Abstract
Heat treatments are very popular methods of food preparation in European countries. Phytosterol present in foodstuffs undergoes oxidative changes during heat treatment, and phytosterol oxides (e.g., 7α-, 7β-hydroxysterol, 5α,6α-, 5β,6β-epoxysterol, 7-ketosterol and triol) are formed. Phytosterol oxidation products (POPs) have been associated with cytotoxic and pro-apoptotic effects in humans. On the other hand, several studies conducted on animals revealed that some phytosterol oxides lower serum triacyglycerol and blood glucose levels. The aim of this study was to evaluate the effect of heat treatment on formation of phytosterol oxidation products in selected foodstuffs. The following products were taken into considerations: minced meat (pork and beef), frozen French fries, frozen fish fillets, frozen fish products (e.g., fish sticks), wheat and egg noodles. Sterols and POPs content was evaluated by GC–MS working in total and selected ion monitoring modes. The phytosterol oxidation rate was higher in French fries and fish fillets (0.20–1.69% of total phytosterol content) than in noodles, minced meats and readymade fish products (in 0.04–0.36% range). Method of POPs determination using GC–MS is reported in this study. Results of this study show also that products of the animal origin might be considered as sources of the phytosterol oxides in the human diet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Practical application
The method reported in this study may be used for determination of phytosterol oxidation products in various food matrices. The developed method is useful for the determination of the following POPs: 5α,6α-epoxycampesterol and 5β,6β-epoxycampesterol, 5α,6α-epoxysitosterol, 7-ketositosterol and 7-ketocampesterol. The reported data on the POP content in selected thermally processed products are crucial for a reliable dietary intake and the resulting biological effects in human’s assessment.
Introduction
Frying is a very popular way of food preparation in European countries. Crunchy and delicious meals may be prepared with panfrying; furthermore, frying is also very convenient and time-saving. It is common to panfry meat, fish, potatoes and other products with a small addition of vegetable oils (olive or rapeseed oil) to improve the heat transfer [1].
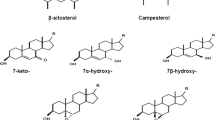
The chemical structures of the phytosterol molecules are similar to the cholesterol. Phytosterols are alicyclic alcohols with an unsaturated bond between C5 and C6, hydroxyl group at C3 and side chain at C17 [2]. Phytosterol present in products of plant origin are β-sitosterol, stigmasterol, campesterol, Δ5-avenasterol and brassicasterol, the latter is a typical compound of rapeseed oil (Fig. 1). The main sources of phytosterol in the human diet are vegetable oils, cereals and nuts. High consumption of plant sterols may reduce blood cholesterol levels [3]. Phytosterols also show anticancer, anti-inflammatory, anti-atherogenicity and antioxidation activities. Consumption of phytosterols may also cause the drop in LDL cholesterol blood levels [4]. Plant sterols are used as biologically active additives for functional foods, for example, yellow fat spreads, which are believed to reduce plasma cholesterol levels when consumed systematically [5].
Due to phytosterol structure, they are susceptible to oxidative changes. The most common products of the phytosterol oxidation reactions occurring in foods are 7α-, 7β-hydroxysterol, 5α,6α-, 5β,6β-epoxysterol, 7-ketosterol and triol [6]. The rate of POPs formation in foods is determined by at least several factors, which are the presence of oxygen, air, water, transition metals, light and higher temperature [5]. During thermal processing, sterols also undergo the autooxidation. A high temperature causes formation of free radicals and lipid peroxide decomposition that promotes the initiation and propagation of lipid autooxidation process [7]. Oxyphytosterols have been found in dried canola seeds, refined and cold-pressed oils, French fries, spread especially enriched products, potato chips, infant formulas and coffees [8]. Many studies concerning phytosterol oxidation in foods of plant origin have been reported previously. Bortolomeazzi et al. [9] stated that POPs content in crude vegetable oils (peanut, sunflower, maize, palm nut and lampante olive oils) ranged from 1.5 ppm in lampante olive to 67.5 ppm in sunflower oils. Dutta investigated the impact of the frying medium on POPs formation in French fries. Potatoes were fried in hydrogenated rapeseed oil/palm oil blend, sunflower oil and high oleic oil, and the total POPs ranged from 2.4 to 4.0 ppm [10]. Lampi et al. heated rapeseed oil at 180 °C and calculated the POPs amount after 6 and 24 h of the treatment. They reported total content of sitosterol and campesterol oxidation products of 266 and 1,068 μg/g after 6 and 24 h of heating, respectively [12].
It has been noticed that high consumption of POPs could have an adverse effect on human health, but on the basis of the available data, this hypothesis could not be unequivocally confirmed [5], even though more attention should be paid to phytosterol oxidation during food processing and meal preparation, because POPs are supposed to have cytotoxic and pro-apoptotic potential [6]. Phytosterol present in vegetable oils subjected to frying may oxidize, and the formed oxidation products can cause damage in an in vitro cell line similar to cholesterol oxides in human body cells [13]. Ryan et al. [14] stated that when comparing to COPs, higher concentrations of POPs are required to elicit the cytotoxicity and modulatory effects on apoptosis in similar extent. On the other hand, some animal studies revealed that specific phytosterol oxides (e.g., oxycampesterol) reduce body weight gain, visceral fat deposition, serum triacyglycerols and blood glucose [6].
The determination of the POPs in foodstuffs is commonly performed by capillary gas chromatography (GC), coupled with flame ionization detector (FID) or mass spectrometer (MS). Appling mass spectrometry allows confirming the analyte identity. Determination of POPs by GC requires its transformation to more volatile silyl ethers [15–18].
The aim of this study was to evaluate the effect of heat treatment on the formation of POPs. Levels of POPs in selected foodstuffs were also assessed. The results of this study might be used to form a general tips how to minimize the formation of heat-induced POPs.
Materials and methods
Materials
5α-Cholestane was purchased from Sigma-Aldrich Company (Poznań, Poland), while the internal standard for POPs quantification 5α-cholestene-3β,16-diol (16-OH) was obtained from Steraloids Co. (London, United Kingdom). The derivatization reagent N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TCMS) was acquired from Sigma-Aldrich Company (Poznań, Poland) and pyridine from Riedel-de Haёn (Seelze, Germany). Reagents including methanol, chloroform, hexane, potassium hydroxide, sodium chloride and anhydrous sodium sulfate were bought from Polskie Odczynniki Chemiczne (Gliwice, Poland). A DB5 ms capillary column (30 × 0.25 mm i.d., 0.25 μm film thickness, 5%-phenyl-95%-dimethylpolysiloxane) came from Phenomenex (Torrance, CA, USA).
Commercial samples
Two different types of minced meat (pork and beef), frozen French fries, fish fillets and frozen fish products (fish sticks, fishburger, Salmon in pastry with spinach), noodles and egg noodles were purchased at a local market in December 2007 in Warsaw, Poland. Minced meat was fresh (with 2-day shelf-life expiration), and it was stored at temperatures between 2 and 5 °C. Frozen French fries, fish fillets and frozen fish products (expiration of shelf-life June 2008) were stored at the temperature of −20 °C. Detailed data on the studied products composition are shown in Table 1.
Thermal processing
Noodles were cooked by boiling for 5–8 min, in accordance with manufacturers’ recommendations. Frozen French fries were heated in oven without addition of vegetable oil at 225 °C for 15 min. Minced meats, fish and readymade fish chops were panfried in a shallow layer (3 ± 0.5 mm) of rapeseed oil for several minutes, except for the salmon with spinach in puff pastry, that was heated in oven at 220 °C for 20 min. After processing, samples were allowed to drain on the kitchen paper for 10 min to eliminate the excess of oil. Boiled, panfried and oven-heated products were analyzed immediately after thermal processing. Samples were prepared in triplicates.
Instrumentation
The GC instrument equipped with a mass spectrometer (GCMS-QP2010S) was obtained from Shimadzu Corporation (Shim-Pol A. M. Borzymowski, Poland). The centrifuge (MPW-210) came from Mechanika Precyzyjna (Poland) and grinder Grindomix GM 200 from Retsch (Haan, Germany).
Fat content determination
About 2 g of well-grounded sample (minced meats, fish fillets, readymade fish chops) was extracted with 16 mL of chloroform/methanol (2:1, v/v) and filtered through the filter paper. Grounded samples of French fries (2 g) and noodles (10 g) were extracted with hexane and also filtered. The solution was washed with 3 mL of a saturated solution of sodium chloride, and then the organic layer was transferred into a flask and centrifuged at 8,000 rpm for the period of 10 min. After centrifugation, the chloroform or hexane layer was collected and filtered through filter paper with 0.5 g of anhydrous sodium sulfate to remove residual water. The filtrate was evaporated to dryness with a gentle nitrogen stream. The total lipid content was determined gravimetrically [19]. Samples were analyzed in triplicates.
Extraction of sterols and POPs
Extracted fat (100–200 mg) was re-dissolved in 2 mL of hexane, and 100 μL of internal standards 19-OH (1 ppm) and 5α-cholestane (4 ppm) were added. The mixture was saponificated with 0.5 mL of sodium hydroxide in methanol (2 N) at room temperature for 1–2 h; 200 μL of the hexane layer was transported into 1.5-mL vial, and after evaporation to dryness with a nitrogen stream, the residue was re-dissolved in 100 μL of pyridine and 100 μL BSTFA with 1% TCMS and left in the dark for 24 h to complete derivatization. Afterward, sample was mixed with additional 1 mL of hexane, and 1 μL was injected into GC–MS. Samples were analyzed in triplicates. The data were statistically analyzed using Statgraphics Plus 4.1 software. To verify the statistical significance of the differences between the determined mean concentrations in particular products, Tuckey’s test was used, at significance level of α = 0.05.
GC–MS analysis of sterols
A DB5-ms capillary column was used to separate cholesterol and the internal standard 5α-cholestane in 28 min with helium as a carrier gas, with a flow rate of 0.6 mL/min. The injector temperature was 230 °C, and split (50:1) injection was applied. The column temperature was programmed as follows: 50 °C at the beginning for 2 min and subsequently increased to 230 °C at the rate of 15 °C/min then to 310 °C at the rate of 3 °C/min maintained for 10 min. The interface temperature for GC–MS was 240 °C. The temperature of ion source was 220 °C, and ionization energy was 70 eV. The total ion current (TIC m/z 100–600) mode was used to quantify phytosterols [20]. The elution order of internal standard (5α-cholestane) and sterol was as follows (molecular ion peaks are given in parenthesis): 5α-cholestane (m/z 217 characteristic ion), cholesterol TMS (m/z 368), brassicasterol TMS (m/z 470), campesterol TMS (m/z 472), stigmasterol TMS (m/z 486) and β-sitosterol (m/z 366). Internal standard method was used for quantification of particular sterols [11]. It was assumed that all studied compounds gave the same chromatographic signal, so GC response factors were set on 1. The detection limits for phytosterol determined using standard solutions were 0.5 mg/100 g of fat. Precision of the method expressed as a RSD was 6.0% for total sterols when 5α-cholestane was used as the internal standard. Samples were analyzed in triplicates.
GC–MS analysis of POPs
A DB5-ms capillary column was used to separate POPs. Conditions for chromatographic analysis of POPs were the same as used for sterols. Samples were, however, introduced using splitless injection. The SIM mode was used for detection and quantification of POPs. For each compound, one target and 3 qualifier ions were monitored (Table 2). As no POPs’ analytical standards were commercially available, laboratory-made standard prepared by thermooxidation (200 °C for 4 h) of sterols was used. The quantification of POPs with a internal standard (19-hydoxycholesterol) method was done indirectly, that is, GC–MS was calibrated using laboratory-made standards and quantified by a previously published GC-FID method using a general RRF of 1.00 [12]. The use of this RRF was confirmed by commercially available structurally analogous cholesterol oxides [12].
The registered mass spectra of POPs were compared with the previous published data [21]. To evaluate the day-to-day repeatability of the method, control sample was analyzed in each sample batch. Due to the lack of POPs standards, the same limits of detection were adopted as determined for cholesterol oxides. Detection limits (S/N > 10) were different for each POP, for example, 11 and 5 ppb for 5α,6α-epoxyphytosterol and 7-ketophytosterol, respectively. Quantification limits were calculated as DL × 5 and were 55 and 25 ppb for 5α,6α-epoxyphytosterol and 7-ketophytosterol, respectively. Precision of the method determined for COPs was in 5–11% range. Samples were analyzed in triplicates.
Results and discussion
POPs content in thermally processed French fries
The data on fat, sterols and POPs contents in French fries are given in Table 3. Similar fat concentrations (4.2–4.8%) were found in the studied frozen French fries samples. The fat content was higher in sample no. 1 and 3 and lower in no. 2 than the concentration declared by manufacturer. Total determined sterol content was in 467.6–533.5 mg/100 g of fat range. After thermal processing (at 225 °C for 15 min in oven) without vegetable oil addition, fat and sterol content decreased about 14–33 and 8–22%, respectively. POPs were not detected in nonheated samples, while in thermally processed French fries, the following POPs were determined: 5α,6α-epoxycampesterol, 5β,6β-epoxycampesterol, 7-ketocampesterol, 5α,6α-epoxysitosterol and 7-ketositosterol. Total determined POPs concentration was in 6.0–68.8 μg/g of fat range. It represented 1.69, 0.5 and 0.2% of phytosterol content in product no. 1, 2 and 3, respectively. 7-Ketositosterol and 7-ketocampesterol have the highest contribution in the POP fraction.
Those results indicate that oven heating causes dynamic oxidation of phytosterols. In products no. 2 and 3, one of the epoxycampesterol, epimers, was detected. That phenomenon is most probably caused by the limited oxidation of campesterol due to the presence of stabilizing additives in the product.
Thermal processing especially frying not heating in the oven of French fries in terms of oxysterol formation was of interest to researchers due to the popularity of the product. Dutta showed that the degree of fat unsaturation, which was used as a medium during frying, has significant impact on the content of POP generated during the process [10, 22]. The higher share of unsaturated fatty acids in fat, the higher total concentration of POPs in the product. Moreover, if French fries were fried with a mixture of vegetable and animal fat, the presence of COPs in the range of 20–24 mg/g of product had been observed [23].
It is known that it is reprehensible repeated frying using the same fat during frying of food. It contributes to formation of harmful substances, for example, peroxides, and also to the high content of POPs [24]. In addition, nonenzymatic browning reactions (e.g., Maillard reaction) during the French fries frying are accompanied by the formation of hydroperoxides and free radicals, which catalyze the oxidation of sterols [25]. Products of the Maillard reactions show, however, an antioxidant potency through scavenging oxygen radicals and are also able to chelate metal ions [26].
POPs in thermally processed noodles
The determined fat, sterols and POPs contents in noodle samples are summarized in Table 4. Two types of the products including wheat noodles (no. 1 and 3) and egg noodles (noodle no. 2) were investigated. Noodles were cooked in boiling water for 5–8 min according to manufacturer’s instructions. Egg noodles contained 3.2% of fat while wheat noodles only 0.3% in product no. 1 and 1.5% in product no. 3. According to the products labels, fat contents were similar in product no. 1 and 3, while no data on the fat contents were given for the product no. 2. The determined drop in the fat contents after processing was about 40%. The total determined sterol contents in the studied samples were in 2,135.7 mg/100 g of fat and 611.2 mg/100 g of fat range for egg noodles and wheat noodles, respectively. Thermal processing caused significant sterol content decreased ranging from 28 to 55% of the initial value, depending on the product type. The highest decrease was observed in egg noodle samples.
The loss of sterol was related to the loss of product fat migrating from the product to the boiling water. In the cooked pasta, 7-ketocampesterol and 7-ketositosterol were found at concentrations ranging from 1.8 to 4.0 μg/g fat, what contributed to 0.06, 0.27 and 0.04% of the total phytosterol contents in products no. 1, 2 and 3, respectively. POPs were observed only in thermally processed noodles, what proofs that they are formed during thermal processing. Any COPs were detected in pasta products. In the bibliography, none of the POPs content data in pasta products were found; therefore, the results were discussed comparing with COPs content. Boselli and coworkers found COPs in Italian egg noodles [27].
In the latter work, the reported total content of cholesterol oxides in commercial and homemade pasta was from 0.6 to 6.5 μg/g and from 1.7 to 3.6 μg/g of raw product, respectively. The percentage of oxidized cholesterol was between 0.10 and 0.35 [27]. Verardo et al. [28] investigated the influence of storage conditions on cholesterol oxidation in dried egg pasta. The total content of COPs was between 43.8 and 52.0 μg/g of fat. Unfortunately, information about percentage of cholesterol oxidation was not given. Observed discrepancies are related to the different heat medium used for processing. The result of other studies evidences also the formation of POPs in an aqueous phase especially when pro-oxidants, for example, transition metals are present. Transition metal ions are able to accelerate lipid oxidation by promoting the decomposition of lipid hydroperoxides with formation of free radicals that could cause further oxidation of lipids [29]. However, more investigations should be performed to confirm this hypothesis.
POPs content in thermally processed animal products
The fat, cholesterol and POPs contents determined in studied animal products (minced meats, fish fillets and readymade fish products) are given in Tables 5, 6 and 7. All products were panfried with rapeseed oil addition at the temperature >200 °C for up to 10 min, except for the salmon samples with spinach in puff pastry, that was heated in oven at 220 °C for 20 min.
Raw pork and beef contained 21.7 and 23.0% of fat, respectively (Table 5). Due to rapeseed oil absorption, panfried pork and beef minced meats contained higher amount of fat (between 25 and 40%). Total sterol concentration in meats after thermal processing was significantly lower. About 30% of the sterols were lost mainly due to its thermal degradation and oxidation.
In processed samples, 7-ketositosterol and 7-ketocampesterol were determined. In the minced meat samples after thermal processing, POPs contributed to 0.04 and 0.36% of the phytosterol content in pork and beef, respectively (Table 5). Cholesterol oxidation products were also found in the processed products, but the detailed data were reported previously [18].
In fish fillets, fat content ranged from 0.6 to 1.7% (Table 6). Increase in the fat concentration after panfrying of fish fillets was also observed (by about 41 and 67%). Also in fish fillets, decrease in total sterol concentration after panfrying was observed. The drop in the pollock and sole fillets sterol concentration was in 21–30% range comparing to the values determined before processing. The high content of total phytosterol oxides was found in pollock fillets—38.7 μg/g of fat and sole fillets—32.3 μg/g of fat (Table 5). POPs in pollock and sole fillets after thermal processing contributed to 0.57 and 0.74% of the total phytosterol content, respectively (Table 6). The higher content of POPs in fish fillets comparing to minced meats after thermal processing can be associated with unsaturation level of the fish fat, which contained 0.6 in pollock and 1.0 g in sole fillets of unsaturated fatty acids in 100 g of the product.
Readymade fish products contained from 5.7 to 7.2% of fat (Table 7). After thermal processing, the fat content in panfried fillet fish fingers and fishburgers has increased by about 50%, and in salmon with spinach in puff pastry, the fat content has decreased by about 5%. It was most probably caused by the specific heat treatment type (oven at 220 °C for 20 min). Total sterol concentration in readymade fish products after thermal processing was lower by about 40–50% in fillet fish fingers and fishburgers and by about 65% in salmon with spinach. The readymade fish products contained 2.5, 3.5 and 13.6 μg of POPs in 1 g of fat in salmon, fillet fish fingers and fishburgers, respectively. Total content of POPs contributed to 0.04, 0.34 and 0.36% of the total phytosterol content in thermally processed readymade products.
Results of this study indicate that the POPs contributed to less than 1% of the total determined phytosterol contents (0.04% in minced pork meat to 0.74% in sole fillets range). The reported data are in concordance with reports of Soupas et al. [30] and Rudzińska et al. [7]. Soupas has studied the influence of the panfrying process on the oxidation of rapeseed oil and rapeseed oil enriched with commercial β-sitosterol at a level of 8%. Samples were panfried at 180 and 200 °C.
For instance in pure rapeseed oil fried at 180 and 200 °C for 5 min, respectively, 0.7 and 0.6% of sitosterol have been oxidized. In rapeseed oil enriched with free, esterified phytosterols or with phytostanyl esters at a level equivalent to 8% sterol/stanol after 10 min of thermal processing at 180 °C, sitosterol oxidation reached 1.8, 1.4 and 0.1%, respectively [30]. Rudzinska et al. [7] have investigated the oxidation process of pure sitosterol mixtures at 180 °C and reported that after 1 h of thermal treatment, the 6% of the phytosterols were transformed into oxysterols. Formation of phytosterol oxides was hastened by the addition of rapeseed oil due to high content of phytosterols, heat treatment (between 180 and 225 °C) and the presence of air (especially during thermal processing in oven), free radicals, peroxides or dyes like heme (only in meat).
Conclusions
Heat treatment (e.g., panfrying, heating in oven) is very popular method of meal preparation. Very commonly, vegetable oil has been used as a heat transfer agent. Heat treatment causes, however, the phytosterol oxidation process, and phytosterol oxides are formed.
In this study, several types of food products were thermally processed, for example, French fries were heated in oven; minced meats, fish fillets and readymade fish products were panfried; and noodles were cooked in boiling water. Thermally processed products contained 5α,6α-epoxycampesterol and 5β,6β-epoxycampesterol, 5α,6α-epoxysitosterol, 7-ketositosterol and 7-ketocampesterol. The observed phytosterol oxidation rate in thermally processed product was higher in French fries and fish fillets (0.20–1.66% of total phytosterol content before thermal processing) than in noodles, minced meats and readymade fish products (0.04–0.36% of the total phytosterol content). Formation of phytosterol oxides was induced by the addition of rapeseed oil due to the high content of phytosterols, heat treatment (between 180 and 225 °C) and the presence of air, free radicals, peroxides or dyes. These factors lead to formation of various phytosterol oxides, but their contribution was only in low 0.04–1.69% range of the total determined phytosterol content. Taking into consideration that phytosterol oxides can be incorporated by the human, and its possible adverse effect on human health, the content of the POPs should be monitored. However, further studies shall not only evaluate the POP levels in products of plant origin but also in phytosterol-enriched foodstuffs especially when thermal treatment is incorporated in the production or the meal preparation process.
Abbreviations
- POPs:
-
Phytosterol oxidation products
- COPs:
-
Cholesterol oxidation products
- GC:
-
Capillary gas chromatography
- FID:
-
Flame ionization detector
- MS:
-
Mass spectrometry
- BSTFA:
-
N,O-Bis(trimethylsilyl)trifluoroacetamide
- TCMS:
-
Trimethylchlorosilane
- TIC:
-
Total ion monitoring
- SIM:
-
Selected ion monitoring
- m/z:
-
Mass-to-charge ratio
- RRF:
-
Relative response factor
References
Chiou A, Kalogeropoulos N, Salta FN, Efstathious P, Andrikopoulos NK (2006) LWT 42:1060–1067
Kmiecik D, Korczak J, Rudzińska M, Gramza-Michałowska A, Hęś M (2006) Eur J Lipid Sci Technol 111:1124–1132
Rozner R, Garti N (2006) Colloids Surf A 282–283:435–456
Salta FN, Kalogeropoulos N, Karavanou N, Andrikopoulos NK (2008) Eur J Lpid Sci Technol 227:361–400
García-Llatas G, Rodríguez-Estrada MT (2011) Chem Phys Lipids 164:607–624
Otaegui-Arrazola A, Menendez-Carreno M, Ansorena D, Astiasarán I (2010) Food Chem Toxicol 48:3286–3303
Soupas L, Juntunen L, Lampi A-M, Piironen V (2004) J Agric Food Chem 52:266–273
Rudzińska M, Przybylski R, Wąsowicz E (2006) J Am Oil Chem Soc 86:651–662
Bortolomeazzi R, Cordaro F, Pizzale L, Conte LS (2003) J Agric Food Chem 51:2394–2401
Dutta PC (1997) JAOCS 74:659–666
Soupas L (2006) Oxidative stability of phytosterols in food models and foods. http://ethesis.helsinki.fi/julkaisut/maa/skemi/vk/soupas/oxidativ.pdf
Lampi A-M, Juntunen L, Toivo J, Piironen V (2002) J Chromatogr B 777:83–92
Adcox C, Boyd L, Oehrl L, Allen J, Fenner G (2001) J Agric Food Chem 46:2060–2065
Ryan E, Chopra J, McCarthy F, Maguire AR, O’Brien NM (2005) Br J Nutr 64:443–451
Conchillo A, Cercaci L, Ansorena D, Rodriquez-Estrada MT, Lercker G, Astiasarán I (2005) J Agric Food Chem 53:7844–7850
Kemmo S, Soupas L, Lampi A-M, Piironen V (2005) Eur J Lipid Sci Technol 107:805–814
Menendez-Carreño M, Ansorena D, Astiasarán I (2008) J Agric Food Chem 56:9997–10002
Janoszka B (2010) Meat Sci 86(4):976–984
Dionisi F, Golay PA, Aeschlimann JM, Fay LB (1998) J Agric Food Chem 46:2227–2233
Derewiaka D, Obiedziński M (2010) Eur J Lipid Sci Technol 10:1130–1137
Dutta PC (2002) Determination of phytosterol oxidation products in foods and biological samples. In: Guardiola F (ed) Cholesterol and phytosterol oxidation products analysis, occurrence, and biological effects. AOCS Publishing, Illinois, pp 335–374
Dutta PC, Appelqvist L-Å (1997) J Am Oil Chem Soc 74:647–657
Savage GP, Dutta PC, Rodriguez-Estrada MT (2002) Asia Pac J Clin Nutr 11:72–78
Rudzińska M, Korczak J, Wąsowicz E (2005) Pol J Food Nutr Sci 14(55):381–387
Angulo AJ, Romera JM, Ramirez M, Gil A (1997) J Agric Food Chem 45:4318–4323
Yilmaz Y, Toledo R (2005) Food Chem 93:273–278
Boselli E, Caboni MF, Frega NG, Lercker G (2004) Eur Food Res Technol 218:410–414
Verardo V, Pasini F, Iafelice G, Messia MC, Marconi E, Caboni MF (2010) J Agric Food Chem 58:3586–3590
Cercaci L, Rodriguez-Estrada MT, Lercker G, Decker EA (2007) Food Chem 102:161–167
Soupas L, Huikko L, Lampi A-M, Piironen V (2007) Food Chem 101:255–264
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Derewiaka, D., Obiedziński, M. Phytosterol oxides content in selected thermally processed products. Eur Food Res Technol 234, 703–712 (2012). https://doi.org/10.1007/s00217-012-1681-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1681-3