Abstract
Hanseniaspora vineae is a non-Saccharomyces yeast used in winemaking to increase the complexity of wines. However, the fermentation rate in sequential inoculations may be challenging, particularly in industrial winemaking settings. This study aimed to assess how different co-inoculation protocols involving H. vineae and S. cerevisiae affect the fermenting performance and aroma of white and red wines. White and red wines were co-fermented with varying H. vineae-to-S. cerevisiae ratios (67%, 80%, 90%, 95%, and 98%). Results were compared to sequential and pure S. cerevisiae inoculation. Co-inoculation mitigated the inhibitory mechanisms associated with sequential inoculation, resulting in a reduction of 30 days and 6 days of fermentation for white and red wines, respectively. Moreover, the fermentation time in co-inoculation was similar to that of the controls, thereby avoiding the slowdowns typically observed in sequential inoculation. Five yeast-derived metabolic markers, two of which characterizing H. vineae metabolism, were studied to evaluate the processes. In white wines, β-phenylethyl acetate and benzyl alcohol were increased by H. vineae up to 64-fold and sevenfold, respectively, while ethyl hexanoate was fourfold higher in S. cerevisiae. In addition, 2-phenylethanol was up to twofold higher in S. cerevisiae. The results for isoamyl acetate varied depending on the co-inoculation ratio. At 67% and 80%, the H. vineae protocols showed the highest concentration, even exceeding that of S. cerevisiae pure inoculation. All compounds correlated linearly with the H. vineae-to-S. cerevisiae ratio at inoculum. The same trends were observed in red wines, but to a different extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hanseniaspora yeasts are among the most important non-Saccharomyces genera linked to grapes [1]. In recent years, different strains belonging to this genus, such as H. uvarum, H. osmophila or H. vineae have been isolated and proposed for use in winemaking [2]. H. vineae Hv205 was initially isolated from Tannat grapes in Uruguay [3], and its genome has been fully sequenced [4, 5]. Its metabolism was explored under different winemaking conditions [6, 7] before its recent introduction to the market in 2022 [1], and different studies have reported H. vineae's contribution in white, red, and rosé winemaking [8, 9]. Compared to conventional Saccharomyces strains, H. vineae has been found to increase wine flavor diversity and improve the juice ecosystem, while avoiding contamination by aerobic bacteria and yeasts [6]. In particular, H. vineae exhibits an intermediate to low fermentation capability, demonstrating the potential to achieve ethanol levels of up to 10% [10], with a moderate competitive capacity during vinification compared to Saccharomyces strains, contributing to increased microbial diversity in the process [9, 10]. Furthermore, H. vineae has a complementary secondary metabolism with Saccharomyces cerevisiae, which can affect the aromatic profile of the resulting wines [11, 12]. H. vineae can lead to the de novo synthesis of benzenoids and other phenylpropanoid compounds, including benzyl alcohol, which confers floral and fruity flavors to wines [13]. It has been demonstrated to overproduce up to 90-fold more β-phenylethyl acetate than S. cerevisiae [1], thereby improving the aroma profile of wines, particularly in non-aromatic grape varieties [8, 14]. Therefore, the presence of these compounds may indicate the involvement of H. vineae in vinification. However, once a particular strain is chosen for its distinctive metabolic traits, its suitability for industrial applications is determined not only by these features but also by its overall capability to produce the desired outcome and meet expectations. The wine industry requires a chosen yeast strain to fulfil fermentation requirements, which include fast fermentation onset, quick sugar consumption kinetics, and complete fermentation of musts containing a high sugar concentration [15, 16].
The oenological attributes of H. vineae are intriguing in this industry. Conversely, the flip side of the coin involves the necessity for precise adjustments to ensure the conclusion of fermentation, especially in highly mature white grapes [1, 17]. This improvement was achieved through sequential inoculation with S. cerevisiae midway through fermentation. Additionally, compared to Saccharomyces strains, H. vineae exhibits a slower rate of ethanol and CO2 production, along with a lower production of fatty acids and branched chain higher alcohols. These characteristics have been proposed to increase yeast diversity during fermentation, allowing native or winery microflora to participate in the fermentation process [6, 18]. Evidence of challenges in achieving satisfactory fermentation performance for industrial purposes under sequential inoculation [17] has led this study to further explore the methods and management of co-inoculated cultures with H. vineae. This work aims to evaluate the effect of different co-inoculation protocols of H. vineae with S. cerevisiae in white and red winemaking in the fermenting kinetic performances while maintaining the aroma features and flavor intensity of the corresponding wines.
Materials and methods
Winemaking
A. White wine vinification.
250 L of grape must were obtained from the pressing (Willmes GmbH, Germany) of Glera grapes under anaerobic conditions, with the use of dry ice, in the de-stemmer and the press, while a continuous free flow of inert gas (Argon) was used in the must collection vessels. To the must, it was then added sulfur dioxide (15 mg/L) as potassium metabisulphite, ascorbic acid (5 mg/L; Dal Cin Gildo S.P.A., Concorrezzo, MB, Italy), and pectolytic enzymes (10 μL/L; Rapidase Clear Extreme; Oenobrands, Montpellier, France). 24 h later, the must was racked and homogeneously divided into twenty-one 9 L aliquots and then inoculated. At the end of alcoholic fermentations, wines were racked and stabilized with 65 mg/L of sulfur dioxide.
B. Red wine vinification.
About 300 kg of grapes of Termantis variety were randomized manually bunch by bunch to limit the normal compositional variability found in the vineyard, into twenty-one aliquots of about 9.5 kg each. Grapes were destemmed, supplemented with 15 mg/Kg of sulfur dioxide, and inoculated with the same procedure as above. Maceration was managed by hand-punch downs, twice a day. At the end of alcoholic fermentations, wines were racked and stabilized with 65 mg/L of sulfur dioxide as potassium metabisulfite.
Inoculation protocols
Musts were fermented in triplicate with seven different inoculation protocols depending on the active dry yeast species used. The inoculation was made at 5⋅106 cells/ml for each trial. Commercial yeast was used, S. cerevisiae (Fermivin® LVCB, Oenobrands, France) and H. vineae (Fermivin® VINEAE, Oenobrands, France). In sequential inoculation protocol, the S. cerevisiae was added at 1/3 of the alcoholic fermentation. Inoculations and abbreviations used are resumed in Table 1.
Yeasts were inoculated after 20 min of rehydration in distilled water at 37 °C (separately or as a mixture). Concurrently with inoculation, 300 mg/L yeast lysates (Natuferm® Bright, Oenobrands, France) and 0.3 mg/L thiamine were added to the musts. After about 48 h of fermentation, the second addition of the same yeast lysates was made at a dose of 300 mg/L, except for the Hv processes, which were supplemented with the yeast autolysates at the sequential inoculation of S. cerevisiae, at 1/3 of the alcoholic fermentation.
Fermentation kinetics
The fermentation kinetics of the wines were evaluated by measuring the density with a portable density meter (DMA 35, Anton Paar, GmbH, Austria). Data were collected twice a day after must homogenization. For each thesis and replicate, the measurement was made in triplicate. The parameterization of the kinetics was performed by comparing kinetics on the percentage of the fermentative course. Percentage points were then chosen that were useful for characterizing fermentation performance. Specifically, the times required to reach 3%, 5%, and 10% were calculated for the evaluation of the beginning of the alcoholic fermentation; 90%, 95%, and 97% for the evaluation of the last steps of fermentation, along with 25%, 50% and 75% for the control of exponential and stationary phases. Values were obtained by interpolation between the two closest experimental points, assuming a linear behavior between them.
Cell count
For verification of the viable cell concentration of the dry yeasts and, consequently, calculations of the corresponding inoculum dose, a cell count was performed on the active dry yeasts used. The diluted samples were spread on a WL Nutrient Agar medium (OXOID, Oxford, UK). Petri dishes were incubated at 25 °C for 4 days, after which yeast cells were quantified according to the OIV-MA-AS4-01: 2010 method.
Yeast evolution monitoring
The evolution of yeast population, and, in particular of H. vineae, was followed by plate and microscopical yeast count, according to the OIV methods (OIV, 2010). More in detail, a microscopic yeast count was performed by diluting 1:10 the sample with peptone-water (mycological peptone, 1 g/L in distilled water. Oxoid, UK). 1 mL of diluted sample was stained, adding 1 mL of methylene blue solution (OIV, 2022), and incubating for 10 min at room temperature in dark conditions. The stained sample was counted utilizing a Burcher chamber and an optic microscope ZL-N400T CF (Optika Science, I) equipped with a 400× objective and a phase contrast apparatus. Plate count was performed utilizing different synthetic media to discriminate among the yeast species utilized in this work. The total yeast population was enumerated onto WL agar medium (Oxoid, UK), after incubation at 25 ± 2 °C for 3 days. H. vineae was discriminated by plating samples onto Lysine Agar (Oxoid, UK), and incubated for 5 days at 25 ± 2 °C. The attribution of species was based on morphological characters of different yeast isolated, according to the description reported on the OIV methods (OIV, 2010).
Fourier-transform infrared spectroscopy (FTIR) measurement of must and wine basic chemical parameters
50 mL of juice, previously centrifuged (5000 rpm, 5 min) and filtered (25 mm × 0.45 μm cellulose acetate syringe cartridge; Alltech, Deerfield, IL, USA), were analyzed for °Brix, pH, and titratable acidity with a WineScan™ FT 120 Type 77,310 (Foss Electric A/S, Hillerød, Denmark), calibrated with the official methods. Wines were prepared in the same way for analysis of ethanol, pH, and titratable acidity.
GC–MS/MS analysis of volatile compounds
The method reported by [19] was used to analyze the volatile organic compounds (VOCs) in wine. Briefly, 50 mL of wine was diluted with Milli-Q water to 100 mL after adding 100 µL of internal standard (mixture of 1‐heptanol at 230 mg/L and ethyl‐3‐hydroxybutyrate at 1000 mg/L in ethanol/water 50:50), the volatile compounds were then extracted by solid-phase extraction (SPE) and finally analyzed by GC–MS/MS. The gas chromatographic system used was a GC Agilent Intuvo 9000 coupled with a Triple Quadrupole MS Agilent 7000 equipped with an electron ionization source operating at 70 eV. Separation was obtained by injecting 2 μL in split mode (1:5) into a DB‐Wax Ultra Inert (20 m × 0.18 mm id × 0.18 μm film thickness) capillary column with constant He flow of 0.8 mL/min. The injector temperature was set at 250 °C. The oven temperature was programmed starting at 40 °C for 2 min, raised to 55 °C by 10 °C/min, then raised to 165 °C by 20 °C/min, and finally raised to 240 °C by 40 °C/ min and held at this temperature for 5 min. The mass spectrum was acquired in dMRM (dynamic multiple reaction monitoring) mode. The transfer line and source temperatures were set at 250 °C, and 230 °C, respectively. To identify and quantify the VOCs, the pure standard of each selected compound was injected at different concentration levels.
Results
Fermentation kinetics
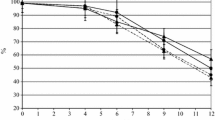
The fermentation kinetics of Glera and Termantis are depicted in Supplementary Material S1, where it can be observed how management with sequential inoculation of H. vineae and S. cerevisiae (Hv) delayed the completion of alcoholic fermentation in both white and red wine vinification. Regarding Glera, Hv prolonged fermentation (~ 40 d) not only compared to Sc (~ 7d) but also to all co-inoculated trials with a mixture of both yeasts. The same differences, but to a different extent, were observed in Termantis, for which the time required for the completion of fermentation in Hv processes (~ 16 d) almost doubled Sc (~ 8 d). Although inhibitory mechanisms cannot be ruled out, this speculation is supported when parametrized kinetics are analyzed (Fig. 1); every Glera process inoculated with H. vineae accelerates sugar consumption up to the middle of fermentation. In addition, at 25% fermentation, the Hv sequential protocol was faster than that for C67. Interestingly, despite the better performance during the first stages, the time needed to achieve 97% sugar consumption significantly increased with the H. vineae ratio and led to an average delay compared to Sc of ~ 72 h, ~ 61 h, ~ 46 h, ~ 31 h, and ~ 24 h for the C98, C95, C90, C80, and C67 processes, respectively. The same trend can be observed in Termantis (Fig. 1), for which Sc was the slowest process, up to 50% of the alcoholic fermentation, even if not significantly differentiated from all of them. The kinetics tended to improve with a lower proportion of H. vineae, leading to a mean delay to achieve 97% of fermentation of ~ 24 h, ~ 33 h, ~ 11 h, and ~ 18 h for C98, C95, C90, and C80 processes, respectively, concerning Sc, with C67 comparable, on average anticipating by ~ 4 h.
Parameterization of the fermentation kinetics of a Glera and b Termantis according to the type of inoculum of Saccharomyces cerevisiae and Hanseniaspora vineae. ▀ Hv: inoculation of Sc upon reaching 1/3 of fermentation; ▀Sc: fermentation managed only with Saccharomyces cerevisiae; The numbers of the remaining theses correspond to the percentage of Hanseniaspora vineae for co-inoculation with Saccharomyces cerevisiae at the start of fermentation: ▀98%, ▀95%, ▀90%, ▀80%, ▀67%. Data are compared with one-way ANOVA followed by Tukey HSD multiple comparison (p < 0.05). Different letters indicate values statistically differentiated. The x-axis represents time in hours (h), while the y-axis represents the percentage of the fermentation process (%)
Viable cells of H. vineae and S. cerevisiae were monitored on days three and six after the initial inoculum, the former before the S. cerevisiae sequential inoculation of the Hv processes (Fig. 2). No H. vineae was found in Sc processes either at day three or at day six, while S. cerevisiae was present in Hv processes only at day six, with a limited number of viable cells (~ 7.7⋅104 CFU/mL), even following S. cerevisiae inoculation with 5⋅106 CFU/mL. At the first monitoring point, the number of H. vineae cells was comparable among the co-inoculated trials, but also to Hv and the viable cells of S. cerevisiae in Sc, which was approximately 2 107 CFU/mL. After three days, the ratio between H. vineae and S. cerevisiae was approximately between 80% (C67) and 98% (C98), slightly increasing the proportion of H. vineae in the lower ratio protocols (C67, C80, and C90) compared to the initial inoculum. This behavior confirms the respect of H. vineae towards S. cerevisiae [1] but also a higher colonization rate of the apiculate yeast [20]. At day six, when the alcoholic fermentation exceeded 50% in all processes, H. vineae was still the main species in Hv and in those protocols in which H. vineae was inoculated with over 90% of the cells (Fig. 2) however, with fewer viable cells in all protocols respect the first sampling point. At this second point, H. vineae cells showed an upward trend with the initial dose employed in the co-inoculated trials, reflecting the initial ratio of H. vineae inoculum. On the other hand, the number of viable S. cerevisiae cells was consistent at both sampling points with the initial number of cells inoculated in the different protocols. Between both species, H. vineae represented ~ 35%, ~ 40%, ~ 60%, ~ 70%, and ~ 82% in C67, C80, C90, C95, and C98, respectively on day six. Despite the higher rate of H. vineae in the first stages, these results indicate a sort of maintenance of the original proportions employed, with no preferential colonization of one species on the other after six days. However, these differences could plausibly be related to the ethanol produced at this stage of fermentation. The number of viable H. vineae cells by day six was considerable, as the alcohol content in wines (8% to 9%) approached the levels of ethanol tolerance reported under laboratory conditions[14, 18].
Mean values (n = 3) ± standard deviation of viable cells (CFU/mL) at day three (left) and day six (right) of alcoholic fermentation of (gray) H. vineae and (black) S. cerevisiae in the fermenting must of Glera. Data are compared with one-way ANOVA followed by Tukey HSD multiple comparison (p < 0.05). Different letters indicate values statistically differentiated
The quality control parameters of wine at the end of fermentation are reported in the supplementary material (S2a, S2b). Despite the differences observed for some of them, it will be focused on those associated with the acid profile (Fig. 3a, b), which has already been reported to be modified by H. vineae under winemaking conditions [17]. Processes inoculated with H. vineae resulted in lower titratable acidity of wines, in both Glera and Termantis, with a negative correlation with the initial H. vineae ratio inoculated. The lowering of titratable acidity was mostly related to the decrease in malic acid concentration, which showed the same downward trend in both varieties. This statement becomes even more evident when evaluating Hv processes, for which malic acid is about half that of Sc in Glera wines and ~ 0.4 g/L lower in Termantis. Contrary to malic acid, tartaric acid presented an unclear trend, and the sole protocol to be influenced in both wine styles is Hv, probably as a consequence of an increased potassium bitartrate precipitation because of the slower fermenting kinetics.
(a) Mean values (n=3) ±standard deviation of the acidic parameters of wines at the end of the alcoholic fermentation of Glera. Titratable acidity (gray); Malic acid (red); Tartaric acid (black) and Volatile acidity (blue). Data are compared with one-way ANOVA followed by Tukey HSD multiple comparison (p < 0.05). Different letters indicate values statistically differentiated. (b) Mean values (n=3) ±standard deviation of the acidic parameters of wines at the end of the alcoholic fermentation. Titratable acidity (gray); Malic acid (red); Tartaric acid (black) and Volatile acidity (blue). Data are compared with one-way ANOVA followed by Tukey HSD multiple comparison (p < 0.05). Different letters indicate values statistically differentiated
Volatile acidity was higher in Sc processes in the white winemaking of Glera and, in contrast, much lower than Hv in Termantis. Within H. vineae co-inoculated protocols, this parameter showed the same upward trend for both wine styles, with the H. vineae ratio being clearer and statistically significant in the red wine vinification of Termantis. H. vineae produces high concentrations of ethyl acetate [23], which, along with acetic acid, represents the volatile acidity of the wines. Nonetheless, ethyl acetate imparts fruity characteristics [24], and wines produced with H. vineae are perceived as fruitier [8, 18].
Regarding aroma compounds, the volatilome of the resulting wines is reported in supplementary material S3a and S3b, respectively for Glera and Termantis. To evince better the effect of yeast species and inoculum protocols, some metabolic markers known to be influenced by yeast species or directly produced due to lipid, nitrogen, and central carbon metabolisms have been selected (Table 2).
Ethyl hexanoate
Ethyl hexanoate in wines is formed by yeast from the esterification of ethanol with hexanoic acid, resulting mainly from lipid metabolism in the production of long-chain fatty acids [25, 26]. It confers wine-ripe fruit notes and presents an odor threshold of only 14 μg/L [27]. These features, along with the higher concentration of this ester normally reported in wines compared to others, convey its importance to wine quality. Ethyl hexanoate concentration was the highest among the main ethyl esters in both Glera and Termantis (Supplementary material S3a, S3b), and values were at least 44% and 55% higher than any other ethyl ester in Sc wines. Between species, Sc was richer than Hv in both white and red wine vinifications: ~ eightfold higher in Glera, and ~ 2.5-fold higher in Termantis. These differences have been reported in several studies that compared the metabolic features of H. vineae in sequential or pure inoculation [17, 28]. Within the co-inoculated processes, a downward trend was observed with a higher H. vineae ratio in both white and red wine vinifications (Fig. 4a and b, respectively), which allowed differentiating C67 from the rest of the processes, C80 from Hv in Glera wines, and C67 from C95 in Termantis.
(a) Correlation between the concentration of a) 2-phenylethanol; b) β-phenylethyl acetate; c) ethyl hexanoate; and d) isoamyl acetate and the H. vineae ratio in co-inoculated fermentations of Glera. The dotted lines represent the 95% confidence interval. (b) Correlation between the concentration of a) 2-phenylethanol; b) β-phenylethyl acetate; c) ethyl hexanoate; and d) isoamylacetate and the H. vineae ratio in co-inoculated fermentations of Termantis. The dotted lines represent the 95% confidence interval
Isoamyl acetate
Among the higher alcohol acetate esters, isoamyl acetate was selected as it is largely the main yeast-derived compound of this family of volatile molecules in S. cerevisiae [29, 30]. It is formed as a result of the esterification of isoamyl alcohol by acetyltransferases, and its content in wine is highly dependent on the nitrogen metabolism of yeast [31, 32]. It characterizes the aroma of young wines with its typical hint of banana and "yellow" fruit [33, 34] because of its low odor threshold of 30 μg/L [23]. Compared with Sc, sequential inoculations of H. vineae (Hv) in Glera and Termantis wines resulted in a lower concentration of this acetate. This difference was more evident in Glera wines (> threefold) than in the red wine vinification of Termantis, where the difference was not statistically significant. A lower production with sequential inoculation has been reported previously [17, 35] nevertheless, the C67 and C80 Glera processes were richer in isoamyl acetate than in Sc. Among the co-inoculation processes, a negative correlation was observed with higher H. vineae ratios in Glera (Fig. 4a), and even if not differentiated, mean values showed the same tendency in Termantis wines (Fig. 4b).
2-phenylethanol
2-phenylethanol in wines is derived from phenylalanine, which is metabolized or produced by yeasts via the Erlich and Shikimate pathways [36]. This alcohol can actively participate in the flavor profile, developing honey-like and rose-like notes when present at concentrations greater than 14 mg/L [37]. It is also a necessary precursor for the production of β-phenylethyl acetate by yeast. This compound was consequently considered as H. vineae presents specific features in the biosynthetic pathways of aromatic amino acids [1], influencing the volatile yeast derivatives. The concentration of 2-phenylethanol was lower in Hv compared to the other processes, both in white and red winemaking. Differences were noticeable in white wines, for which Sc concentrations were approximately twice as high as in Hv however, the absolute values were higher in red wines. These results have already been observed in both pure [18, 38] and sequential H. vineae inoculation in winemaking [8], probably because S. cerevisiae can activate the phenylpyruvate pathway under nutritional starvation conditions, overproducing this alcohol in YAN-limited fermenting musts [12]. The YAN of Glera and Termantis musts was low (30 mg/L and 61 mg/L, respectively), probably explaining the exceeding of the odor threshold in Sc wines. For the inoculated processes, two statistically differentiated groups were observed: C67 was separated from the rest by its higher concentration in Glera wines, and C95 and C98 in Termantis. For both wine styles, 2 phenylethanol showed the same downward trend with the increase in the H. vineae ratio as for the previous metabolites (Fig. 4a, b, respectively). Some authors have explained the lower concentration of 2-phenylethanol in wines through the overproduction of the corresponding ester by yeasts [5], which is a compound that characterizes H. vineae wines [38]. However, this explanation fails to fully explain the difference in concentration in molar terms [17] and may be the result of the different competing metabolic pathways employed by H. vineae in the catabolism and metabolism of aromatic amino acids [1].
β-Phenylethyl acetate
Among the metabolic yeast markers studied, β-phenylethyl acetate is probably the most important feature of H. vineae [21, 38], in terms of both concentration and aroma characteristics [1]. This compound exhibits a marked rose-like scent [39], for which wines produced with S. cerevisiae rarely exceed the odor threshold [40]. The results confirm this assertion, and Sc wines did not exceed 0.25 mg/L of this acetate ester, both in white and red wines. Hv processes were characterized by increased amounts of β-phenylethyl acetate compared to Sc, more clearly in white wines, for which the concentration was up to64-fold higher. These results confirm the remarkable production of this ester by H. vineae, as previously reported by several authors [14, 18, 21]. β-phenylethyl acetate values were consistent with those of previous studies on red wine production with sequential inoculation with H. vineae [41]. Regardless of the absolute values and diversly of the previous metabolites, the concentration of β-phenylethyl acetate showed an upward trend with the H. vineae ratio (Fig. 4a and b).
Benzyl alcohol
Benzyl alcohol is considered a metabolic marker of H. vineae, as it can be also de novo synthesized by this yeast in the absence of grape precursors [13]. In Termantis wines, the benzyl alcohol levels were comparable among processes (Table 2). In Glera, the sole differences appeared between Hv and Sc, for which the former was ~ sevenfold higher, according to previous studies on pure fermentation with H. vineae [18]. Despite the low content, an upward trend was observed with increasing H. vineae ratio, as previously observed for β-phenylethyl acetate.
Discussion
Indigenous non-Saccharomyces species have often been associated with the appearance of sensory faults in wines [42, 43]. Besides, non-Saccharomyces yeasts present a lower tolerance to ethanol, which does not allow normally to complete fermentation independently [44, 45]. Thus, under industrial conditions, the completion is normally accomplished by S. cerevisiae. The increased knowledge carried out during the last 30 years has led non-Saccharomyces to be in the spotlight of researchers and winemakers, owing to some specific, overexpressed, or silenced features to be exploited in wine production [46,47,48]. In the case of H. vineae, some strains have been selected because of their ethanol tolerance thanks to the higher glycolytic activity [49] and copy number of alcohol dehydrogenase genes [5], that allows to reach up to 10% v/v under controlled conditions [10]. Along with the alcohol tolerance [12, 22], the Hv205 strain used was further selected from other H. vineae strains because of the specific aroma features that impart in wines [50]. Once selected, suitability in industrial contexts depends not only on these attributes but rather on the overall attitude to result in the desired product and fulfill expectations. These also include the possibility of foreseeing the final characteristics of wines and scheduling the production. This is particularly important in winemaking, as fermentation is condensed in just a few weeks. In addition, the aroma features given by yeasts must comply with varietal aspects that play an important role in the perception of wine typicality.
In our study, the use of H. vineae in sequential inoculation resulted in slower fermentation kinetics compared to S. cerevisiae in both white and red wine vinification, confirming this feature in semi-industrial wines [17]. Regarding red wine vinification, Hv doubled the time required for the completion of fermentation, and in white wine vinification, needed over five times the time employed by S. cerevisiae. Usually, winemakers that use non-Saccharomyces species assume different fermentation kinetics only if the metabolic features that they are looking for emerge. In the case of H. vineae, its use has been proposed to increase the aroma complexity of wines through the overproduction of some compounds that are mainly involved in the metabolism of aromatic amino acids [5]. However, it is not limited to these compounds and its features allow for increased terpenes and some norisoprenoids in wines [8, 51], probably as a consequence of the exocellular activity of β-glycosidases secreted by H. vineae. Recently, it has also been reported the production of safranal [51] during the aging of Albillo produced with H. vineae or the enhanced concentration of yeast mannoproteins [8, 51], as a consequence of its faster autolysis [52, 53]. Co-inoculation of H. vineae with S. cerevisiae in Glerashortened fermentation compared to Hv, and even if with slower kinetics than Sc, some protocols could reasonably comply with most industrial needs during harvest. The shortened time showed a downward trend with an increasing H. vineae inoculum ratio. At day three, the number of viable H. vineae cells was comparable among the different protocols and with S. cerevisiae in Sc. However, at day six, differences were substantial but coherent with the initial inoculum -both H. vineae and S. cerevisiae- increasing the viable cell ratio in those processes initially poorer than the apiculate yeast (C67, C80, and C90). These results might be interpreted as an advantage of H. vineae development during the first stages of the alcoholic fermentation towards S. cerevisiae. To date, H. vineae has not been reported to produce toxins against S. cerevisiae [1], similar to other Hanseniaspora spp. [54], supporting previous speculations regarding the vantage of nutrient assimilation [17].
Some species of the genus Hanseniaspora have been proposed for the degradation of malic acid during winemaking [55] and the degradation of malic acid by Hv205 was previously noted in sparkling wine production [17]. Although malate dehydrogenases are present in a vast number of Hanseniaspora spp., H. vineae lacks most malate cell transporters found in S. cerevisiae [1]. However, H. vineae contains JEN family genes that can metabolize fumarate and succinate, which are in turn involved in malate degradation by other microorganisms, such as Pichia kudriavzevii [56]. The decreasing content of malic acid with an increasing number of viable cells inoculated with H. vineae in white and red winemaking provides further evidence of its ability to degrade malate. Nonetheless, further studies are required to elucidate the malate degradation mechanisms of H. vineae.
Regarding the production of aroma compounds, every volatile metabolic marker studied in white and red wines was significantly correlated with the H. vineae ratio in the co-inoculated wines (Table 3), positively only with β-phenylethyl acetate and benzyl alcohol. The linearity of the results with the H. vineae ratio at inoculum allowed for modulation of the overall volatile profile in wines, increasing the main H. vineae feature (β-phenylethyl acetate production) compared to S. cerevisiae, even at the lowest ratio (C67), both in Glera and Termantis wines. This has resulted in a 44-fold to 64-fold increase compared to Sc, confirming the remarkable production of this ester by H. vineae [18, 50]. The concentration of β-phenylethyl acetate in mixed fermentations was moreover comparable to that of Hv from C90 in Glera and C67 in Termantis. Thus, even if significantly lower, the aroma characteristics of H. vineae emerged even at the lower co-inoculation ratio in mixed fermentations. These results are in agreement with previous studies that have highlighted this metabolic feature in pure and sequential inoculations [1, 57]. However, comparison with Hv processes might be misleading, as acetate esters in wines are subject to acid hydrolysis [58] arising in 2-phenylethanol. Thus, the longer fermentation kinetics of Hv may have increased β-phenylethyl acetate degradation. Considering the sum of β-phenylethyl acetate and 2-phenylethyl alcohol, both of which are mainly derived from the same metabolic pathways, protocols managed with H. vineae were not differentiated. Sc was the sole showing different amounts of the sum of phenylalanine derivatives, higher than any other protocol. Differences may be then remitable to the acetylation ratio, for which that found in Sc was much lower (barely 0.01) than in any co-inoculated protocol. This parameter increased linearly with the H. vineae ratio: from 0.46 ± 0.01 in C67 to 0.90 ± 0.04 in C98, indicating its dependence on the metabolic activity of H. vineae during fermentation.Differences in absolute values between wine styles could be partially attributable to the inhibition of the expression of alcohol acetyltransferases in aerated media [59], such as red winemaking, which could have resulted in only Termantis C98 exceeding the odor threshold in red wines. However, these differences could be also the result of different population dynamics during fermentation. H. vineae has been demonstrated to not impede S. cerevisiae growth [1], but under anaerobic conditions, it can limit its development during the first stages of fermentation. However, H. vineae growth benefits from moderate microaerobic conditions [60], largely exceeding the red winemaking of Termantis, for which aeration conditions are known to increase cell growth [61]. The absence of differences from sequential inoculation suggests that aerobic management applied during maceration may have affected H. vineae metabolism, as already observed in Sc [62]. The increased expression of the acetyltransferases of H. vineae [38] seems to be somehow specific for aromatic higher alcohols as confirmed by the overproduction of β-phenylethyl acetate, its positive correlation with the increasing inoculum ratio, and the negative correlation with the same found for isoamyl acetate. Not only, but the increased concentration of isoamyl acetate in C67 and C80 white wines suggests a sort of synergy that the presence of S. cerevisiae would determine in the formation of this compound. This speculation is supported by the negative correlation found with the H. vineae inoculation ratio and the lower concentration found in Hv wines, that may have varied the kinetics of 2-phenylethanol release in the fermenting media, acting as a substrate for acetylation, as previously reported for other non-Saccharomyces multistarters used in winemaking [63].
Overall, the results reported for white wines are comparable to those of red wine vinification protocols, but to different extents. These differences might be explained by the concurrent features of wine style production that are intrinsically linked to the aeration conditions in red winemaking. Aeration is known to affect yeast metabolism, and H. vineae prefers moderate aeration conditions to best express its enological features [60]. S. cerevisiae is a facultative anaerobe that could increase competition against H. vineae during the population dynamics of the first steps of alcoholic fermentation [18, 64], thus limiting the effect on the metabolic profile of wines. Lastly, the expression of acetyltransferases is inhibited by oxygen [59, 61], further limiting acetate ester production.
Regarding benzyl alcohol, despite its low content, an upward trend was observed with increasing H. vineae ratio as for β-phenylethyl acetate. The increased production of phenylpropanoid derivatives by H. vineae is dependent on the nutritional status of musts [65]. In Glera, a slowdown of the alcoholic fermentation kinetics reported in Hv processes during the second part of alcoholic fermentation could have activated the mandelate and 4-hydroxy mandelate pathways[65], leading to a higher production of this aromatic amino acid derivative, not observed co-inoculated trials.No difference were found between processes for any of the terpenes or norisoprenoids analyzed (Supplementary material S3a, S3b); however, compounds belonging to these families were present only in limited concentrations in wines.
Conclusions
This work has demonstrated that different co-inoculation strategies of H. vineae with S. cerevisiae might be a promising alternative for the use of this apiculate yeast in winemaking industrial conditions. Every parameter studied was correlated with the initial H. vineae inoculum ratio, starting from the fermentation kinetics, for which the time needed to complete the alcoholic fermentation was reduced with a lower ratio in both winemaking styles and significantly diminished with respect to the sequential protocol. Interestingly, despite the presence of S. cerevisiae at the onset of alcoholic fermentation, H. vineae was the main species in the first stages of fermentation and did not limit S. cerevisiae contribution.
The metabolic features of H. vineae were clearly expressed in co-fermentation, resulting in increased production of β-phenylethyl acetate compared to S. cerevisiae, while it remained comparable to the sequential inoculation with the highest studied ratios. The lower ratios resulted in the amelioration of other aroma compounds, better than S. cerevisiae for isoamyl acetate in Glera wine. Regarding the acidic parameters, the co-inoculation strategies limited the natural degradation of malic acid, and even if white wines could not be sought, this feature could be exploited in red winemaking in cool climates or vinification of early harvested grapes.
These results underscore the potential impact of strategically managing the co-inoculation ratio to achieve industrial requirements, demonstrating its ability to influence wine quality and increase aroma complexity and product diversity.
Data availability
I don't have any research data outside the submitted manuscript file.
References
Carrau F, Dellacassa E, Boido E, Medina K, Valera MJ, Fariña L, Perez G, Martin V, Alvarez-Valin F, Balestrazzi L (2023) Biology and physiology of Hanseniaspora vineae: metabolic diversity and increase flavour complexity for food fermentation. FEMS Yeast Res 23:1–18. https://doi.org/10.1093/femsyr/foad010
Badura J, Kiene F, Brezina S, Fritsch S, Semmler H, Rauhut D, Pretorius IS, von Wallbrunn C, van Wyk N (2023) Aroma profiles of Vitis vinifera L. cv. gewürztraminer must fermented with co-cultures of saccharomyces cerevisiae and seven Hanseniaspora spp. Fermentation 9:2. https://doi.org/10.3390/fermentation9020109
Medina K, Ferreri L, Fariña L, Boido E, Gaggero C, Carrau FM (2007) Aplicacion de la levadura Hanseniaspora vineae en cultivos mixtos con Saccharomtces cerevisiae en la vinificacion. Enologia 2:1–6
Giorello F, Berná L, Greif G, Camesasca L, Salzman V, Medina K, Robello C, Gaggero C, Aguilar PS, Carrau F (2014) Genome sequence of the native apiculate wine yeast Hanseniaspora vineae T02/19AF. Genome Announc 2:3. https://doi.org/10.1128/GENOMEA.00530-14
Giorello F, Valera MJ, Martin V, Parada A, Salzman V, Camesasca L, Fariña L, Boido E, Medina K, Dellacassa E, Berna L, Aguilar PS, Mas A, Gaggero C, Carrau F (2019) Genomic and transcriptomic basis of Hanseniaspora vineae’s impact on flavor diversity and wine quality. Appl Environ Microbiol 85(1):1–20. https://doi.org/10.1128/AEM.01959-18
Carrau F, Henschke PA (2021) Hanseniaspora vineae and the concept of friendly yeasts to increase autochthonous wine flavor diversity. Front Microbiol 12(August):1–8. https://doi.org/10.3389/fmicb.2021.702093
Del Fresno M, Escott C, Loira I, Herbert-Pucheta JE, Schneider R, Carrau F, Cuerda R, Morata A (2020) Impact of hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality whitewine. Fermentation. https://doi.org/10.3390/FERMENTATION6030066
Del Fresno M, Escott C, Loira I, Carrau F, Cuerda R, Schneider R, Bañuelos MA, González C, Suárez-Lepe JA, Morata A (2021) The impact of Hanseniaspora vineae fermentation and ageing on lees on the terpenic aromatic profile of white wines of the albillo variety. International Journal of Molecular Sciences Article. https://doi.org/10.3390/ijms22042195
Martin V, Fariña L, Medina K, Boido E, Dellacassa E, Mas A, Carrau F (2019) Oenological attributes of the yeast Hanseniaspora vineae and its application for white and red winemaking. BIO Web Conf 12:02010. https://doi.org/10.1051/bioconf/20191202010
Lleixà J, Manzano M, Mas A, del Portillo M (2016) Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01959
Lleixà J, Martín V, Giorello F, Portillo MC, Carrau F, Beltran G, Mas A (2019) Analysis of the NCR mechanisms in Hanseniaspora vineae and Saccharomyces cerevisiae during winemaking. Front Genet 10:1–9. https://doi.org/10.3389/fgene.2018.00747
Martin V, Boido E, Giorello F, Mas A, Dellacassa E, Carrau F (2016) Effect of yeast assimilable nitrogen on the synthesis of phenolic aroma compounds by Hanseniaspora vineae strains. Yeast 33:323–328. https://doi.org/10.1002/YEA.3159
Martin V, Giorello F, Fariña L, Minteguiaga M, Salzman V, Boido E, Aguilar PS, Gaggero C, Dellacassa E, Mas A, Carrau F (2016) De novo synthesis of benzenoid compounds by the yeast hanseniaspora vineae increases the flavor diversity of wines. J Agric Food Chem 64:4574–4583. https://doi.org/10.1021/acs.jafc.5b05442
Viana F, Belloch C, Vallés S, Manzanares P (2011) Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: impact on 2-phenylethyl acetate production. https://doi.org/10.1016/j.ijfoodmicro.2011.09.005
Novo M, Gonzalez R, Bertran E, Martínez M, Yuste M, Morales P (2014) Improved fermentation kinetics by wine yeast strains evolved under ethanol stress. LWT Food Sci Technol 58(1):166–172. https://doi.org/10.1016/J.LWT.2014.03.004
Peltier E, Bernard M, Trujillo M, Prodhomme D, Barbe JC, Gibon Y, Marullo P (2018) Wine yeast phenomics: a standardized fermentation method for assessing quantitative traits of Saccharomyces cerevisiae strains in enological conditions. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0190094
Gallo A, Larcher R, Cappello N, Paolini M, Moser S, Carrau F, Schneider R, Nardin T, Roman T (2023) Insights into the grape must composition effect on Hanseniaspora vineae performance and metabolic aroma compounds in Chardonnay base wine for sparkling wine production. J Food Compos Anal 123:105514. https://doi.org/10.1016/j.jfca.2023.105514
Lleixà J, Martín V, Portillo MC, Carrau F, Beltran G, Mas A (2016) Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00338
Paolini M, Tonidandel L, Moser S, Larcher R (2018) Development of a fast gas chromatography–tandem mass spectrometry method for volatile aromatic compound analysis in oenological products. J Mass Spectrom 53(9):801–810. https://doi.org/10.1002/jms.4259
van Wyk N, Badura J, von Wallbrunn C, Pretorius IS (2023) Exploring future applications of the apiculate yeast Hanseniaspora. Critical reviews in biotechnology. Taylor and Francis Ltd, Singapore. https://doi.org/10.1080/07388551.2022.2136565
Martin V, Jose Valera M, Medina K, Boido E, Carrau F (2018) Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—a review. In: Fermentation, vol. 4. MDPI AG. https://doi.org/10.3390/fermentation4030076
Carrau F, Gaggero C, Aguilar PS (2015) Yeast diversity and native vigor for flavor phenotypes. Trends Biotechnol 33(3):148–154. https://doi.org/10.1016/j.tibtech.2014.12.009
Escott C, López C, Loira I, González C, Bañuelos MA, Tesfaye W, Suárez-Lepe JA, Morata A (2021) Improvement of must fermentation from late harvest cv. Tempranillo grapes treated with pulsed light. Foods 10:6. https://doi.org/10.3390/foods10061416
Cliff M, Pickering G (2006) Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J Wine Res 17(1):45–52. https://doi.org/10.1080/09571260600633234
Boss PK, Pearce AD, Zhao Y, Nicholson EL, Dennis EG, Jeffery DW (2015) Potential grape-derived contributions to volatile ester concentrations in wine. Molecules 20(5):7845–7873. https://doi.org/10.3390/molecules20057845
Restrepo S, Espinoza L, Ceballos A, Urtubia A (2019) Production of fatty acids during alcoholic wine fermentation under selected temperature and aeration conditions. Am J Enol Vitic 70(2):169–176. https://doi.org/10.5344/ajev.2018.18030
Ferreira V, López R, Cacho JF (2000) Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric 80(11):1659–1667. https://doi.org/10.1002/1097-0010(20000901)80:11%3c1659::AID-JSFA693%3e3.0.CO;2-6
Viana F, Gil JV, Genovés S, Vallés S, Manzanares P (2008) Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol 25(6):778–785. https://doi.org/10.1016/j.fm.2008.04.015
Fukuda K, Yamamoto N, Kiyokawa Y, Yanagiuchi T, Wakai Y, Kitamoto K, Inoue Y, Kimura A (1998) Balance of activities of alcohol acetyltransferase and esterase in saccharomyces cerevisiae is important for production of isoamyl acetate. Appl Environ Microbiol 64(10):4076. https://doi.org/10.1128/AEM.64.10.4076-4078.1998
Quilter MG, Hurley JC, Lynch FJ, Murphy MG (2003) The production of isoamyl acetate from amyl alcohol by Saccharomyces cerevisiae. J Inst Brew 109(1):34–40. https://doi.org/10.1002/J.2050-0416.2003.TB00591.X
Plata C, Millán C, Mauricio JC, Ortega JM (2003) Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol 20(2):217–224. https://doi.org/10.1016/S0740-0020(02)00101-6
Rollero S, Mouret JR, Sanchez I, Camarasa C, Ortiz-Julien A, Sablayrolles JM, Dequin S (2016) Key role of lipid management in nitrogen and aroma metabolism in an evolved wine yeast strain. Microbial Cell Fact 15:1. https://doi.org/10.1186/s12934-016-0434-6
Guth H (1997) Identification of character impact odorants of different white wine varieties. https://pubs.acs.org/sharingguidelines
Pérez-Coello MS, González-Viñas MA, Garća-Romero E, Díaz-Maroto MC, Cabezudo MD (2003) Influence of storage temperature on the volatile compounds of young white wines. Food Control 14(5):301–306. https://doi.org/10.1016/S0956-7135(02)00094-4
Dutraive O, Benito S, Fritsch S, Beisert B, Patz CD, Rauhut D (2019) Effect of sequential inoculation with non-saccharomyces and saccharomyces yeasts on riesling wine chemical composition. Fermentation 79:5. https://doi.org/10.3390/FERMENTATION5030079
Hua D, Xu P (2011) Recent advances in biotechnological production of 2-phenylethanol. https://doi.org/10.1016/j.biotechadv.2011.05.001
Carrau FM, Medina K, Farina L, Boido E, Henschke PA, Dellacassa E (2008) Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res 8(7):1196–1207. https://doi.org/10.1111/j.1567-1364.2008.00412.x
Valera MJ, Olivera V, Boido E, Dellacassa E, Carrau F (2021) Wine aroma characterization of the two main fermentation yeast species of the apiculate genus hanseniaspora. https://doi.org/10.3390/fermentation7030162
Francis IL, Newton JL (2005) Determining wine aroma from compositional data. In: Australian journal of grape and wine research (Vol. 11, Issue 2, pp. 114–126). Australian Society of Viticulture and Oenology. https://doi.org/10.1111/j.1755-0238.2005.tb00283.x
Viana F, Gil JV, Vallés S, Manzanares P (2009) Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int J Food Microbiol 135(1):68–74. https://doi.org/10.1016/J.IJFOODMICRO.2009.07.025
Medina K, Boido E, Dellacassa E, Carrau F (2018) Effects of non-saccharomyces yeasts on color, anthocyanin, and anthocyanin-derived pigments of tannat grapes during fermentation. Am J Enol Vitic 69(2):148–156. https://doi.org/10.5344/AJEV.2017.17055
Romano P, Braschi G, Siesto G, Patrignani F, Lanciotti R (2022) Role of yeasts on the sensory component of wines. In: Foods, vol. 11. MDPI. https://doi.org/10.3390/foods11131921
Tufariello M, Fragasso M, Pico J, Panighel A, Castellarin SD, Flamini R, Grieco F, Biasioli F, Capozzi V, Khomenko I, Borruso L, Silcock P (2021) Influence of non-saccharomyces on wine chemistry: a focus on aroma-related compounds. Molecules. https://doi.org/10.3390/molecules26030644
Ciani M, Comitini F, Mannazzu I, Domizio P (2010) Controlled mixed culture fermentation: a new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res 10(2):123–133. https://doi.org/10.1111/j.1567-1364.2009.00579.x
Jolly NP, Varela C, Pretorius IS (2014) Not your ordinary yeast: non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res 14(2):215–237. https://doi.org/10.1111/1567-1364.12111
Becerra M, Aranda A, Maicas S, Juan Mateo J (2023) The life of saccharomyces and non-saccharomyces yeasts in drinking Wine. Microorganisms 11(5):1178. https://doi.org/10.3390/MICROORGANISMS11051178
Padilla B, Gil JV, Manzanares P (2016) Past and future of non-Saccharomyces yeasts: from spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front Microbiol 7:185047. https://doi.org/10.3389/FMICB.2016.00411/BIBTEX
Roudil L, Russo P, Berbegal C, Albertin W, Spano G, Capozzi V (2019) Non-Saccharomyces commercial starter cultures: scientific trends, recent patents and innovation in the wine sector. Recent Pat Food Nutr Agric 11(1):27–39. https://doi.org/10.2174/2212798410666190131103713
Valera MJ, Boido E, Dellacassa E, Carrau F (2020) Comparison of the glycolytic and alcoholic fermentation pathways of Hanseniaspora vineae with saccharomyces cerevisiae wine yeasts. Fermentation. https://doi.org/10.3390/fermentation6030078
Medina K, Boido E, Fariña L, Gioia O, Gomez ME, Barquet M, Gaggero C, Dellacassa E, Carrau F (2013) Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem 141(3):2513–2521. https://doi.org/10.1016/j.foodchem.2013.04.056
Del Fresno JM, Escott C, Carrau F, Herbert-Pucheta JE, Vaquero C, González C, Morata A (2022) Improving aroma complexity with Hanseniaspora spp.: terpenes, acetate esters, and safranal. Fermentation 8(11):654. https://doi.org/10.3390/FERMENTATION8110654
Alexandre H, Guilloux-Benatier M (2006) Yeast autolysis in sparkling wine—a review. Aust J Grape Wine Res 12(2):119–127. https://doi.org/10.1111/j.1755-0238.2006.tb00051.x
Martínez JM, Cebrián G, Álvarez I, Raso J (2016) Release of mannoproteins during saccharomyces cerevisiae autolysis induced by pulsed electric field. Front Microbiol. https://doi.org/10.3389/FMICB.2016.01435
Alturki SN, Al-saud NS, Alhejin AM, Hussan Amasha R (2019) Killer phenomenon in yeast: an overview. J Am Sci. https://doi.org/10.7537/marsjas150419.08
van Wyk N, Scansani S, Beisert B, Brezina S, Fritsch S, Semmler H, Pretorius IS, Rauhut D, von Wallbrunn C (2022) The use of Hanseniaspora occidentalis in a sequential must inoculation to reduce the malic acid content of wine. Appl Sci 12(14):6919. https://doi.org/10.3390/APP12146919
Xi Y, Zhan T, Xu H, Chen J, Bi C, Fan F, Zhang X (2021) Characterization of JEN family carboxylate transporters from the acid-tolerant yeast Pichia kudriavzevii and their applications in succinic acid production. Microb Biotechnol 14(3):1130–1147. https://doi.org/10.1111/1751-7915.13781
Gallo A, Roman T, Paolini M, Cappello N, Guzzon R, Carrau F, Schneider R, Larcher R (2024) Aroma features of Hanseniaspora vineae Hv205 wines in sequential and co-inoculation strategies. Fermentation 10(4):191. https://doi.org/10.3390/fermentation10040191
Ramey DD, Ough CS (1980) Volatile ester hydrolysis or formation during storage of model solutions and wines. J Agric Food Chem 28(5):928–934. https://doi.org/10.1021/JF60231A021/ASSET/JF60231A021.FP.PNG_V03
Fujii T, Kobayashi O, Yoshimoto H, Furukawa S, Tamai Y (1997) Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl Environ Microbiol 63(3):910–915. https://doi.org/10.1128/aem.63.3.910-915.1997
Yan G, Zhang B, Joseph L, Waterhouse AL (2020) Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vineae and Saccharomyces cerevisiae. Food Microbiol 90:103460. https://doi.org/10.1016/J.FM.2020.103460
Verbelen PJ, Saerens SMG, Van Mulders SE, Delvaux F, Delvaux FR (2009) The role of oxygen in yeast metabolism during high cell density brewery fermentations. Appl Microbiol Biotechnol 82(6):1143–1156. https://doi.org/10.1007/s00253-009-1909-8
Day MP, Schmidt SA, Smith PA, Wilkes EN (2015) Use and impact of oxygen during winemaking. Aust J Grape Wine Res 21:693–704. https://doi.org/10.1111/ajgw.12199
Zhang B, Tang C, Yang D, Liu H, Xue J, Duan C, Yan G (2022) Effects of three indigenous non-Saccharomyces yeasts and their pairwise combinations in co-fermentation with Saccharomyces cerevisiae on volatile compounds of Petit Manseng wines. Food Chem. https://doi.org/10.1016/j.foodchem.2021.130807
Rintala E, Jouhten P, Toivari M, Wiebe MG, Maaheimo H, Penttilä M, Ruohonen L (2011) Transcriptional responses of saccharomyces cerevisiae to shift from respiratory and respirofermentative to fully fermentative metabolism. OMICS 15(7–8):461. https://doi.org/10.1089/OMI.2010.0082
Valera MJ, Boido E, Ramos C, Manta E, Radi R, Dellacassa E (2020) The Mandelate pathway, an alternative to the phenylalanine ammonia Lyase pathway for the synthesis of benzenoids in ascomycete yeasts. Appl Environ Microbiol 86:17
Funding
Open access funding provided by Fondazione Edmund Mach - Istituto Agrario di San Michele all'Adige within the CRUI-CARE Agreement. The authors declare that they have not received any financial support or funding for this research.
Author information
Authors and Affiliations
Contributions
A.G. conceptualised, investigated, analysed data, and wrote a draft. T.R. conceptualised, supervised, wrote the main manuscript text and administered the project. M.P., S.S. and R.G. did the formal analysis. D.C and N.C investigated and prepared samples F.C. reviewed and edited R.S. conceptualized and reviewed R.L. reviewed and administrated. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallo, A., Roman, T., Paolini, M. et al. The co-inoculation ratio of Hanseniaspora vineae-to-Saccharomyces cerevisiae correlates with aroma metabolic features in wine. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04588-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04588-8