Abstract
Potato glycoalkaloids can reach levels that are harmful to human health. A rapid and reliable microwave assisted extraction method for quantitative analysis of α-solanine and α-chaconine content in raw potato and potato based products is presented. A chemical microwave was used to determine optimal temperature and pressure conditions for the extraction of α-solanine and α-chaconine from Idaho grown tubers and six commercially available mashed potato products. Recovery efficiency of glycoalkaloids was 37% greater by microwave assisted extraction (19.92 mg/kg glycoalkaloid) as compared to conventional solid/liquid methods (12.51 mg/kg glycoalkaloid). Optimal extraction of glycoalkaloids from potato samples dissolved in methanol was achieved using a microwave reactor set to 90 °C for ten minutes. The interior of Idaho grown tubers was determined to contain lower levels of glycoalkaloids (19.92 mg/kg dry weight; 6.5 ± 1.78 mg α-solanine and 13.40 ± 1.65 mg α-chaconine), as compared to commercial potato products (33.86–81.59 mg/kg).
Resumen
Los glicoalcaloides de la papa pueden alcanzar niveles dañinos a la salud humana. Aquí se presenta un método rápido y confiable de extracción asistida por microondas para análisis cuantitativo del contenido de α-solanina y α-chaconina en papa cruda y en productos a base de papa. Se usó una microonda química para determinar las condiciones óptimas de temperatura y presión para la extracción de α-solanina y α-chaconina, de tubérculos cultivados en Idaho y de seis productos comerciales disponibles de puré de papa. La eficiencia en la recuperación de los glicoalcaloides fue 37% mayor mediante la extracción asistida por microondas (19.92 mg de alcaloide/kg) en comparación a métodos convencionales de sólido/líquido (12.51 mg alcaloide/kg). La extracción óptima de glicoalcaloides de muestras de papa disueltas en metanol se logró usando un reactor de microondas a 90 °C por diez minutos. Se determinó que el interior de los tubérculos cultivados en Idaho contenían niveles más bajos de glicoalcaloides (19.92 mg/kg de peso seco; 6.5 ± 1.78 mg α-solanina y 13.40 ± 1.65 mg α-chaconina), al compararse con productos comerciales (33.86–81.59 mg/kg).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glycoalkaloids are secondary metabolites in Solanum tuberosum L. and other members of the Solanaceae family, which serve as chemical defense against fungi, nematodes, herbivores and other stress conditions (Majeed et al. 2014). Glycoalkaloids are found in vegetables including potato, tomato, eggplant, pepper and non-food plants like tobacco (Friedman 2015; Passam and Karapanos 2008; Weissenberg et al. 1998). Most commercial potato tubers have been reported to contain between 20 to 60 mg of glycoalkaloids per 100 g of freeze dried material, and 4 to 12 mg per 100 g on fresh weight basis (Sotelo and Serrano 2000). Examples of potato tubers with higher glycoalkaloid content have been reported in literature with as much as 33 mg per 100 g on fresh weight basis (Widmann et al. 2008). Concentrations of glycoalkaloids are typically 3 to 10 times greater in the potato peel than flesh. Variation in potato glycoalkaloid quantity is dependent on crop variety, geographic location of crop growth, post-harvest storage conditions, and type of fertilizer applied to soil (Gosselin and Mondy 1989; Mondy and Munshi 1990). At concentrations above 20 mg per 100 g fresh weight, potatoes will have an undesirable bitter taste that can cause nausea, vomiting, diarrhea, stomach and abdominal cramps, headache, fever, rapid and weak pulse, hurried breathing, hallucination, delirium, and in extreme cases coma (Friedman and McDonald 1997). For food safety purposes, an upper limit for glycoalkaloid content of 20 mg per 100 g of potato (fresh weight) has been established by the United States Department of Agriculture (USDA) (Aziz et al. 2012).
Glycoalkaloids are resistant to degradation by household or industrial food processing methods, including exposure to boiling water, oven baking, deep frying, and microwave irradiation. Determination of glycoalkaloid content in food is generally not actively monitored, however incidences of potato glycoalkaloid poisoning have been reported as far back as the 1800’s (Smith 2013). Even though literature reports for total glycoalkaloid content in several potato varieties are available, the total glycoalkaloid content in commercial potato products is not reported and its determination is often complicated by complex constituent mixtures of food ingredients.
Common methods for the determination of glycoalkaloids in potatoes involve three steps: 1) extraction with a suitable polar solvent like water or methanol, 2) purification by precipitation or chromatography, and 3) quantification by spectrophotometry. Methods for glycoalkaloid extraction include steam distillation or Soxhlet reflux. The solvent systems most commonly employed for glycoalkaloid extraction consist of aqueous acetic acid or combinations of acetic acid with methanol/chloroform, tetrahydrofuran, or acetonitrile–methanol; some literature reports specify only methanol (Driedger and Sporns 2001; Nikolic et al. 2005; Jensen et al. 2007).
The purification of glycoalkaloids from potatoes is particularly challenging. Several reported methods implement neutralization with ammonium hydroxide to precipitate glycoalkaloids from polar solvents, and/or partitioning with butanol (Shanker et al. 2011). These techniques were described as substantially limited due to variable glycoalkaloid losses from partial solvent solubility and incomplete recovery as a result of multistep processes (Gregory et al. 1981).
The quantitative determination of glycoalkaloid content in potato has been reported by colorimetric, gravimetric and titrimetric measurement. High performance liquid chromatography (HPLC) with ultraviolet to visible (UV-Vis) detection at 200 nm wavelength is the method most suitable for quantitative determination of glycoalkaloids when used in combination with calibration curves and co-elution of glycoalkaloid standards. In summary, conventional extraction and purification techniques require highly refined skills to accurately quantitate glycoalkaloids from potatoes.
Here we describe a robust, highly reproducible, and efficient method to extract and purify glycoalkaloids from potatoes using microwave assisted extraction (MAE) (Talebpour et al. 2009; Bekdeser et al. 2014). Microwave radiation provided consistent, non-ionizing electromagnetic energy at frequencies between 300 MHz and 300 GHz that evenly heat polar molecules (Cardoso-Ugarte et al. 2013). Conventional heating uses conduction to transfer heat from the outside to the inside of a vessel, whereas microwave irradiation creates internal heat within a sample vessel, thereby minimizing heat loss to the environment (Man and Shahidan 2007). MAE processes occur in a closed system that allows parameter control over temperature and pressure, which results in significantly reduced extraction times as compared to conventional solid/liquid extraction or Soxhlet reflux processes (Huie 2002). In this work we report a MAE method that is optimized for efficient and rapid extraction of glycoalkaloids from potatoes and potato products.
Materials and Methods
Materials
Sodium biphosphate (sodium dihydrogen phosphate monohydrate, ACS grade, J.T. Baker, USA), sodium phosphate (dibasic, anhydrous, ACS grade, Acros, USA), acetonitrile (HPLC grade, Fischer Scientific), methanol (Sigma-Aldrich, HPLC grade), and commercial potato products and Idaho grown potatoes (local market, Boise, ID) were used as received. Nanopure (18 MΩ) water was used for MAE and HPLC. The glycoalkaloid standards (α-solanine and α-chaconine) were provided by an area chemical laboratory (Boise, ID) and validated for purity (>98%) and identity by HPLC-diode array detector (DAD)-mass spectrometry (MS) detection in our laboratory. A glycoalkaloid stock solution (1 mg/mL) was prepared by dissolving α-solanine and α-chaconine in methanol; serial dilutions were performed to generate calibration curves. The chromatogram showed a single peak for each standard and the mass of the single component was consistent with pure α-solanine and α-chaconine. All solvents were HPLC grade.
Methods
Quantitative and qualitative determination of glycoalkaloids from Idaho grown potato tuber and commercially available potato products was performed by MAE and compared to conventional solid/liquid extraction.
Sample Preparation
Idaho grown potato tubers were washed and peeled by hand. The peeled potatoes were boiled in water for 60 min to ensure thorough cooking, followed by mashing. The pulpy slurry was dried in a laboratory oven at 50 to 55 °C for 14 h. The dried samples were milled into powders by mortar and pestle, and then extracted as described below. Commercial instant mashed potato products were all powders and were used as purchased.
Extraction
Microwave Assisted Extraction of Glycoalkaloids
Each sample, including mash dried Idaho potato sample (1500 mg each) was mixed with 20 mL of methanol in a 100 mL Ultra Prep Teflon microwave vessel equipped with a stir bar and capped securely. The reaction vessel was heated from room temperature to the desired extraction temperature over four minutes in a MARS 5, CEM brand chemical microwave. The desired temperature was held constant for one to ten minutes using a power of 100 W. MAE was evaluated at temperatures ranging from 70 to 120 °C, and extraction times from 2 to 12 min to identify the optimal time and temperature conditions for maximum glycoalkaloid recovery. A subsequent 10 min cool-down period lowered the solution temperature to below 55 °C. Particulate was removed from the extraction mixture by filtration and the methanol removed by rotatory evaporation. The residual solid containing the glycoalkaloids was reconstituted in 1 mL of methanol, followed by filtration (0.45 μm) and direct injection into the UPLC.
Solid/Liquid Extraction of Glycoalkaloids
The solid/liquid glycoalkaloid extraction method of Aziz et al. 2012 was performed, with slight variation, to serve as a comparison to our MAE method. To 1500 mg of each potato sample was added 20 mL of hot methanol in a 100 mL Erlenmeyer flask containing a magnetic stir bar; the mixture was stirred vigorously for 30 min. The solid was separated by vacuum filtration and the eluent collected into a separate flask. The solid residue was added back into the Erlenmeyer flask and combined with 20 mL of methanol, to be stirred vigorously for 30 min, followed by filtration. This process was repeated a total of four times. The four eluent fractions were combined and transferred to a 150 mL separatory funnel. The pH was adjusted to 11 by addition of ammonium hydroxide, resulting in the precipitation of glycoalkaloids. It was discovered that glycoalkaloid solubility and extraction efficiency was superior in methanol as compared to aqueous solvent, so the butanol extraction procedural step utilized by Aziz et al. was not required. The alkaline extract was partitioned four times with 10 mL aliquots of water saturated with butanol. The butanol extracts containing the glycoalkaloids were combined and the solvent evaporated to dryness under nitrogen stream. The alkaloid residue was dissolved in 1 mL of methanol, filtered, and analyzed using HPLC as described below.
Purification
Ultra-High Performance Liquid Chromatography- Mass Spectroscopy (UPLC-MS)
Qualitative analysis of the product was conducted using UPLC coupled to a mass selective detector (Thermo scientific MSQ plus) operating in electrospray ionization (ESI) mode. The UPLC method was adapted from a similar potato glycoalkaloid separation reported by Aziz et al. 2012. Quantitative analysis of glycoalkaloids was performed by UPLC-DAD (Dionex Ultimate 3000, Thermo Scientific, USA) monitoring at 200 nm. Samples were separated across an Acclaim™ 120 C18 column with 3 μm particle size and column dimensions of 2.1 μm × 150 mm i.d. Acetonitrile: phosphate buffered saline (PBS) 0.05 M (30:70, v/v) was used as mobile phase at pH 6.5 and a flow rate of 0.4 mL/min. The column temperature was maintained at 40 °C and the injection volume was 40 μL. Recovery calculations were based on the area under glycoalkaloids peaks at retention times of 7.39 and 8.68 min for α-solanine and α-chaconine, respectively. ESI positive ion mode was used for spectral analysis at a cone voltage of 75 V, and a probe temperature at 350 °C. Spectral data were analyzed between a mass range of 100–1000 m/z. Sample extraction and analysis were performed in triplicate.
Glycoalkaloid Recovery Validation
An experiment was conducted to establish the extent of recovery of added glycoalkaloids from potato product by spiking the original material with with α-solanine and α-chaconine. To 1500 mg of mashed potato product powder, sample #5, was added 40 μg each of α-solanine and α-chaconine. The sample was thoroughly mixed, extracted, and analyzed by HPLC for recovery of added glycoalkaloids.
Results and Discussions
Validity Study
The comparative efficiency of MAE to solid/liquid extraction with respect to recovery of potato glycoalkaloids was assessed. Idaho grown potatoes were selected for this investigation due to the following qualities: 1) very low glycoalkaloid content, thus quantitative methods of analysis that work for the Idaho grown potatoes will presumably work for most varieties of potato, 2) the Idaho grown potato is a major contributor to the vitality of the Idaho potato industry, 3) literature data on glycoalkaloid composition are available for comparison and validation of results. This investigation determined the glycoalkaloid content in the flesh of Idaho grown potatoes to be 19.92 mg/kg, which is consistent with literature reports of 20 to 120 mg/kg total glycoalkaloids (Glorio-Paulet and Durst 2000; Friedman and Dao 1992). Glycoalkaloids are predominantly contained in the potato peel, with lower levels in the flesh.
Quantitative and Qualitative Analysis of α-Solanine and α-Chaconine
Figure 1 shows the structures of the two most abundant potato glycoalkaloids, α-solanine and α-chaconine. Figure 2 illustrates a representative HPLC chromatogram for both glycoalkaloid standards separated in accordance with the method of Aziz et al. 2012; the retention times for α-solanine and α-chaconine were 7.39 and 8.68 min, respectively. The identity of each standard was confirmed by mass spectrometry (Fig. S1 & S2). Both α-chaconine (molecular weight 851.5 Da) and α-solanine (molecular weight 867.49 Da) were tetra-protonated. MS spectrum of sample #5 was also given (Fig. S3 & S4) for comparison purposes to show that the compounds eluting at retention times 7.39 and 8.68 min have been definitively identified as α-chaconine and α-solanine. A linear relationship between light absorbance at 200 nm and alkaloid concentration in the range of 1–50 μg/mL was observed for both α-solanine (R2 = 0.99) and α-chaconine (R2 = 0.98) (Fig. 3).
Optimization of Microwave Assisted Extraction of Glycoalkaloids
The factors evaluated to optimize glycoalkaloid extraction efficiency included solvent polarity and volume, microwave power, matrix characteristics such as sample type, extraction time and temperature. Our preliminary experiments with water and other aqueous solvents including 5% acetic acid (Aziz et al. 2012) or 90% methanol (Hossain et al. 2015) caused swelling in potato samples that resulted in solvent loss. Loss of solvent was minimized when neat methanol was used, and literature reports support the use of methanol due to its favorable extraction efficiencies when coupled with pressurized liquid extraction processes (Hossain et al. 2015; Luthria 2012). Since glycoalkaloids are completely miscible in methanol, using this solvent allowed avoidance of an additional glycoalkaloid precipitation step from the extraction mixture. Similarly, addition of excess solvent compared to the sample amount nullified potential issues of solute solubility associated with reduced solvent volume on extraction. In this study, all samples consisted of mashed potato products and Idaho grown potatoes that were mashed consistent in texture to commercial potato products before analysis. All samples were treated identically for sample preparation in an effort to eliminate matrix effects.
Temperature
Temperature dramatically affects glycoalkaloid extraction efficiency. In general, elevated solvent temperature increases solute solubility provided the solute does not decompose. Moreover, since the microwave reactor is a closed system, MAE has a provision for higher temperatures beyond the boiling point of the solvent, provided the vessel is compatible with the autogenous pressure. The critical temperature for the decomposition of alkaloids in cooked potatoes has been reported to be 243 °C (Zitnak and Johnston 1970). The Ultraprep vessels used for this investigation are designed to achieve temperature and pressure conditions up to 300 °C and 1500 psi, respectively. In order to study the influence of temperature on the glycoalkaloid extraction efficiency, MAE was performed at temperatures ranging from 70 to 120 °C. The effect of temperature on glycoalkaloid recovery was observed to reach a maximum for α-chaconine at 90 °C (Fig. 4). The corresponding profile for α-solanine paralleled that of α-chaconine. Reactor contents for MAE experiments that exceeded 90 °C were discolored, indicating the combination of reactor temperature and pressure may have led to alkaloid degradation accounting for reduced product yield.
Effect of Microwave Power and Time
Microwave power and irradiation time were two additional factors that can influence MAE efficiency. Increased irradiation power is expected to reduce the time required to achieve maximum extraction of alkaloids until the point where degradation of alkaloids occurs. Combinations of low to moderate power coupled with longer irradiation time are commonly used to identify optimal conditions for MAE (Liu et al. 2012). In order to minimize the factors affecting alkaloid extraction, microwave power was kept constant at 100 W and alkaloid extraction was monitored from 0 to 12 min. At 100 W power, the optimal extraction efficiency was determined to occur at a reaction time of 10 min (Fig. 5).
Solid/liquid extraction of glycoalkaloids from potatoes has been well-documented (Maldonado et al. 2014; Wang et al. 1972; Distl et al. 2009). However, when glycoalkaloid content is very low, as in the case of the flesh of Idaho grown potatoes, loss of glycoalkalids due to multi-step methods often exceeds the amount of alkaloid present, making quantitative determination exceedingly challenging. In this study, we demonstrated the advantage of MAE as compared to solid/liquid extraction using the flesh of Idaho grown potatoes. From the optimized MAE method, the total recovery of glycoalkaloids, based on triplicate trials was 19.92 mg/kg, which consisted of 6.5 ± 1.78 mg α-solanine and 13.40 ± 1.65 mg α-chaconine. In contrast, solid/liquid extraction yielded total glycoalkaloids of 12.51 mg/kg; a 37% reduction in extraction efficiency. The higher yield achieved by MAE can be attributed to a combination of parameter optimizations (temperature, pressure, time) and a reduction in the number of steps in the solid/liquid extraction method where sample loss occurs (filtration, solvent wash, transfers).
Glycoalkaloid Recovery Validation
MAE of glycoalkaloids from sample #5 spiked with 40 μg α-solanine and 40 μg α-chaconine yielded an 88.19 ± 1.88% recovery of added α-solanine and 76.50 ± 2.17% recovery of added α-chaconine. The UPLC chromatogram of spiked and unspiked sample #5 is provided in the supplemental information (Fig. S5).
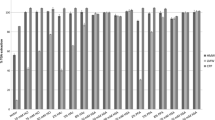
Total Glycoalkaloid Content in Commercially Available Potato Products
Table 1 lists the alkaloid content for a sampling of instant mashed potato products in milligrams alkaloid per kilogram product. UPLC chromatograms for all samples used in this study are shown in Fig. 6. The flesh of Idaho grown potatoes were determined to contain the lowest glycoalkaloid content at 19.92 mg/kg as compared to commercial products with alkaloid quantities that ranged from 33.86–81.59 mg/kg. The difference in glycoalkaloid quantity among the commercial potato products evaluated may be due to factors such as potato variety, inclusion of potato peel in the product, or increases in alkaloid content known to correlate with storage times prior to processing.
In conclusion, the reported procedure uses MAE for faster and more efficient extraction of glycoalkaloids from potato and potato products with respect to traditional solid/liquid extraction techniques. Moreover, microwave irradiation provides uniform temperature adjustment throughout the sample, contributing to higher alkaloid recovery. From the optimized MAE process (power = 100 W, time = 10 min, temperature = 90 °C, solvent = methanol), the total glycoalkaloid content in the flesh of Idaho grown potato was determined to be 19.92 mg/kg (6.5 ± 1.78 mg α-solanine and 13.40 ± 1.65 mg α-chaconine), which was a 37% improvement over traditional solid/liquid extraction. Analysis of six commercial potato samples showed that alkaloid content varied by product from 33.86–81.59 mg/kg. In all cases, the glycoalkaloid content was well within safe consumption limits based on serving size.
DAD, Diode Array Detector; HPLC, High performance liquid chromatography; MAE, Microwave assisted extraction; UPLC, Ultra–high performance liquid chromatography.
References
Aziz, A., M.A. Randhawa, M.S. Butt, A. Asghar, M. Yasin, and T. Shibamoto. 2012. Glycoalkaloids (alpha-chaconine and alpha-solanine) contents of selected pakistani potato cultivars and their dietary intake assessment. Journal of Food Science 77(3): T58–T61.
Bekdeser, B., N. Durusoy, M. Ozyurek, K. Guclu, and R. Apak. 2014. Optimization of microwave-assisted extraction of polyphenols from herbal teas and evaluation of their in vitro hypochlorous acid scavenging activity. Journal of Agricultural and Food Chemistry 62(46): 11109–11115.
Cardoso-Ugarte, G.A., G.P. Juarez-Becerra, M.E. Sosa-Morales, and A. Lopez-Malo. 2013. Microwave-assisted extraction of essential oils from herbs. Journal of Microwave Power and Electromagnetic Energy 47(1): 63–72.
Distl, M., M. Sibum, and M. Wink. 2009. Combination of on-line solid-phase extraction with LC-MS for the determination of potentially hazardous glycoalkaloids in potato products. Potato Research 52(1): 39–56.
Driedger, D.R., and P. Sporns. 2001. Immunoaffinity sample purification and MALDI-TOF MS analysis of alpha-solanine and alpha-chaconine in serum. Journal of Agricultural and Food Chemistry 49(2): 543–548.
Friedman, M. 2015. Chemistry and anticarcinogenic mechanisms of glycoalkaloids produced by eggplants, potatoes, and tomatoes. Journal of Agricultural and Food Chemistry 63(13): 3323–3337.
Friedman, M., and L. Dao. 1992. Distribution of glycoalkaloids in potato plants and commercial potato products. Journal of Agricultural and Food Chemistry 40(3): 419–423.
Friedman, M., and G.M. McDonald. 1997. Potato glycoalkaloids: chemistry, analysis, safety, and plant physiology. Critical Reviews in Plant Science 16(1): 55–132.
Glorio-Paulet, P., and R.A. Durst. 2000. Determination of potato glycoalkaloids using a liposome immunomigration, liquid-phase competition immunoassay. Journal of Agricultural and Food Chemistry 48(5): 1678–1683.
Gosselin, B., and N.I. Mondy. 1989. Effect of packaging materials on the chemical-composition of potatoes. Journal of Food Science 54(3): 629–631.
Gregory, P., S.L. Sinden, S.F. Osman, W.M. Tingey, and D.A. Chessin. 1981. Glycoalkaloids of wild, tuber-bearing Solanum species. Journal of Agricultural and Food Chemistry 29(6): 1212–1215.
Hossain, M.B., A. Rawson, I. Aguilo-Aguayo, N.P. Brunton, and D.K. Rai. 2015. Recovery of steroidal alkaloids from potato peels using pressurized liquid extraction. Molecules 20(5): 8560–8573.
Huie, C.W. 2002. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Analytical and Bioanalytical Chemistry 373(1–2): 23–30.
Jensen, P.H., B.J. Harder, B.W. Strobel, B. Svensmark, and H.C.B. Hansen. 2007. Extraction and determination of the potato glycoalkaloid alpha-solanine in soil. International Journal of Environmental Analytical Chemistry 87(12): 813–824.
Liu, Y., L. Yang, Y. Zu, C. Zhao, L. Zhang, Y. Zhang, Z. Zhang, and W. Wang. 2012. Development of an ionic liquid-based microwave-assisted method for simultaneous extraction and distillation for determination of proanthocyanidins and essential oil in Cortex cinnamomi. Food Chemistry 135(4): 2514–2521.
Luthria, D.L. 2012. Optimization of extraction of phenolic acids from a vegetable waste product using a pressurized liquid extractor. Journal of Functional Foods 4(4): 842–850.
Majeed, A., Z. Chaudhry, and Z. Muhammad. 2014. Changes in foliar glycoalkaloids levels of potato (Solanum tuberosum) triggered by late blight disease severity. International Journal of Agriculture and Biology 16(3): 609–613.
Maldonado, A.F.S., E. Mudge, M.G. Ganzle, and A. Schieber. 2014. Extraction and fractionation of phenolic acids and glycoalkaloids from potato peels using acidified water/ethanol-based solvents. Food Research International 65: 27–34.
Man, A.K., and R. Shahidan. 2007. Microwave-assisted chemical reactions. Journal of Macromolecular Science, Part A 44(4–6): 651–657.
Mondy, N.I., and C.B. Munshi. 1990. Effect of nitrogen-fertilization on glycoalkaloid and nitrate content of potatoes. Journal of Agricultural and Food Chemistry 38(2): 565–567.
Nikolic, N.C., M.Z. Stankovic, and D.Z. Markovic. 2005. Liquid-liquid systems for acid hydrolysis of glycoalkaloids from Solanum tuberosum L. tuber sprouts and solanidine extraction. Medical Science Monitor 11(7): BR200–BR205.
Passam, H.C., and I.C. Karapanos. 2008. Eggplants, peppers and tomatoes: factors affecting the quality and storage life of fresh and fresh-cut (minimally processed) produce. The European Journal of Plant Science and Biotechnology 2(1): 156–170.
Shanker, K., S. Gupta, P. Srivastava, S.K. Srivastava, S.C. Singh, and M.M. Gupta. 2011. Simultaneous determination of three steroidal glycoalkaloids in Solanum xanthocarpum by high performance thin layer chromatography. Journal of Pharmaceutical and Biomedical Analysis 54(3): 497–502.
Smith, K.A. 2013. Horrific tales of potatoes that caused mass sickness and even death. http://www.smithsonianmag.com/arts-culture/horrific-tales-of-potatoes-that-caused-mass-sickness-and-even-death-3162870/?no-ist. Accessed 5 March 2016.
Sotelo, A., and B. Serrano. 2000. High-performance liquid chromatographic determination of the glycoalkaloids alpha-solanine and alpha-chaconine in 12 commercial varieties of Mexican potato. Journal of Agricultural and Food Chemistry 48(6): 2472–2475.
Talebpour, Z., A. Ghassempour, M. Abbaci, and H.Y. Aboul-Enein. 2009. Optimization of microwave-assisted extraction for the determination of glycyrrhizin in menthazin herbal drug by experimental design methodology. Chromatographia 70(1–2): 191–197.
Wang, S.L., N.R. Thompson, and C.L. Bedford. 1972. Determination of glycoalkaloids in potatoes (S. tuberosum) with a bisolvent extraction method. American Journal of Potato Research 49(8): 302–308.
Weissenberg, M., A. Levy, J.A. Svoboda, and I. Ishaaya. 1998. The effect of some Solanum steroidal alkaloids and glycoalkaloids on larvae of the red flour beetle Tribolium castaneum, and the tobacco hornworm, Manduca sexta. Phytochemistry 47(2): 203–209.
Widmann, N., M. Goian, I. Ianculov, D. Dumbrava, and C. Moldovan. 2008. Determination of the glycoalkaloids content from potato tubercules (Solanum tuberosum). Lucrări stiintifice Zootehnie si Biotehnologii 41(1): 807–813.
Zitnak, A., and G.R. Johnston. 1970. Glycoalkaloid content of B5141-6 potatoes. American Journal of Potato Research 47(7): 256–260.
Acknowledgements
The authors wish to thank Dr. Jeffri Bohlscheid, Senior Principal Scientist at J. R. Simplot Company for consultation and material support for this project. The chemical microwave was purchased using funds provided by the state of Idaho Technology Incentive Grant program and the separation and analysis of products was conducted using a UPLC-DAD-MS purchased with funds obtained from the Idaho Department of Commerce Global Entrepreneurial Mission grant program. The financial support of Boise State University College of Arts and Sciences and Office of Sponsored Programs made this work possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing financial interests.
Electronic supplementary material
ESM 1
(DOCX 223 kb)
Rights and permissions
About this article
Cite this article
Kondamudi, N., Smith, J.K. & McDougal, O.M. Determination of Glycoalkaloids in Potatoes and Potato Products by Microwave Assisted Extraction. Am. J. Potato Res. 94, 153–159 (2017). https://doi.org/10.1007/s12230-016-9558-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-016-9558-9