Abstract
The aim of the industrial trial was to monitor the contents of the glycoalkaloids α-solanine, α-chaconine and their aglycon solanidine along the production process from unpeeled raw tubers to potato crisps. This should identify process factors that reveal possible process-related reduction or enrichment of these substances. The successful validation of an LC–MS/MS method using an external calibration for the determination of the glycoalkaloids α-solanine, α-chaconine and its aglycone solanidine met the requirements of the monitoring recommendation EU 2022/561, both, in terms of sensitivity and in view of the required enzyme inactivation. For the tested potato variety, none of the 15 industrially produced samples exceeded the proposed indicative value of 100 mg total glycoalkaloids/kg product mass (range: 13.2 mg/kg for peeled tuber to 67.6 mg/kg for potato crisps). Finally, solanidine contents were measured for the first time in-line a potato crisps production, ranging from 0.03 to 0.14 mg/kg product mass. The glycoalkaloid contents were additionally calculated as fat-free potato dry mass (ffpdm) to enable a standardized comparison of analytical values. With regard to this newly introduced reference value in this kind of process control, the ffpdm, a significant change of the glycoalkaloid contents during the production process of potato crisps after the peeling process could be excluded. This finding refutes current literature knowledge dealing with non-standardized results based on pure product weight ignoring water loss and/or fat intake along the production process. Starting from the unpeeled tuber to the final potato crisps, a glycoalkaloid reduction of 62% on average related to ffpdm could be achieved during the process, mainly related to the peeling. In contrary, the frying process contributed only insignificantly to the glycoalkaloid reduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potatoes are among the world's most important staple foods because they contain nutritionally important ingredients such as high-quality proteins, are rich in vitamins and contain numerous minerals such as potassium, magnesium and manganese [1–3]. It is therefore plausible that in 2021, approximately 376 million tons were harvested worldwide and approximately 11 million tons in Germany, the largest potato producer in the EU [4]. In Germany, an average of 56 kg of potatoes per capita were consumed in 2021/2022 [2]. However, it is not only the potato itself that is a popular food, but also potato-based products such as potato crisps.
The most commonly grown potato variety is Solanum tuberosum, which belongs to the nightshade family (Solanaceae). This plant family contains toxicologically relevant secondary metabolites, the so-called glycoalkaloids (GA). These are steroid glycosides which protect the plant against pests and pathogens. GAs belong to the group of nitrogen-containing steroid glycosides and consist of two components, a nitrogen-containing steroidal alkaloid skeleton and the trisaccharides of α-solanine solatriose (consisting of the monosaccharides: d-glucose, d-galactose and l-rhamnose) rsp. the trisaccharide of α-chaconine chacotriose (consisting of the monosaccharides d-glucose and two units l-rhamnose). The steroid alkaloid skeleton of GA is also known as the so-called aglycon solanidin. The two quantitatively most common glycoalkaloids in potatoes are α-solanine and α-chaconine. α-GA are degraded under certain conditions to β- and γ-metabolites, which are also present in small amounts in potatoes. In the β-,and γ-forms, one and two of the three sugar molecules attached to the aglycone are split off [5].
However, in general, the concentrations of these other GA in tubers are less than 5% of the total GA content [6, 5].The aglycone, solanidine, is not counted as a GA due to the lack of sugar building blocks, but is also toxicologically relevant [5,6,7]. Furthermore, it has recently been shown that solanine and chaconine degrades to their aglycons solanidine by the intestinal pig cecal microbiota with a dependence of the degradation rate on the linked sugar side chain [8].
To give special expression to their poisonous effect, the potato was selected as the “poisonous plant of the year 2022” by the Botanical Special Garden Wandsbekim. To prevent possible toxicological effects of GA, consumption of green, sprouting, or damaged potatoes is discouraged [9]. Mild intoxications may result in nausea, abdominal pain, vomiting, or diarrhea. Severe intoxications may result in disturbances of consciousness up to unconsciousness as well as lead to disturbances of respiration or brain function [9].
GA occur in varying concentrations within the potato plant. For example, sprouts, berries, flowers, and leaves contain significantly (up to 100 times in the case of sprouts) higher levels than roots or tubers. GA also vary within the tuber. The skin of the tuber contains three to ten times more GA than the flesh [10]. In general, GA content in the tuber depends on various factors such as storage conditions, cultivar, tuber size, growing conditions (climate), insect damage, fertilization, or ripeness [5, 7, 11].
GA are considered to be thermally very stable [12] and are hardly destroyed during cooking, baking, frying, and microwave cooking [6, 13, 14]. Solanine breaks down at temperatures between 260 and 270 °C [15].
Legal regulations
For many decades, an indicative level of less than 200 mg total glycoalkaloids (TGA, sum α-solanine and α-chaconine)/kg in the fresh weight of unpeeled, uncooked potatoes was considered safe. This general statement is often found in the scientific literature and can be traced back for decades [7]. However, there was one GA intoxication case in Germany in 2015, in which potatoes with a GA content of 236 mg/kg were consumed. Due to the fact that this value is very close to the previous indicative value, the German Federal Office for Risk Assessment (BfR) used this case as an opportunity to set a new NOAEL (No Observed Adverse Effect Level) of 0.5 mg per kg body weight and a LOAEL (Lowest Observed Adverse Effect Level) of 1 mg GA per kg body weight [9]. To ensure that these health-based guidance values, HBGVs, are not exceeded, the BfR recommends a new safety/guideline level of 100 mg/kg GA as sum of α-solanine and α-chaconine in potatoes and processed potato products. The LOAEL of 1 mg GA per kg body weight was confirmed by EFSA in 2020 [7].
Subsequently, in 2022, the European Commission called for monitoring of glycoalkaloids, their degradation products, and the aglycone solanidine in potatoes and potato products in Recommendation (EU) 2022/561 [16]. In addition, the factors leading to high GA levels are to be identified, especially with regard to processing procedures. The main objective is to find measures to reduce GA levels in potatoes and potato-based products. Member States, with the active participation of food business operators, should identify the factors leading to levels exceeding the indicative value of 100 mg/kg as the sum of solanine and chaconine in potatoes and processed potato products [16].
Influence of processing on GA in potato crisps production
In the production process of potato crisps, the raw potato tubers are first washed and the peels are removed using suitable peeling devices. In the next step, the tubers are cut into very thin slices (1.2–3 mm). The potato slices are again washed to remove starch residues from the surface. Residual water is removed and the potato slices are deep fried for approx. 1.5–3 min at a maximum temperature of 190 °C, whereby the outlet temperature should not exceed 168 °C. The outlet temperature is the temperature in the last zone of the fryer. Here, the crisps are still in the oil and most of the water has already evaporated. The crisps are cooled on belts where excess fat is removed. Immediately after frying, the moisture content should range between 1 and 3% [17]. Finally, the crisps are flavoured in a spice drum. The production process of potato crisps is depicted in Fig. 1 [1, 3, 17].
There are numerous, sometimes ambivalent, statements in the available literature about the behavior of GA during the potato processing. Due to the accumulation of GA on the outer layers of the potato tuber, peeling undoubtedly leads to a significant reduction in the contents of GA by 25%–75%, on average 48% of the total GA [18,19,20]. When slicing and washing potato slices, GA reductions of 71% have been reported compared to unpeeled potatoes [21]. Although it was reported that GA breaks down at rather high temperature of 260–270 °C, several literature studies report that frying is the most effective method to reduce GA content, and decreases of GA contents between 20 and 90% in the final fried potato product compared to the peeled potato are reported [13, 20,21,22]. It should be noted here, however, that all of these literature references relate GA loss to the respective dry mass of the product. Corrections for water loss and fat intake during the frying process were not made in the respective investigations.
Analytical methods
Many different analytical techniques have been applied over the last century for the detection and quantification of GA. With older analytical methods (gravimetric, colorimetric), it was often not possible to distinguish between the individual GA. These non-specific methods are currently no longer used for quantitative purposes.
The most common analytical method for the determination of GA is liquid chromatography (LC) and detection by UV–Vis (ultraviolet–visible light) or (tandem) mass spectrometry (MS) resp. MS/MS. Quantification is performed using external standards where possible, since isotopic labelled internal standards do not exist. However, except for α-solanine, α-chaconine, and solanidine, there are currently no commercially available standards for the other GA in necessary analytical purity [7, 8, 16].
To prevent enzymatic degradation of the sugar moieties of GA, especially when testing raw (unpeeled/peeled) potatoes, a solution of 1% formic acid in methanol in a ratio of 1:2 (volume:weight) should be added to the potatoes during mixing and homogenization, according to Monitoring Recommendation EU 2022/561, before extraction and purification. The limit of quantification (LOQ) for the determination of individual GA should preferably be around 1 mg/kg and not exceed 5 mg/kg [16].
Aims and procedure of the industrial trial
The aim of the industrial trial was to analyse samples along the industrial process for the analytes α-solanine, α-chaconine and solanidine and to check how their contents change during the processing from unpeeled raw tubers to potato crisps. This should identify process factors that reveal possible process-related reduction or enrichment of these substances.
To identify the possible process factors, the fat-free potato dry mass, or ffpdm, was determined for all samples to have a uniform, comparable analytical dataset throughout the trial. For this purpose, in addition to analysing for solanine, chaconine, and solanidine, the dry matter contents of all samples and the fat contents of potato crisps were determined. For raw potato samples, the fat content was neglected, since the average fat content is about 0.1% [1].
Furthermore, the main objective of this study was to validate a suitable selective and sensitive routine method by means of LC–MS/MS for the analysis of solanine, chaconine and solanidine. This method should also be checked for compliance with the criteria of the Monitoring Recommendation [16].
Materials and methods
Solvents and reagents
α-Solanine (CAS No. 20562–02-01) and α-chaconine (CAS No. 20562–03-02) were ordered with a purity of ≥ 95% each and solanidine (CAS No. 80–78-4) with a purity of ≥ 97% from PhytoLab (Vestenbergsgreuth, Germany). All other chemicals and solvents had purity for analysis grade or HPLC grade and were purchased from Th. Geyer (Lohmar, Germany) or Merck (Darmstadt, Germany).
Standard preparation
Stock solutions with a concentration of 1 mg/mL of α-solanine, α-chaconine, and solanidine were prepared in methanol. Working standard solutions of 5 ng/mL for α-solanine and α-chaconine and of 1 ng/mL for solanidine were obtained by dilutions using methanol. The stock solutions and the standard mixes were stored at 4 °C.
Sampling
The sample pool is composed of all relevant production steps of potato crisps. These were the raw tubers as a raw material, the unflavoured potato crisps as a finished product, and various intermediate stages along the production process (see Fig. 1).
The samples were quickly taken from an industrial production line depicting the production process in time at five different process points and three subsamples of approx. 1 kg each. To avoid batch-to-batch fluctuations, samples were taken from one production lot representing the real process time and originated from the following process steps:
I. Potato tuber, unpeeled, washed, variety Priska.
II. Tuber, peeled.
III. Sliced potatoes.
IV. Washed and drained potato slices
V. Potato crisps, unflavoured (frying oil: high-oleic sunflower oil, HOSO).
In Fig. 1, these five sampling steps are marked with a star. Samples II, III and IV were ground and deep-frozen directly on site using an electric knife mill (Retsch Grindomix GM200™, Haan, Germany). Samples I and V were crushed promptly in the laboratory (Retsch Grindomix GM200™, Haan, Germany) and the fresh sample material of samples I–IV was frozen, whereas samples V were stored dry. After samples I–IV were frozen, they were freeze-dried (CHRIST alpha 1–2 LSCbasic™, Osterode am Harz, Germany), and the freeze-dried samples were finely ground again.
The moisture content as well as the contents of α-solanine, α-chaconine, and solanidine were determined in all samples. In sample V, the unflavoured potato crisps, the fat content was analysed in addition. The moisture content was determined using the drying oven method. For this purpose, the sample material was weighed in a glass crucible, mixed with sea sand and the weight loss was determined after drying in an oven at 103 °C for 4 h [23]. The fat content of the potato crisps samples was analysed following the official method according to Stoldt Weibull [24].
Sample preparation
The raw tuber samples (I) were ground as quickly as possible after sampling using a laboratory mill (Retsch Grindomix GM200™, Haan, Germany) and frozen (– 20 °C) for at least 2 days. This was followed by freeze drying (CHRIST alpha 1–2 LSCbasic™, Osterode am Harz, Germany) for at least 48 h at 0.3 bar (main drying), followed by mortaring and subsequent drying for 24 h at 0.03 bar. For homogenization, the freeze-dried samples were ground again and the unpeeled potato samples were crushed in an Ultracentrifugal Mill ZM 200 (sieve 0.5 mm, Retsch™, Haan, Germany). The samples (I–IV) were then weighed directly into PP centrifuge tubes to the nearest 2 g (± 0.01 g). The unflavoured potato crisps (V) were ground using a laboratory mill and weighed to 2 g (± 0.01 g). 80 mL of n-hexane was used for degreasing.
Sample extraction was done according to Matsuda et al. [25]. 10 mL of ethanol (EtOH) and extraction solvent consisting of 5% acetic acid were added to the samples, followed by extraction in an ultrasonic bath for 30 min at 50 °C. As the next step, another 10 mL of extraction solvent and EtOH were added. The sample extracts were centrifuged for 10 min at 3000 rpm and 4 °C. The supernatant was membrane filtered directly into a vial (nylon, 0.45 µm, Th.Geyer, Lohmar, Germany) and analysed using an LC–MS/MS system.
LC–MS/MS analysis
For the quantification of the described analytes in parallel, the HPLC system 1260 Infinity II™ from Agilent (Agilent Technologies, Waldbronn, Germany) was used coupled with a TripleQuad™ 4500 mass spectrometer (Sciex, Darmstadt, Germany). The results were evaluated using Analyst data system (version 1.7.1; Sciex, Darmstadt, Germany). The HPLC column PerfectSil Target ODS™-3 3 μm 125 × 4.0 mm (MZ Analysetechnik, Mainz, Germany) was applied to separate the analytes. 0.1% acetic acid was used as eluent A and acetonitrile was used as eluent B. The flow was 0.5 mL/min and the column temperature was 35 °C. In MRM mode (multiple reaction monitoring), α-solanine, α-chaconine, and solanidine were identified using electron spray ionization (ESI) in positive mode.
Two ion transitions were selected to monitor the analytes. For α-solanine, m/z 868 → 398 was used as a quantifier and m/z 868 → 722 as a qualifier. For α-chaconine, the quantifier m/z 852 → 398 and the qualifier m/z 852 → 706 were used, and for solanidine the quantifier m/z 398 → 98 and the qualifier m/z 398 → 150 were used. The ion transitions of the quantifier were applied to quantify the analytes.
Eluent A was a solution of 0.1% acetic acid in water, while acetonitrile was used as eluent B. A multi-step gradient was optimized to obtain satisfying peak shapes as follows: during the first minute, the column was kept at 10% eluent B, before raising to 50% in 3 min, maintaining this condition for 9 min and further going to 10% B until min 13. Then, an isocratic column-purging phase at 10% eluent B (from 13.1 to 15 min) was appended to restore the initial conditions.
To quantify the samples for α-solanine and α-chaconine or solanidine, an external calibration curve with a total of ten dilutions in the concentration range 0.5—50 ng/mL or 0.01—1 ng/mL was prepared and these were measured every working day.
Method validation
Prior to analysis of α-solanine and α-chaconine and its aglycon solanidine, the LC–MS/MS method was validated for the matrices potato and potato crisps following the guidelines laid down by EURAChem to ensure the quality of the data [26]. To unambiguously identify the target analytes in the LC–MS/MS system, the parameters selectivity and specificity had to be validated. This is first done by directly injecting a standard of the highest possible purity and relatively high concentration into the mass spectrometer. Then the retention times of the analyte and standard mixture are compared via the LC–MS/MS system and thus clearly assigned. In addition, ratios between the mass transitions used per analyte were characterized, and their transferability to the samples was determined and used to confirm the identity of the respective analyte.
Precision, or the degree of dispersion of independent analytical results around the mean, can be divided into intra-day accuracy and inter-day precision. In both cases, two samples were measured 12 times. The first sample was a fresh potato, freeze-dried, and the second sample was a potato crisp sample, which had natural contents of α-solanine, α-chaconine, and solanidine. The results obtained were first tested for normal distribution and outliers according to Grubbs.
For the determination of correctness, both samples were sent to an analytical laboratory with experience in GA analytics. In addition, the recovery rate of a spiked analyte-free peanut snack sample was determined in a double determination. Five different concentrations in the range of 0–100 mg/kg (α-solanine, α-chaconine) and 0–5 mg/kg (solanidine) were considered. The recoveries obtained were between 102 and 118% for chaconine and solanine, respectively, and for solanidine a recovery rate of 112% was established. According to the final draft of the sampling and analysis of plant toxins in food, the average recovery should be between 70 and 120% [27].
To determine the robustness of the method, four different method parameters were changed during the analytical processing of the samples, fresh potato, freeze-dried, and potato crisp. These results were then compared with two regular workups carried out at the same time. The relative repeatability limit served as a parameter for the robustness.
To assess the linearity, an analyte-free peanut snack sample was measured in duplicate and ten further samples were spiked; five different concentrations were spiked in two samples each. The method showed good linearity in the range of LOD—768 μg/kg for α-solanine and LOD—920 μg/kg for α-chaconine with correlation coefficients from 0.9998 and 0.9998 and in the range of LOD – 4.9 mg/kg for solanidine with a correlation coefficient from 0.9999 for both fresh potatoes and potato crisps. The determination of the limit of detection (LOD) and the limit of quantification (LOQ) was carried out using a calibration curve approach. For this purpose, an analyte-free peanut snack sample was processed in duplicate determination for unspiked and with different ascending spiked concentrations twice. The results for the validation for the three analytes, α-solanine, α-chaconine, and solanidine are summarized in Table 1 for the fresh potatoes (freeze-dried) and Table 2 for the potato crisps.
The limit of quantification (LOQ) for the individual GA was well below 5 mg/kg and therefore meets the requirements of the Monitoring Recommendation (EU) 2022/561 [16].
Sample preparation for enzyme inactivation
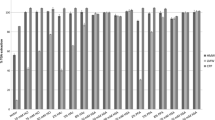
According to the Monitoring Recommendation (EU) 2022/561, a specific solvent is recommended for the pretreatment of fresh potato samples. To prevent enzymatic degradation of the GA during sample workup, a solution of 1% formic acid in methanol in a ratio of 1:2 (volume:weight) should be added when mixing and homogenizing the fresh potato samples before the extraction and purification take place [16]. In this study, all fresh potato samples were frozen immediately after crushing and then freeze-dried. To check whether these different sample preparations lead to the same analytical results, two independent fresh potato samples were treated as suggested by the monitoring recommendation. Aliquots of the same fresh samples were freeze-dried using the method used in this study. The results of these analyses are shown in the Fig. 2.
As a result, it was found that both methods ensure enzyme inactivation and lead to the same results for α-solanine and α-chaconine within the limits of the relative standard deviation of repeatability. For this reason, the method used in this work meets the requirements of the Monitoring Recommendation EU 2022/561 with regard to enzyme inactivation.
Results and discussion
To ensure the comparability of the analysed GA levels in the different potato products generated in this work with literature data, in a first step all values were expressed with reference to the fresh/product weight (mg glycoalkaloide/ kg fw) in the products (uncorrected). Table 3 shows the analysed levels of the GA α-solanine and α-chaconine and their sum expressed as total GA (TGA) as well as the solanidine content in the samples studied, with reference to the fresh weight (fw) in the products (uncorrected). In fresh unpeeled tubers of the Priska variety, α-solanine and α-chaconine levels reached levels of 14.5 and 26.3 mg/kg, respectively, and TGA level of 40.8 mg/kg was calculated from these results. In the course of the subsequent peeling process, a significant reduction of the TGA levels was achieved. The resulting industrially peeled tuber samples contained an average of 18.8 mg/kg TGA. In the next production process, the peeled tubers were sliced and then watered for starch removal, which slightly reduced the average TGA content to first 16.6 mg/kg and then 14.8 mg/kg. The unflavoured potato crisps prepared from these tuber slices contained a mean TGA level of 63.6 mg/kg.
None of the samples examined in this study exceeded the TGA indicative level of currently 100 mg/kg proposed in the Monitoring Recommendation (EU) 2022/561.
According to the literature, the levels of TGA in tubers depends mainly on the variety, growing conditions, and postharvest conditions (mechanical injury, physiological stress, too much light, storage conditions) [28,29,30]. GA are also not equably distributed in the potato tuber. Within a tuber, the highest concentrations are found in the skin, below the skin, and at the germination areas [9].
As also reported in literature, most commercially available potato varieties show GA levels below 100 mg/kg fresh weight [6, 28, 31]. In 177 potato samples of German origin, a mean value of 47.7 mg/kg fw and a maximum of 270.7 mg/kg fw were found [9].
Numerous studies have also been published on the reduction of TGA levels during the peeling process. This reduction depends on the potato variety and beyond this on the depth and peeling technique used. Decreases of 30–58% based on fresh weight as part of the peeling process are reported [32].
According to Lui 2014, ready-to-eat potato crisps contain between 16 and 61 mg/kg fw TGA [33], and Friedman and Dao [10] determined TGA levels of 23.5–109 mg/kg fw in potato crisps [10].Thus, the TGA levels found in this study with respect to fresh weight compared well with literature data.
In the unpeeled, washed tubers, the aglycone solanidine was analysed with mean values of 0.06 mg/kg fw in this study. The peeled tubers prepared from these tubers contained 0.03 mg/kg solanidine. The subsequent slices and the watered slices contained 0.05 and 0.10 mg/kg solanidine, respectively.
An explanation for this higher range in solanidine levels would be biochemical degradation in the time between cutting and frying. There is more time for the slices for enzymatic reactions after the washing and watering process and they therefore show increased values compared to the samples taken directly under the cutting basket. Anyhow, these levels are negligible compared to the natural GA levels, being orders of magnitudes higher. The biological activity is subsequently stopped in the frying process. Mean solanidine contents of 0.14 mg/kg were measured in the potato crisps produced from this material.
Data on the occurrence of solanidine in samples along the potato crisp production process are scarce in the available literature. However, in accordance with the monitoring recommendation, manufacturers are explicitly requested to provide content data on solanidine to the European Commission.
The main objective of the present work was to investigate the potential reduction in TGA as well as solanidine during the manufacturing process of potato crisps. For this purpose, the next step was to select a uniform reference point—the fat-free potato dry mass (ffpdm)—to which all products were converted. Table 3 shows the fat-free potato dry masses (ffpdm) as mean values of three individual determinations examined in this study. These dry masses [g/100 g] were used for the conversion of the GA and the solanidine contents to comparable analytical results covering the entire production process.
Only by using this standardized approach, it is possible to identify absolute variations in the analytes that can be assigned to the processing procedure and is not a consequence of concentration-dependent reduction of water or—simultaneous—intake of fat.
Figure 3 shows the result of this calculation for the samples described in Table 4.
Corrected for this fat-free potato dry mass, unpeeled, washed tubers contain an average of 166.1 mg/kg TGA; as expected, the subsequent peeling process resulted in a substantial decrease in TGA levels of 48% to a value of 86.4 mg/kg ffpdm. The subsequent process step of slicing led to a minimal decrease of TGA of 3% to 83.6 mg/kg ffpdm. Another clear decrease in TGA levels was observed by the process step of watering, which was quantified to 19% or 67.7 mg/kg ffpdm. The final frying of the washed and drained potato slices had an insignificant influence on the contents of TGA. TGA contents of 63.6 mg/kg ffpdm on average were determined in the unflavoured potato crisps, which corresponds to a decrease of only 6% due to the frying process.
It can therefore be stated at this point that based on the standardized fat-free potato dry mass, the peeling process leads to the most significant reduction in TGA content in the course of the potato crisp production process, followed by the watering. The other process steps do not lead to any further significant reduction in TGA. Since the GA are thermally very stable compounds, a significant reduction is anyhow not to be expected during the frying process.
In summary, a decrease in TGA levels during the potato crisp production process of 62% with respect to ffpdm was achieved, which is derived from peeling and washing, not from the subsequent frying process.
A limitation of the present study is the fact that it was only carried out in one production plant and on one potato variety. An extension of the investigations is currently being considered. Nevertheless, since crisps production is a standardized process throughout the snack industry, the variation to be expected is limited to the initial load of GA in different varieties and by that a proportional issue. This will result in different GA levels at the productions steps inspected, but the fundamental conclusions of this study are not expected to be compromised.
Conclusion
A successful method validation for the quantification of the GA α-solanine, α-chaconine, and the aglycone solanidine by means of LC–MS/MS with external calibration has been proven to meet the requirements of the Monitoring Recommendation EU 2022/561, both, in terms of sensitivity and with respect to the required enzyme inactivation.
This method was used to check the potential reduction in the TGA levels during the manufacturing process of potato crisps in industrial application. For this purpose, the analysed GA levels were first calculated in the samples of the industrial trial with reference to the fresh weight or product weight and thereafter to the respective fat-free potato dry mass (ffpdm). This calculation method leads to a standardized basis for the evaluation of the alteration in the TGA levels during the processing process, which was not the case in literature so far. In contrast to numerous literature studies, this reasonable conversion was able to show that the TGA levels remain stable in the production process of potato crisps after the peeling and washing processes are completed. Starting from the unpeeled potatoes to the final potato crisps, a TGA reduction of an average of 62% based on ffpdw was achieved over the course of the process. However, the frying process led to a proportionate reduction of 6% only, which must be regarded as insignificant taking the measurement uncertainty into account. The thermal stability of the GA was therefore once again proven.
In view of the current discussions at EU Commission level regarding the setting of indicative/maximum levels for GA, these results are of utmost relevance. As a consequence of these data collected for the first time it must be stated, that processing factors are no meaningful tool define achievable GA levels in the crisps production process.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Biesalski H-K (2005) Die Kartoffel – kalorienarmer Nährstofflieferant mit wertvollen Inhaltstoffen. LCI - Modere Ernährung heute:1–6
Bundesinformationszentrum Landwirtschaft (2022) Kartoffeln. https://www.landwirtschaft.de/landwirtschaftliche-produkte/wie-werden-unsere-lebensmittel-erzeugt/pflanzliche-produkte/kartoffeln. Accessed 6 Feb 2024
Deutsche Lebensmittelbuch Kommission (ed) (1997) Leitsätze für Kartoffelerzeugnisse
FAOSTAT (2023) Crops and livestock products. https://www.fao.org/faostat/en/#data/QCL/visualize. Accessed 6 Feb 2024
Milner SE, Brunton NP, Jones PW, O’Brien NM, Collins SG, Maguire AR (2011) Bioactivities of glycoalkaloids and their aglycones from solanum species. J Agric Food Chem 59:3454–3484. https://doi.org/10.1021/jf200439q
Friedman M, McDonald GM, Filadelfi-Keszi MA (1997) Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Crit Rev Plant Sci 16:55–132. https://doi.org/10.1080/07352689709701946
Schrenk D, Bignami M, Bodin L, Chipman JK, Del Mazo J, Hogstrand C, Hoogenboom LR, Leblanc J-C, Nebbia CS, Nielsen E, Ntzani E, Petersen A, Sand S, Schwerdtle T, Vleminckx C, Wallace H, Brimer L, Cottrill B, Dusemund B, Mulder P, Vollmer G, Binaglia M, Ramos Bordajandi L, Riolo F, Roldán-Torres R, Grasl-Kraupp B (2020) Risk assessment of glycoalkaloids in feed and food, in particular in potatoes and potato-derived products. EFSA J 18:e06222. https://doi.org/10.2903/j.efsa.2020.6222
Kasimir M, Wolbeck A, Behrens M, Humpf H-U (2023) Intestinal metabolism of selected steroidal glycoalkaloids in the pig cecum model. ACS Omega 8:18266–18274. https://doi.org/10.1021/acsomega.3c01990
Bundesinstitut für Risikobewertung, Speisekartoffeln sollten niedrige Gehalte an Glykoalkaloiden (Solanin) enthalten: Stellungnahme Nr. 010/2018 des BfR vom 23. 2018. https://doi.org/10.17590/20180423-085250
Friedman M, Dao L (1992) Distribution of glycoalkaloids in potato plants and commercial potato products. J Agric Food Chem 40:419–423
Morris SC, Petermann JB (1985) Genetic and environmental effects on levels of glycoalkaloids in cultivars of potato (Solanum tuberosum L.). Food Chem 18:271–282
Jadhav SJ, Salunkhe DK (1975) Formation and control of chlorophyll and glycoalkaloids in tubers of Solanum tuberosum and evaluation of glycoalkaloid toxicity. Adv Food Res 21:307–354
Friedman M (2006) Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J Agric Food Chem 54:8655–8681
Machado RMD, Toledo MCF, Garcia LC (2007) Effect of light and temperature on the formation of glycoalkaloids in potato tubers. Food Control 18:503–508. https://doi.org/10.1016/j.foodcont.2005.12.008
Porter WL (1972) A note on the melting point of α-solanine. Am Potato J 49:403–406
Commission Recommendation (EU) 2022/561 of 6 April 2022 on monitoring the presence of glycoalkaloids in potatoes and potato-derived products. In: Official Journal of the European Union
Commission Regulation (EU) 2017/2158 of 20 November 2017 establishing mitigation measures and benchmark levels for the reduction of the presence of acrylamide in food. In: Official Journal of the European Union
Haase NU (2010) Glycoalkaloid concentration in potato tubers related to storage and consumer offering. Potato Res 53:297–307. https://doi.org/10.1007/s11540-010-9162-1
Tajner-Czopek A, Jarych-Szyszka M, Lisinska G (2008) Changes in glycoalkaloids content of potatoes destined for consumption. Food Chem 106:706–711
Tajner-Czopek A, Rytel E, Kita A, Peksa A, Hamouz K (2012) The influence of thermal process of coloured potatoes on the content of glycoalkaloids in the potato products. Food Chem 133:1117–1122
Rytel E, Tajner-Czopek A, Kita A, Miedzianka J, Bronkowska M (2015) The influence of washing and selection processes on the contents of glycoalkaloid and other toxic compounds during industrial chip production. Int J Food Sci Technol 50:1737–1742. https://doi.org/10.1111/ijfs.12840
Peksa A, Lubowska G, Anilowski K, Lisinska G, Rytel E (2006) Changes of glycoalkaloids and nitrate contents in potatoes during chip processing. Food Chem 97:151–156
Beuth Verlag (1996) DIN EN 12145:1996-10 Fruit and vegetable juices - Determination of total dry matter - Gravimetric method with loss of mass on drying; German version
AOAC (1973) AOAC Official Method 925.07: Fat in Cacao Products
Matsuda F, Morino K, Miyazawa H, Miyashita M, Miyagawa H (2004) Determination of potato glycoalkaloids using high-pressure liquid chromatography-electrospray ionisation/mass spectrometry. Phytochem Anal PCA 15:10. https://doi.org/10.1002/pca.755
EURACHem (2014) The fitness for purpose of analytical methods. https://www.eurachem.org/index.php/publications/guides/mv. Accessed 6 Feb 2024
Commission Implementing Regulation (EU) 2023/2783 of 14 December 2023 laying down the methods of sampling and analysis for the control of the levels of plant toxins in food and repealing Regulation (EU) 2015/705
Barceloux DG (2009) Potatoes, tomatoes, and solanine toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis Mon 55:391–402
Knuthsen P, Jensen U, Schmidt B, Larsen IK (2009) Glycoalkaloids in potatoes: Content of glycoalkaloids in potatoes for consumption. J Food Compos Anal 22:577–581
Nema PK, Ramayya N, Duncan E, Niranjan K (2008) Potato glycoalkaloids: formation and strategies for mitigation. J Sci Food Agric 88:1869–1881
Ruprich J, Rehurkova I, Boon PE, Svensson K, Moussavian S, van der Voet H, Bosgra S, van Klaveren JD, Busk L (2009) Probabilistic modelling of exposure doses and implications for health risk characterization: glycoalkaloids from potatoes. Food Chem Toxicol 47:2899–2905
Rytel E (2012) Changes in the levels of glycoalkaloids and nitrates after the dehydration of cooked potatoes. Am J Potato Res 89:501–507
Liu W, Zhang N, Li B, Fan S, Zhao R, Li L-P, Wu G-H, Zhao Y (2014) Determination of α-chaconine and α-solanine in commercial potato crisps by QuEChERS extraction and UPLC-MS/MS. Chem Pap 68:1099. https://doi.org/10.2478/s11696-014-0617-8
Acknowledgements
We would like to thank the Intersnack Group GmbH & Co KG, The Lorenz Bahlsen Snack-World GmbH & Co KG and Agrarfrost GmbH & Co KG for the intensive support of our project, for numerous discussions on the topic of GAs and for the opportunity to carry out the industrial trial and providing the samples along the production chain for the production of potato crisps.
We would also like to thank Ms. Lisa Weltermann for her very helpful assistance in the laboratory and the statistical evaluations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Compliance with ethics requirements
This research does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Raters, M., Strohmaier, R. & Heckel, F. Glycoalkaloid transformation during potato crisps production? Method validation and industrial trial. Eur Food Res Technol 250, 1341–1351 (2024). https://doi.org/10.1007/s00217-023-04463-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04463-y