after healthy subjects stood on an inclined surface. We also measured muscle activation to determine whether leaning can be explained by after-contraction effects that maintain EMG activity when surface inclination changes. In a second experiment, we prevented subjects’ legs from leaning during the post-incline period to more explicitly test whether the adaptive mechanism responsible for leaning acts only on local postural variables, such as the position of the ankle joint or the level of activity in specific muscles, or instead, acts on more global postural variables. Preliminary results of this work have been reported as an abstract (Kluzik et al. 2000).
Methods
Subjects
Seven healthy subjects (5 F and 2 M, mean age 36.1 ± 10.7 years, range 22–49 years) participated in two experiments. In a previous study that showed that the duration of the post-incline lean varies along a continuum in healthy subjects, some subjects did not lean at all or leaned for only a few seconds, while others leaned for up to 4 min, and half (27/51) of the tested subjects leaned for over 1 min (Kluzik et al. 2005). Since the goal of the present study was to understand how different postural variables contribute to the leaning after-effect, we chose to study a group of subjects who demonstrated long-duration after-effects. Thus, we screened subjects for persistence of leaning and excluded subjects who did not lean for at least 1 min after they stood on a 5° toes-up-inclined surface for 2.5-min. Of the seven subjects who participated in the present study, four participated in a previous study of stance on an inclined surface. The remaining three subjects were naïve and were selected after screening four subjects. Subjects were healthy and free of musculoskeletal, neurological, or other health impairments that could affect posture or balance. The experimental protocol conformed to the standards set by the Declaration of Helsinki and was approved by the Institutional Review Board at Oregon Health & Science University. All subjects gave their informed consent prior to participation in experiments.
Protocol for Experiment 1
We studied the postural alignment and postural muscle activity of subjects before, during, and after they stood on a toes-up inclined surface. Subjects were blindfolded to eliminate visual information and wore headphones to remove auditory information that could influence postural orientation. In addition, a short story audiotape was played through the headphones in order to distract each subject’s attention away from postural alignment. Subjects were instructed to: ‘stand in a relaxed way; listen to the story and try not to pay attention to your posture. Do not resist any pull or tendency to lean if it occurs’. Subjects stood with head facing forward, arms crossed in front of the chest, and feet spaced a comfortable, self-selected distance apart, approximately hip width. Foot placement for each subject was kept constant across all trials.
Figure 1a shows the surface conditions for a single trial. Trials were 8.5 min in duration and consisted of a pre-incline period of 1 min when the support surface was horizontal, a during-incline period of 2.5 min when the surface was inclined in the toes-up direction, and a post-incline period of 5 min when the surface was again horizontal. Transitions between the horizontal and inclined conditions were accomplished by rotating the surface at a constant velocity of 1°/s, with the axis of rotation at approximately ankle height. Surface rotations were slow in order to avoid sharp accelerations that could trigger fast proprioceptive-triggered balance responses.
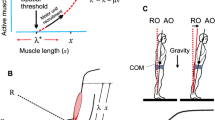
Experimental protocol and dependent variables. a Shows that each trial consisted of a pre-incline period, a during-incline period in which amplitude of inclination was varied, and a post-incline period. Gray shading indicates the period during which the surface was inclined. In the post-incline period of Experiment 1, the legs were free to lean. In the post-incline period of Experiment 2, the legs were blocked from leaning by a stable horizontal support. b Shows a drawing of the device that was used to block forward hip movement in the post-incline period. c The marker set-up (black circles) for motion analysis data capture and defines body segment angles (head, trunk, leg) and joint angles (ankle, knee, hip). Arrows indicate the positive going direction for segment angles
Subjects were exposed to four incline-amplitude conditions (2.5°, 5°, 7.5°, and 10°). Each subject participated in 3 trials of each incline-amplitude condition, for a total of 12 trials. For each experiment, the three trials of the same amplitude condition were conducted within a single day, with trials scheduled at least 2 h apart to minimize carry-over effects. The order in which the different amplitude conditions were presented to each subject was randomized.
Protocol for Experiment 2
The protocol for Experiment 2 was similar to that of Experiment 1, with the addition of a device used for preventing leg movement during the post-incline period (Fig. 1a). Subjects were exposed to three trials of three amplitudes of toes up surface inclinations (2.5°, 5°, and 10°). Forward leaning of the lower body was prevented by a rigid, horizontal bar placed in front of the hips at the height of the greater trochanter (Fig. 1b). The rigid bar was placed in front of the hips only during the post-incline period. To be able to move the bar out of the way during the pre- and during-incline periods, the restraint bar was attached at one end to a stable, perpendicular bar by means of a low-friction device that allowed the bar to glide in a forward–backward direction relative to the subject. At the start of each trial, we marked the location of the bar necessary to hold the subject at his or her baseline postural alignment and to prevent forward leaning. We then moved the bar out of the way for the pre- and during-incline periods. Thus, while subjects stood upon the inclined surface, their stance was unrestricted and conditions were identical for the ‘legs blocked’ and the ‘legs free’ experiments. When the surface began to rotate from the inclined to horizontal positions at the beginning of the post-incline period, the bar was moved to the marked baseline location and locked into place where it stayed for the remainder of the trial. The bar prevented forward leaning of the lower body during the post-incline period, as can be seen in the data that are shown in Fig. 7.
Data collection and analysis
Changes in postural orientation activity were measured through kinematic, force-plate, and electromyographic data. The following variables that reflect postural orientation were calculated: angular orientation of the head, trunk, and leg (ankle-to-hip) segments with respect to gravity and with respect to the surface, joint angles of the ankle, knee and hip, angular orientation of the body’s CoM with respect to the ankle joint, and location of CoP underneath the feet. All data were analyzed in the sagittal plane. For each variable, we analyzed how the amplitude of surface inclination and the presence or absence of the lower-body restraint affected the amplitude of post-incline leaning. We also analyzed how duration of leaning was affected by determining the time constant of the decay of leaning measured in the orientation of the trunk and the CoM and in the location of the CoP.
Kinematics
To determine segment and joint angles and to determine the orientation of the body’s CoM, we used a four-camera motion analysis system (Motion Analysis, Santa Rosa, CA, USA) to record eight reflective markers placed on the left side of the body (Fig. 1c). Position data were sampled at 10 Hz. The derived variables of segment orientation and joint angles were low-pass filtered at 0.1 Hz to characterize slow changes in postural orientation.
Body segments were defined as shown in Fig. 1c, using the following landmarks: (1) head segment: forehead to external auditory meatus, with the forehead marker placed on a 5.5 cm extender to increase the length of the head segment, (2) trunk segment: hip to shoulder, (3) leg segment: ankle to hip, and (4) shank segment: ankle to knee. Orientation of each body segment in the sagittal plane was calculated with respect to gravity (space) and with respect to the support surface. Segment-re-space orientation was defined with respect to gravity-vertical, with ‘0’ representing vertical and positive angles representing forward lean. Segment-re-surface orientation was defined as the angle between the body segment and the surface, with ‘0’ representing the mean segment-to-surface position during the baseline period and positive angles representing forward lean.
Ankle, knee and hip angles were calculated based on the location of three markers, with the middle marker placed approximately at the axis of joint motion (Fig. 1c). The ankle angle was calculated using knee-ankle-toe markers, the knee angle was calculated using hip-knee-ankle markers, and the hip angle was calculated using shoulder-hip-knee markers. Hip and knee flexion and ankle dorsiflexion were defined as positive-going into the more acute direction, from a ‘0’ based on the subject’s mean postural alignment during the pre-incline period.
Whole body CoM location in the sagittal plane was calculated as a weighted sum of the CoM location of individual body segments using a five-segment model (head, trunk-arms, thighs, shanks, feet) of a subject standing with arms crossed in front of the chest. The mass of individual body segments was estimated using anthropometric measures and methods proposed by Vaughan et al. (1991) and Chandler et al. (1975). Ratios for calculating the location of the CoM as a percentage of the segment length are from studies performed by Dempster, as reported in a table of anthropometric data that was compiled by Winter (1990). Symmetry was assumed for left and right lower extremity alignment. CoM data are reported as angular changes with respect to the ankle joint.
Center of pressure
The CoP was calculated from vertical ground reaction forces recorded at 50 Hz. Forces were recorded from a custom, dual-plate, force platform with eight vertical sensors. The CoP signal was separated into a low-frequency component (<0.1 Hz) to assess slow changes in the CoP signal related to the postural set point, and a high frequency component (0.1–10 Hz) to assess the faster, corrective control around the set point (Gurfinkel et al. 1995; Lestienne and Gurfinkel 1988; Fransson et al. 2000). First, the low frequency CoP was extracted from the raw data with a 0.1 Hz low-pass, recursive, second order Butterworth filter. The 0.1 Hz filter cut-off rate optimally preserved the slow-rate peaks and valleys of the CoP with minimal distortion, while still eliminating the fast fluctuations of stabilizing postural corrections. Next, we extracted the high frequency component of the CoP by low-pass filtering the raw data at 10 Hz with a recursive, second order Butterworth filter, and then subtracting the low frequency component.
EMG
EMG activity was recorded differentially using surface electrodes that were placed 2 cm apart over the following muscles on the left side of the body: soleus, medial gastrocnemius, anterior tibialis, medial hamstrings, quadriceps (rectus femoris), paraspinals (iliac crest level), and rectus abdominis. A ground electrode was placed over C7. EMG data were amplified 2,000–10,000 X, band-pass filtered between 15 and 2,000 Hz, full-wave rectified, integrated at a cut-off frequency of 100 Hz amplitude, and then sampled at 240 Hz. Prior to data collection, subjects were asked to selectively contract each muscle while EMG activity was monitored to test electrode placement and to make sure that there was minimal cross-talk between muscles.
Maximum lean
The maximum amplitude that subjects leaned in the post-incline period was used to quantify the magnitude of the postural after-effect of stance on an incline. The maximum lean was defined as the peak displacement in the post-incline period with respect to the mean position during the last 30 s of stance on the incline surface. We waited until 5 s after the surface rotated to a new position before identifying the peak displacement because we were interested in the reference position subjects were trying to hold rather than transient body movements made to compensate for instability imposed by the surface rotation. Maximum lean values were calculated for body segment angles (head, trunk, leg, shank), the CoM, and the CoP. The maximum lean for CoP data was normalized as a percentage of foot length: Maximum Lean CoP = 100 × (Peak − Baseline)/(Foot Length).
Decay time constant
To quantify the duration of the post-incline lean for each trial, we modeled the post-incline data as a first order exponential and calculated the dominant decay time constant for the return from a leaned to an upright postural alignment (Kluzik et al. 2005). The mean square error of the exponential fit to the post-incline data was used to assess the goodness of the exponential fit.
Mean postural alignment
To compare how changes in surface orientations affected postural orientation, we calculated the mean position at five different times during each trial: (1) pre-incline period; (2) beginning of the during-incline period; (3) end of the during-incline period; (4) beginning of the post-incline period; and (5) end of the post-incline period. Mean position was calculated across 15-s time periods, except for the pre-incline period, for which mean position was calculated across a 30-s time period.
Stability around the postural set point
In order to quantify stability around the postural set point, we calculated the RMS and mean velocity of the high frequency component (0.1–10 Hz) of the CoP (Prieto et al. 1996). RMS and mean velocity were calculated for consecutive 15-s epochs during the pre-, during-, and post-incline periods to identify the time when stability around the postural set point returned to pre-incline values after the surface rotated to a new orientation.
Statistical analysis
Statistical analyses were performed using Statistica software with significance set at α < 0.05 for all comparisons. Repeated Measures ANOVA with Sheffé post-hoc analyses were used to compare how the amplitude of surface inclination affected the decay time constants and the maximum lean amplitudes. The effects of incline amplitude on trunk and head maximum lean amplitudes were evaluated using MANOVA to bypass problems of compound symmetry and sphericity (Statsoft 1994). Head and trunk maximum lean variables were log-transformed prior to MANOVA analyses to equalize variance, which increased as incline amplitude increased. Linear regression was performed to determine the slope of the relationship between incline amplitude and maximum lean for body segment angles. Whenever group means are reported to compare differences across condition, we also report the standard error.
Results
Effects of surface inclination on kinematic postural variables
When subjects returned to standing on a horizontal surface after they had stood on a toes-up inclined surface, they leaned forward, with a postural after-effect observed at all body segments (Fig. 2). Figure 2a shows how postural alignment changed before, during and after a representative subject stood on a 5°-toes-up inclined surface. When the surface changed from a horizontal to an inclined orientation, the body segments maintained their pre-incline postural alignment with respect to gravity vertical. However, when the surface returned from an inclined to a horizontal orientation, a large-amplitude lean away from gravity was observed in the head, the trunk, the legs, and the CoM.
The large amplitude, long lasting whole body lean after 2.5 min. of stance on a 5°-toes-up inclined surface is shown in reconstructed stick figures and in segment angle, CoM and CoP data for a representative subject in a. The stick figures compare body alignment before, during, and after the subject stood on the inclined surface. To construct the stick figures, position data were averaged across the first 15 s of the pre-incline period, the first 15 s in the during-incline period, and the first 5 s of the post-incline period. b, c Show the group’s average (± SE) postural alignment before, during, and after the subjects stood on a 5°-toes-up inclined surface. The average was performed across the seven subjects, with three repetitions per subject. Body segment orientation is shown in b and joint angle position is shown in c
Figure 2b, c compare the group average changes in segment and joint angles before, during, and after subjects stood on a 5°-inclined surface. When the surface changed from the pre-incline horizontal position to the inclined position, only small changes were observed in joint angles and segment orientation, except for dorsiflexion of the ankle joint. Furthermore, subjects maintained consistent postural alignment while on the inclined surface: there was no significant difference in joint angles between the first and last 15 s of the period of surface inclination (P > 0.05). When subjects stood on the 5°-inclined surface, ankle joint dorsiflexion nearly matched the amplitude of surface inclination (5.7 ± 0.4°), the knees and hips flexed a small amount (1.0 ± 0.4° and 1.6 ± 0.4°, respectively), and the trunk leaned slightly forward (1.2 ± 0.4°).
In the post-incline period, when the surface returned from the inclined to the horizontal position, large amplitude forward leaning was observed in the orientation of the body’s CoM and all of the body segments (Fig. 2b). On average, after subjects stood on the 5°-inclined surface, the maximum amplitude of leaning in the post-incline period was 4.0 ± 0.4° for the body’s CoM, 7.2 ± 1.6° for the head, 6.1 ± 0.9° for the trunk, and 3.5 ± 0.6° for the legs. This whole body lean was achieved in part by distributed changes in the ankle (1.8 ± 0.6°), knee (−0.3 ± 0.9°), and hip (3.2 ± 0.8°) joints with respect to baseline postural alignment.
The leaned postural orientation decayed toward upright similarly for each postural variable. For example, for the 5°-incline condition, the average decay time constants for leaning of the trunk, the legs, the CoM, and the CoP were 57.7 ± 8.4, 58.9 ± 7.7, 57.9 ± 7.4, and 59.9 ± 7.4 s, respectively. The mean square errors of the exponential fits associated with these time constants, averaged across subjects, were 0.55 ± 0.15 cm2 for the trunk, 0.40 ± 0.12 cm2 for the legs, 0.34 ± 0.10 cm2 for the CoM, and 0.78 ± 0.23 cm2 for the CoP. Thus, the decay time constant of any one of these postural variables provides a representative measure of the persistence of the leaning after-effect. The approximately 60 s time constant indicates that, on average, subjects leaned for a duration of 3 min (three times the decay time constant).
Effects of surface inclination on the CoP
The low and high frequency components of the CoP were affected differently by exposure to stance on an incline, as shown by data from a representative subject in Fig. 3a. The high frequency CoP displacements showed transient changes in RMS (Fig. 3b) and in mean velocity (Fig. 3c) after the surface changed orientation. The RMS and mean velocity of the high frequency CoP returned to baseline values within 15 s. In contrast, the low frequency CoP, reflecting the position subjects were trying to maintain, returned toward baseline gradually and slowly, taking well over 1 min (Fig. 3d). Thus, although stance on an incline led to a long-lasting after-effect on postural orientation, the more dynamic, corrective activity around the set point, represented by the high-frequency component of the CoP, was only briefly affected.
The duration of after-effects differed between the high (0.1–10 Hz) and the low (<0.1 Hz) frequency components of the CoP data. a Shows the decomposition of a representative subject’s CoP data into high and low frequency components. Data is shown before, during and after a representative subjects stood on a 5°-inclined surface. In the post-incline period, the low frequency CoP data, representing the postural set point, took minutes to decay back to baseline values. In contrast, the high frequency CoP data, representing the stabilizing activity around the postural set point, was affected only transiently by the preceding incline condition and returned to baseline values within 15 s. b–d Shows how the high (b and c) and low (d) frequency components of the COP changed across time in the post-incline period, with group averaged data binned in 15 s time intervals. b, c Show changes in the average RMS and average mean velocity of the high frequency COP. d Shows changes in the average location of the low frequency CoP with respect to the heel of the foot and normalized to foot length
Effects of surface inclination on EMG activity
In order to determine whether leaning after exposure to an incline was due to muscle after-contraction effects, we examined how EMG activity changed across the pre-, during-, and post-incline periods. Figure 4a shows EMG activity for a representative subject when tested under the 5°-incline condition. The tibialis anterior and rectus abdominis muscles were inactive throughout the trial, including when the subject stood on the inclined surface. An exception to this was a brief period of increased tibialis activity when the surface initially rotated to a toes-up inclined position. Tonic activity in the postural extensors, including soleus, gastrocnemius, hamstrings, and paraspinals, showed little change from the pre-incline to the during-incline period, remaining at low levels of activity. In the post-incline period, when the subject leaned forward, the postural extensors showed a large increase in activity, which gradually decayed as the subject returned toward baseline postural alignment. Figure 4b shows that for all but one subject, the tibialis muscle was active only in the initial period and was quiet for most of the period while the surface was inclined. Thus, the after-incline effect of forward leaning was not a result of tibialis muscle activity that persisted when the surface rotated from inclined to horizontal. Figure 4c shows that, like the representative subject, all subjects showed increased gastrocnemius activity during the post-incline period of forward lean.
Comparison of EMG activity across the pre-, during-, and post-incline periods. a Shows EMG, trunk orientation, and ankle angle data for a representative subject, tested under the 5°-incline condition. b, c Show how the activity of each subject’s Tibialis (b) and Gastrocnemius (c) muscles changed across the pre-, during-, and post-incline periods. Each subject’s EMG data is the average of three trials under the 5°-incline condition. b Shows that the tibialis was quiet throughout most of the trial for six out of seven subjects, but increased during the period of surface inclination in one subject. c Shows that subjects varied in how their gastrocnemius activity changed from the pre- to during-incline periods, but that all seven subjects showed increased gastrocnemius activity in the post-incline period
Effect of varying amplitude of surface inclination
Figure 5a shows how varying the amplitude of surface inclination affected the orientation of the trunk, the legs, and the CoM for a representative subject. Regardless of the amplitude of surface inclination, leaning occurred only in the post-incline period. While subjects stood on the inclined surface, the alignment of the body segments changed very little with respect to alignment in the baseline, pre-incline period. In contrast, there was a large effect of the amplitude of surface inclination on the amplitude of the trunk’s post-incline lean. The averaged data in Fig. 5b show that the trunk and head, but not the leg maximum lean, increased systematically and significantly as the amplitude of surface inclination increased (P < 0.05). The mean CoM maximum lean showed a small increase with amplitude, but this increase was not significant (P > 0.05). On average, the amplitude of trunk lean in the post-incline period nearly matched the amplitude of the prior surface inclination, however the amplitude varied widely across subjects. The variability of lean amplitude was greater for the head than the trunk. The slope of the relationship between the amplitude of lean and the amplitude of surface inclination provides a gain estimate of the postural after-effect. When averaged across subjects, this gain estimate was near 1 for both the trunk (0.88 ± 0.37) and the head (0.93 ± 0.49). The gain estimate varied widely across individual subjects (Table 1). In contrast to the increasing amplitude of head and trunk lean, the post-incline lean of the leg and body CoM saturated under larger incline amplitude conditions (Fig. 5b).
Effects of varying the amplitude of surface inclination. As the amplitude of surface inclination increased, the amplitude of lean in the upper body, but not the lower body, increased, as shown in data from trials of a representative subject (a) and in the group mean maximum lean (b). In a, the orientation of the CoM, trunk, and leg are shown before, during, and after a representative subject stood on an inclined surface, with the different colors representing different conditions of incline-amplitude. b Shows that on average, as surface inclination increased, the maximum lean of the head and trunk increased linearly with a slope near 1, while the maximum lean of the lower body saturated at the 5°-incline condition. c Shows saturation of the magnitude of displacement the location of the CoP underneath the feet when the surface was inclined at amplitudes beyond 5°. The gray shaded area indicates the region beyond estimated stability limits
Figure 5c shows that, like the CoM, the maximum displacement of the CoP in the post-incline period saturated at amplitudes of surface inclination greater than 5°, near the estimated stability limits for healthy subjects. The gray area in Fig. 5c shows the range of reported stability limits for healthy young subjects who were asked to perform a maximal voluntary lean (Blaszczyk et al. 1994; Murray et al. 1975; Schieppati et al. 1994).
Figure 6 shows how several different postural variables scaled to the four amplitudes of inclination when the surface transitioned from incline to horizontal. The data shown in Fig. 6a, from a representative subject before, during, and after stance on a 10°-incline, illustrate that the trunk-to-surface relationship, but not the position of the ankle joint, was held constant when the surface orientation changed from inclined to horizontal. For all amplitudes of surface inclination, the trunk-to-surface relationship was held constant from late in the during-incline period to early in the post-incline period, as shown in the group-averaged data in Fig. 6b. The changes in the trunk-to-surface relationship with respect to baseline scaled linearly to the amplitude of surface inclination for both the during- and post-incline periods. Similar results were found for the head-to-surface relationship (not shown). In contrast, the change in ankle position from baseline scaled to incline amplitude only during stance on the incline but not in the post-incline period when it quickly returned to baseline values (Fig. 6c). Figure 6d–g shows that the kinematic alignment of the body segments relative to space (gravity) and the position of the CoP were also not held constant from the during- to the post-incline periods. Further, of these variables, scaling with respect to surface inclination was observed only for the magnitude of post-incline leaning of the trunk with respect to space (Fig. 6d).
Post-incline leaning maintained the same trunk-to-surface relative alignment, but not the same ankle angle, trunk-in-space, leg-in-space alignment, or the location of the body’s CoM or CoP, as subjects had adopted while they stood on the inclined surface. a Shows data from a representative subject before, during, and after stance on a 10°-inclined surface. The plots in b–g compare how the mean value of different postural variables changed between the pre- and during incline periods and between the pre- and post-incline periods for all four incline amplitude conditions. The mean positions were calculated for 15-s epochs at the end of the during-incline period and at the beginning of the post-incline period. The mean of the first 15-s of the pre-incline period was then subtracted. Dashed lines indicate a slope of 1, indicating a 1:1 relationship between the adapting incline angle and change in alignment as compared to the pre-incline period. Each data point represents the group mean data for seven subjects, three trials each
Effects of preventing the legs from leaning
Subjects’ trunks leaned in the post-incline period, even when a stable postural support prevented their legs from leaning. Figure 7a compares the postural alignment of a representative subject when the subject’s legs were blocked and when the subject’s legs were free to lean forward in the post-incline period. The amplitude and the decay of the trunk’s lean were similar across conditions. Figure 7b shows that the amplitude of the post-incline trunk lean increased linearly as the amplitude of surface inclination increased, with a slope that was close to 1 both when the legs were blocked (0.93 ± 0.13) and when the legs were free to lean (0.87 ± 0.36). Similarly, the slopes for the head’s maximum lean (not shown) were also close to 1 for the both the legs blocked (0.95 ± 0.20) and the legs free (0.87 ± 0.47) conditions. On average, the maximum lean of the trunk and head were significantly larger when the legs were prevented from leaning than when they were free to lean (F (1,6) = 6.35, P < 0.05 for the trunk and F (1,6) = 6.69, P < 0.05 for the head). The restraint was effective in preventing forward lean of the leg and dorsiflexion at the ankle in the post-incline period, as can be seen in the shank segment data shown in Fig. 7c.
Effect of blocking the legs from leaning in the post-incline period. a Compares the alignment of the trunk, leg, shank, and whole-body CoM between the hips blocked (black traces) and hips free (gray traces) conditions for a representative subject who experienced standing on 5°-incline surface. The effects of preventing the legs from leaning on the scaling of the amplitude of the post-incline lean to the amplitude of prior surface inclination are shown for the trunk maximum lean in b and for the shank maximum lean in c. Dark traces represent average data for the leg blocked condition and light traces represent average data for the legs free condition. The dashed lines show the predicted amplitude of the maximum lean for a 1:1 relationship between the amplitude of surface inclination and the amplitude of the post-incline lean. d Shows how blocking leg movement affected the time constant of decay from the leaned to the upright, baseline alignment of the trunk
Subjects’ trunks leaned for minutes, regardless of whether the hips were blocked or free to lean forward and regardless of the amplitude of surface inclination as shown by the decay time constants in Fig. 7d. For the 2.5° and 5° incline-amplitude conditions, the decay time constants were significantly longer for the legs blocked condition (78.0 ± 13.3 and 83.0 ± 11.4 s) than for the legs free condition (53.7 ± 9.0 and 57.7 ± 8.4 s) (P < 0.01). For the largest incline-amplitude condition of 10°, the decay time constants did not differ significantly between the legs blocked (81.4 ± 10.1 s) and the legs free (79.2 ± 7.2 s) conditions. The mean square errors of the exponential fits, averaged across subjects and incline-conditions, were 0.86 ± 0.20 cm2 for the hips-free condition and 1.05 ± 0.17 cm2 for the hips-blocked condition.
Perceptual after-effects
When trials ended, subjects reported that they were aware that they had leaned during the experiment, but their leaned posture had felt like the ‘right place to be’. Three subjects reported a feeling that the support surface had moved to a toes-down-inclined position when the surface changed position from inclined to horizontal. Toward the end of the trial, late in the post-incline period, subjects reported that they believed they were upright, but were surprised by subsequent feelings of additional ‘backward pulls’ or ‘corrections’. When the stationary restraint bar blocked leg forward movement in the post-incline period, subjects reported a sensation that the bar was pushing them backward.
Discussion
We previously demonstrated that a persistent postural after-effect of leaning occurred in many, though not all, healthy subjects when they returned to standing on a horizontal surface after they stood on an inclined surface (Kluzik et al. 2005). The direction of the post-incline lean depended upon the direction of surface inclination, such that leaning preserved the relative alignment between the upper body and the support surface. The direction-dependence of the post-incline lean suggested that the underlying mechanism of the lean involves an adaptive modification of postural orientation to the altered surface inclination.
In the present study we investigated how different postural variables are affected when subjects adapt to standing on inclined surfaces of varied amplitudes. We found that changing the amplitude of surface inclination resulted in more systematic after-effects on orientation of the trunk and head than on orientation of the legs or the body’s CoM, the position of individual joint angles, or the location of foot CoP. Leaning in the post-incline period preserved the relative spatial alignment between the head and the trunk and the support surface such that it was similar to when subjects stood on the inclined surface (Fig. 6b). Preventing the legs from leaning did not abolish and may have enhanced the long-lasting post-incline lean in the upper body. Furthermore, the leaning after-effect was strong enough to over-ride otolith, trunk graviceptor, and load receptor feedback information and conscious perception about postural alignment with respect to gravity-vertical. These results suggest that the experience of standing on the inclined surface led to a sensorimotor adaptation within the neural pathways involved in postural orientation of the upper body with respect to the support surface.
Which postural variables undergo adaptation?
To understand how and why subjects leaned after exposure to the incline, we examined which postural variables remained constant from the end of the incline period to the beginning of the post-incline period. The persistence of a postural variable after a change in surface conditions, suggests a neural storage or memory of some aspects of postural orientation based on prior experience of stance on the incline (Earhart et al. 2001; Weber et al. 1998). The only postural variables that were held constant from the incline period to the post-incline period were the trunk- and head-to-surface relationships (Fig. 6), consistent with the hypothesis that the adaptive mechanism underlying the post incline lean affects the regulation of the global postural orientation variables of trunk and head alignment with respect to the surface.
Our results suggest that leaning is not due to a change in the regulation of local postural variables related to the position of a single joint, such as ankle angle, or the activation of a single muscle. First, when the legs were prevented from leaning in the post-incline period, leaning of the upper body was enhanced, not abolished. Second, when the legs were free to move in the post-incline period, neither ankle angle, nor any other leg joint angle, was maintained constant from during to immediately after stance on an incline. While the surface was inclined, the ankle joint dorsiflexed near to the same amount as the surface was inclined. When the surface returned to horizontal, the ankle joint rapidly returned about half way toward its baseline alignment (Fig. 2c). Thus, when subjects leaned, they were not maintaining the immediately prior dorsiflexion alignment of the ankle joint. Instead, leaning was accomplished by changes that were distributed throughout the ankle, knee, and hip and joints. Third, we found no evidence from ankle muscle EMG recordings to support an explanation of ankle muscle after-contraction effects for the post-incline lean. After-contraction effects are unintended movements that occur when a period of sustained, isometric muscle contraction ceases and are thought to be due to adaptive effects related to muscle spindles (Duclos et al. 2004; Hagbarth and Nordin 1998; Ghafouri et al. 1998; Gurfinkel et al. 1989). While subjects stood on the incline, we saw no evidence of sustained, isometric Tibialis muscle activity either during or after subjects stood on the inclined surface, showing that subjects did not lean because of after-contraction effects in ankle dorsiflexors (Fig. 4).
While we found no evidence that subjects lean after exposure to an incline because of adaptations in the control of the ankle or any other single joint or muscle variables, our results suggest that leaning is due to adaptation in the regulation of the alignment of global, kinematic postural variables of the trunk and/or head to the support surface. When subjects leaned forward after stance on the toes-up incline, they maintained a consistent relationship between the global variables of trunk (Fig. 6) and head orientation with respect to the surface. The amplitude of the post-incline lean of the head and trunk, but no other postural variables, depended linearly upon the immediately prior amplitude of surface inclination (Figs. 5 and 6). On average, subjects leaned their trunks approximately 5° after standing on a 5°-inclined surface and approximately 10° after standing on a 10°-inclined surface, suggesting a highly specific kinematic effect on upper body orientation. Furthermore, preventing the legs from leaning forward after subjects stood on a toes-up incline surface did not abolish the forward lean of the upper body in the post-incline period. In fact, when the legs were blocked from leaning, the upper body continued to scale linearly with incline-amplitude (Fig. 7). In addition, when subjects leaned in the post-incline period, the lean was accompanied by a large forward shift of the CoM and a large increase in the activity of postural extensors, suggesting that maintaining the body-to-surface relationship took precedence over preserving the location of the projection of the CoM near the center of the support base and over minimizing postural muscle activity. This finding is similar to the finding that when a load is added to a part of the body in cats standing on an incline, they maintain the same geometric alignment at the expense of an altered projection of CoM (Lacquaniti et al. 1990). All of these findings suggest that subjects leaned after they stood on an incline because of an adaptive change affecting regulation of the kinematic orientation of the upper body to the surface.
Other studies of postural orientation in cats and humans have drawn similar conclusions to ours, that postural orientation involves regulation of global, kinematic variables. When cats stood on a surface that was inclined, intra-limb geometry varied, but the legs stayed oriented to gravity-vertical and leg length remained near constant, while the trunk stayed parallel to the surface (Lacquaniti et al. 1990). When human subjects stood on a slowly tilting surface, EMG activity in ankle muscles correlated more highly with trunk orientation than with ankle joint position (Gurfinkel et al. 1981). When human subjects were given galvanic vestibular stimulation prior to a postural perturbation by surface translation, postural responses were consistent with the vestibular input altering the reference position for body orientation and could not be explained only by direct effects of vestibulospinal input on ankle muscle activity (Horak and Hlavacka 2002). Proprioceptive input by vibration to postural muscles, even eye muscles, results in direction-specific altered postural alignment or illusions of altered postural alignment that are context-dependent and cannot be explained only by local proprioceptive effects on the muscle that is vibrated, but rather, involves integration of proprioceptive information from all body segments (Roll et al. 1989). The neural substrate for the coding of global kinematic variables exists early in the processing of sensory information, for example, limb position and length are coded in the nervous system by second order spinocerebellar neurons (Bosco and Poppele 1997; Bosco et al. 2000).
Our findings that the trunk-to-surface relationship was held constant from the during-incline to the post-incline periods and that the amplitude of trunk lean scaled with the amplitude of surface inclination suggest that the trunk may be an especially important variable for controlling postural orientation. Constraining the trunk-to-surface geometry might enable the central nervous system to simplify regulation of the body’s CoM, since the trunk is nearly 50% of body mass and controlling trunk alignment would have a large effect on whole body CoM location and stability (Fung and Macpherson 1995; Horak and Macpherson 1996; Massion 1994). Other studies that have manipulated surface orientation have also found evidence of surface-referenced trunk orientation in humans. When subjects stood on a surface that tilted very slowly (ramps of 0.04°/s or sinusoids of less than 0.1 Hz frequency) and with small amplitude (0.25°–1.5°), they kept their trunks aligned to the surface rather than to gravity (Creath et al. 2002; Gurfinkel et al. 1981, 1995; Walsh 1973). Like these prior studies, our subjects aligned their trunks with respect to the surface rather than to gravity when the surface orientation changed (Fig. 6b), but only in the post-incline period, after having adapted to the inclined surface (Fig. 2). It is not apparent why subjects leaned only after stance on an incline and did not show a similar leaning effect when the surface initially tilted from a horizontal to an incline position. One possible explanation may be related to the biomechanical demands for maintaining stability that are imposed when subjects stand on a surface that is inclined at the amplitudes of 2.5°–10° used in our studies (Kluzik et al. 2005).
In our experiments, the upper body tended to behave as a head-trunk unit and, therefore, our results cannot distinguish between the relative importance of the trunk or the head as a control variable for surface-referenced postural orientation. Figure 5 shows that the head showed similar post-incline postural effects as the trunk, though it was more variable, suggesting that in this task, head position may be less tightly regulated than trunk position. Vestibular and visual sensors are located within the head and the importance of head orientation and stabilization for postural and locomotor tasks is well known (Pozzo et al. 1995).
Although the relationship between surface inclination and upper body orientation suggest that the adaptation affected the postural control variable of the foot-to-trunk relationship, alternative postural variables that could have adapted to the incline condition are the CoM position (Massion 1994) or the level of postural tonus (Mori et al. 1982). It is possible that subjects leaned their trunks forward in the post-incline period in an effort to bring the CoM forward. Although we observed only small changes in the amplitude of the post-incline lean of the CoM as the amplitude of surface inclination was systematically increased, larger changes in the CoM location may have been constrained by postural stability requirements. Subjects leaned near their stability limits after standing on an incline with an amplitude of 5° or more, never bringing their CoM projections beyond the limits of their foot support.
Alternatively, subjects may have leaned their upper bodies in order to produce an overall state of postural tonus or effort that was similar to when subjects stood on the incline. Brainstem areas that can set postural tonus to achieve a particular body orientation have been identified (Mori et al. 1982). Further, after-effects upon resting posture have been shown to follow a sustained period of holding of a particular postural configuration with an isometric muscular contraction, an effect called the Kohnstamm effect (Duclos et al. 2004; Ghafouri et al. 1998; Gurfinkel et al. 1989; Gurfinkel and Levik 1991) or a sustained period of muscle vibration (Wierzbicka et al. 1998). Our recordings of surface EMG do not support such an explanation because when our subjects stood on the incline they did not demonstrate sustained muscle contractions needed to produce a Kohnstamm-like effect. Further, leaning in the post-incline period did not reproduce the immediately prior level of muscle activity, but instead involved a substantially different pattern of activity. However, it is possible that adaptive re-setting of postural tonus occurred in deep postural muscles, from which we did not record.
Sensorimotor adaptation
The underlying mechanism responsible for the post-incline lean is unknown. We speculate that an altered pattern of proprioceptive input, particularly from muscle spindles, may be the critical feature that drives the adaptation responsible for the lean. Muscle spindle information is known to have a powerful influence on actual and perceived body orientation (Roll et al. 1989) and altering patterns of muscle spindle input through muscle vibration or isometric contraction are known to result in postural and perceptual after-effects (Duclos et al. 2004; Ghafouri et al. 1998; Gilhodes et al. 1992; Gurfinkel et al. 1989; Wierzbicka et al. 1998). When subjects stood on the toes-up inclined surface, upright head and trunk alignment were achieved largely through ankle dorsiflexion that nearly matched the amplitude of surface inclination. Thus, upright posture was maintained by ankle muscle postural activity at new muscle lengths, which could alter the pattern of muscle spindle input during the incline-adaptation period. Asymmetrical input from cutaneous and pressure somatosensory receptors has also been shown to affect perception of postural vertical (Bisdorff et al. 1996) and perceived visual vertical (Wade 1970), and could also contribute to the post-incline lean, since it is likely that the incline-condition would alter the distribution of pressure underneath the feet.
We do not think that the post-incline lean could be explained solely by history-dependent properties of muscle spindles (Gregory et al. 1988; Hagbarth and Nordin 1998; Proske et al. 1993), although they can persist for minutes (Proske et al. 1993). It has been argued that these properties explain after-contraction effects such as errors in position sense (Gregory et al. 1988), errors in force estimation (Hutton et al. 1987), and the Kohnstamm effect (Hagbarth and Nordin 1998). In our study, a post-incline lean mechanism restricted to peripheral adaptation is not compatible with the findings. Instead, the results of this study point toward involvement of central mechanisms (Duclos et al. 2004; Ivanenko et al. 2006; Gilhodes et al. 1992). Local after-effects of ankle muscle spindles on ankle muscle activity are unlikely because upper body orientation showed more systematic post-incline lean effects than ankle joint position or ankle muscle activation and upper body lean persisted even when a stable support prevented the legs from leaning. In addition, postural orientation and postural stability had different time courses over which they displayed after-effects (Fig. 3). A peripheral adaptation would be expected to affect both orientation and stability for a similar time course. Postural orientation was altered for minutes in the post-incline period, while measures that reflect postural stability and frequency of postural correction, such as the RMS and mean velocity of the high-frequency component of the CoP, showed only transient disturbances, lasting under 15 s. This suggests that relatively unchanged stabilizing mechanisms were acting around an altered, adapted, set point for postural orientation that had been modified by the experience of standing on the inclined surface (Gurfinkel et al. 1995).
The incline condition altered the relative alignment between two references for postural orientation: a foot-up postural reference, based on the orientation of the support and a head-down reference, based on the direction of gravitational forces (Mergner and Rosemeier 1998; Mergner et al. 2003). An adaptive change in the calibration or weighting of proprioceptive information for preferred postural orientation may have occurred to reconcile conflicting sensory information. Similar adaptive plasticity in the regulation of the foot-to-trunk relationship has been suggested by studies of podokinetic after-rotation (Earhart et al. 2001; Weber et al. 1998) and of pointing movements made with trunk rotation when the support surface is simultaneously rotated in the same or opposite direction as the trunk movement (Hudson et al. 2005).
Perceptual effects
Similar to results of body tilt that occurs during galvanic vestibular stimulation (Fitzpatrick et al. 1994; Horak and Hlavacka 2002), subjects were aware that they were leaning, yet felt driven to adopt the leaned posture. Thus, although preferred postural orientation adapted to stance on an inclined surface, perception of postural orientation with respect to gravity-vertical appears to have been unaffected, or at least, less affected. This finding suggests a separation of the perceived gravitational vertical and the automatic set point for postural orientation, similar to the previously reported separation of the subjective visual vertical and subjective postural vertical (Anastasopoulos et al. 1997; Bronstein 1999; Karnath et al. 2000).
When leaning was restrained with a stationary bar, several subjects reported a sensation of being pushed backward by the bar. Subjects’ perceptions of being pushed backward might explain why their trunks leaned further forward when their legs were blocked than when they were free to lean. Subjects might have leaned farther in order to avoid backward disequilibrium. The perception of backward pushing by the stationary bar in the period when the support surface changed orientation is reminiscent of illusory sensations reported by subjects who are supported by a stationary support during muscle vibration (Gurfinkel et al. 1988) or galvanic vestibular stimulation (Fitzpatrick et al. 1994; Wardman et al. 2003). These illusory sensations show the strong context-dependence of central interpretation of sensory information for postural orientation and are compatible with the idea of a central representation of posture that integrates multisensory input to determine the orientation of the body segments relative to one another and relative to multiple references within the environment (Gurfinkel et al. 1995; Lestienne and Gurfinkel 1988; Quoniam et al. 1990; Roll et al. 1989). This unified central representation of posture has been referred to as a ‘body scheme’ (Gurfinkel et al. 1988; Gurfinkel and Levik 1991; Lestienne and Gurfinkel 1988).
Conclusions
We previously showed that an after-effect of leaning occurred in many, though not all, healthy subjects after a period of stance on an inclined surface and that the direction of leaning depended on the direction of surface inclination (Kluzik et al. 2005). In the present study of subjects who lean, we showed that the amplitude of leaning depended upon the amplitude of surface inclination. Further, we showed that global kinematic postural variables, such as the relative alignment between the trunk and the surface, were more systematically affected by changes in surface inclination than were local postural variables, such as the position of the ankle joint or muscle activity. These results are consistent with the hypotheses that (1) a central adaptive mechanism gradually adjusts the surface-referenced ‘set point’ for postural orientation when a change occurs in the spatial orientation of the surface with respect to gravity and (2) the central nervous system regulates postural orientation with respect to the support surface by controlling global, whole body postural kinematic variables.
References
Anastasopoulos D, Haslwanter T, Bronstein A, Fetter M, Dichgans J (1997) Dissociation between the perception of body verticality and the visual vertical in acute peripheral vestibular disorder in humans. Neurosci Lett 233:151–153
Aniss AM, Diener HC, Hore J, Gandevia SC, Burke D (1990) Behavior of human muscle receptors when reliant on proprioceptive feedback during standing. J Neurophysiol 64:661–670
Anstis S (1995) Aftereffects from jogging. Exp Brain Res 103:476–478
Bisdorff AR, Wolsley CJ, Anastasopoulos D, Bronstein AM, Gresty MA (1996) The perception of body verticality (subjective postural vertical) in peripheral and central vestibular disorders. Brain 119:1523–1534
Blaszczyk JW, Lowe DL, Hansen PD (1994) Ranges of postural stability and their changes in the elderly. Gait Posture 2:11–17
Bosco G, Poppele RE (1997) Representation of multiple kinematic parameters of the cat hindlimb in spinocerebellar activity. J Neurophysiol 78:1421–1432
Bosco G, Poppele RE, Eian J (2000) Reference frames for spinal proprioception: limb endpoint based or joint-level based? J Neurophysiol 83:2931–2945
Bronstein AM (1999) The interaction of otolith and proprioceptive information in the perception of verticality. The effects of labyrinthine and CNS disease. Ann NY Acad Sci 871:324–333
Chandler RF, Clauser CE, McConville JT, Reynolds HM, Young JW (1975) Investigation of inertial properties of the human body. U.S. Department of Transportation, Washington
Creath R, Kiemel T, Horak F, Jeka JJ (2002) Limited control strategies with the loss of vestibular function. Exp Brain Res 145:323–333
Duclos C, Roll R, Kavounoudias A, Roll JP (2004) Long-lasting body leanings following neck muscle isometric contractions. Exp Brain Res 158:58–66
Earhart GM, Melvill Jones G, Horak FB, Block EW, Weber KD, Fletcher WA (2001) Forward versus backward walking: transfer of podokinetic adaptation. J Neurophysiol 86:1666–1670
Fitzpatrick R, Burke D, Gandevia SC (1994) Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol 478(Pt 2):363–372
Fransson P-A, Johansson R, Hafström A, Magnusson M (2000) Methods for evaluation of postural control adaptation. Gait Posture 12:14–24
Fung J, Macpherson JM (1995) Determinants of postural orientation in quadrupedal stance. J Neurosci 15:1121–1131
Ghafouri M, Thullier F, Gurfinkel VS, Lestienne FG (1998) Muscular after-contraction and ongoing postural reactions in standing and sitting humans. Neurosci Lett 250:61–65
Gilhodes JC, Gurfinkel VS, Roll JP (1992) Role of Ia muscle spindles in post-contraction and post-vibration motor effect genesis. Neurosi Lett 135:247–251
Gordon CR, Fletcher WA, Melvill Jones G, Block EW (1995a) Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res 102:540–545
Gordon CR, Spitzer O, Doweck I, Melamed Y, Shupak A (1995b) Clinical features of mal de debarquement: adaptation and habituation to sea conditions. J Vestib Res 5:363–369
Gregory JE, Morgan DL, Proske U (1988) Aftereffects in the responses of cat muscle spindles and errors of limb position in man. J Neurophysiol 59:1220–1230
Gurfinkel VS, Levik YS (1991) Perceptual and automatic aspects of the postural body scheme. In: Paillard J (ed) Brain and space. Oxford University Press, New York, pp 147–162
Gurfinkel VS, Lipshits MI, Mori S, Popov KE (1981) Stabilization of body position as the main task of postural regulation. Fiziol Cheloveka 7:400–410
Gurfinkel VS, Levik YS, Popov KE, Smetanin BN, Shlikov VY (1988) Body scheme in the control of postural activity. In: Gurfinkel VS, Ioffe J, Massion J, Roll JP (eds) Stance and motion. Facts and concepts. Plenum, New York, pp 185–193
Gurfinkel VS, Levik YS, Lebedev MA (1989) Immediate and remote postactivation effects in the human motor system. Neirofiziologiya 21:343–351
Gurfinkel VS, Ivanenko YP, Levik YS, Babakova IA (1995) Kinesthetic reference for human orthograde posture. Neuroscience 68:229–243
Hagbarth KE, Nordin M (1998) Postural after-contractions in man attributed to muscle spindle thixotropy. J Physiol 506(Pt 3):875–883
Horak FB, Hlavacka F (2002) Vestibular stimulation affects medium latency postural muscle responses. Exp Brain Res 144:95–102
Horak FB, Macpherson JM (1996) Postural orientation and equilibrium. In: Rowell LB, Shepherd JT (eds) Handbook of physiology section 12: Exercise: regulation and integration of multiple systems. Oxford University Press, New York, pp 255–292
Hudson T, Lackner JR, DiZio P (2005) Rapid adaptation of torso pointing movements to perturbations of the base of support. Exp Brain Res 165:283–293
Hutton RS, Kaiya K, Suzuki S, Watanabe S (1987) Post-contraction errors in human force production are reduced by muscle stretch. J Physiol 393:247–259
Ivanenko YP, Wright GW, Gurfinkel VS, Horak F, Cordo P (2006) Interaction of involuntary post-contraction activity with locomotor movements. Exp Brain Res 169:255–260
Karnath HO, Ferber S, Dichgans J (2000) The origin of contraversive pushing: evidence for a second graviceptive system in humans. Neurology 55:1298–1304
Kavounoudias A, Roll R, Roll J-P (1998) The plantar sole is a ‘dynamometric map’ for human balance control. NeuroReport 9:3247–3252
Kluzik J, Peterka RJ, Horak FB (2000) Adaptive plasticity in control of postural orientation revealed by stance and stepping on an incline. Soc Neurosci Abstr 26:169
Kluzik J, Horak FB, Peterka RJ (2005) Differences in preferred reference frames for postural orientation shown by after-effects of stance on an inclined surface. Exp Brain Res 162:474–489
Lackner JR, DiZio P (2000) Human orientation and movement control in weightless and artificial gravity environments. Exp Brain Res 130:2–26
Lacquaniti F, Maioli C (1994) Independent control of limb position and contact forces in cat posture. J Neurophysiol 72:1476–1495
Lacquaniti F, LeTaillanter M, Lopiano L, Maioli C (1990) The control of limb geometry in cat posture. J Physiol 426:177–192
Lestienne FG, Gurfinkel VS (1988) Posture as an organizational structure based on a dual process: a formal basis to interpret changes of posture in weightlessness. Prog Brain Res 76:307–313
Maioli C, Poppele RE (1991) Parallel processing of multisensory information concerning self-motion. Exp Brain Res 87:119–125
Martin JP (1965) Tilting reactions and disorders of the basal ganglia. Brain 88:855–873
Massion J (1994) Postural control system. Curr Opin Neurobiol 4:877–887
Mergner T, Rosemeier T (1998) Interaction of vestibular, somatosensory and visual signals for postural control and motion perception under terrestrial and microgravity conditions—a conceptual model. Brain Res Brain Res Rev 28(1–2):118–135
Mergner T, Maurer C, Peterka RJ (2003) A multisensory posture control model of human upright stance. Prog Brain Res 142:189–201
Mori S, Kawahara K, Sakamoto T, Aoki M, Tomiyama T (1982) Setting and resetting of level of postural muscle tone in decerebrate cat by stimulation of the brain stem. J Neurophysiol 48:737–748
Murray MP, Seireg AA, Sepic SB (1975) Normal postural stability and steadiness: quantitative assessment. J Bone Joint Surg Am 57:510–516
Nachum Z, Shupak A, Letichevsky V, Ben-David J, Tal D, Tamir A, Talmon Y, Gordon CR, Luntz M (2004) Mal de debarquement and posture: reduced reliance on vestibular and visual cues. Laryngoscope 114:581–586
Nashner LM, Black FO, Wall C 1982) Adaptation to altered support and visual conditions during stance: Patients with vestibular deficits. J Neurosci 2:536–544
Pozzo T, Levik Y, Berthoz A (1995) Head and trunk movements in the frontal plane during complex dynamic equilibrium tasks in humans. Exp Brain Res 106:327–338
Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM (1996) Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng 43:956–966
Proske U, Morgan DL, Gregory E (1993) Thixotropy in skeletal muscle and in muscle spindles: a review. Progr Neurobiol 41:705–721
Quoniam C, Roll JP, Deat A, Massion J (1990) Proprioceptive induced interactions between segmental and whole body posture. In: Brandt T, Paulus W, Bles W, Dieterich M, Krafczyk S, Straube A (eds) Disorders of posture and gait. Georg Thieme Verlag Stuttgart, New York, pp 194–197
Rieser JJ, Pick HL, Ashmead DH, Garing AE (1995) Calibration of human locomotion and models of perceptual-motor organization. J Exp Psychol Hum Percept Perform 21:480–497
Roll J-P, Vedel J-P, Roll R (1989) Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Prog Brain Res 80:113–123
Schieppati M, Hugon M, Grasso M, Nardone A, Galante M (1994) The limits of equilibrium in young and elderly normal subjects and in parkinsonians. Electroencephalogr Clin Neurophysiol 93:286–298
Statsoft (1994) Statistica for the Macintosh, vol 1. Tulsa, OK, pp 391–392
Vaughan CL, Davis BL, O’Connor JC (1991) Dynamics of human gait. Human Kinetics, Champaign
Wade NJ (1970) Effect of prolonged tilt on visual orientation. Q J Exp Psychol 22:423–439
Walsh EG (1973) Standing man, slow rhythmic tilting, importance of vision. Agressologie 14C:79–85
Wardman DL, Taylor JL, Fitzpatrick RC (2003) Effects of galvanic vestibular stimulation on human posture and perception while standing. J Physiol 551(Pt 3):1033–1042
Weber KD, Fletcher WA, Gordon CR, Melvill Jones G, Block EW (1998) Motor learning in the ‘podokinetic system’ and its role in spatial orientation during locomotion. Exp Brain Res 120:377–385
Wierzbicka MM, Gilhodes JC, Roll JP (1998) Vibration-induced postural posteffects. J Neurophysiol 79:143–150
Winter DA (1990) Biomechanics and motor control of human movement. Wiley, New York, pp 56–57
Acknowledgments
The authors thank V. Gurfinkel for insightful discussions related to this project. We also thank S. Clark-Donovan, J. Roth, and S. Stapley for assistance in data collection and C. Russell and A. Owings for technical assistance. This project was supported by an APTA Foundation for Physical Therapy Scholarship to J. Kluzik, NASA Grant NAG5-7869 and NIH Grant AG17960 to R. Peterka, and NIH Grants DC04082 and AG06457 to F. Horak.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kluzik, J., Peterka, R.J. & Horak, F.B. Adaptation of postural orientation to changes in surface inclination. Exp Brain Res 178, 1–17 (2007). https://doi.org/10.1007/s00221-006-0715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0715-0