Abstract
This study evaluated the extraction yield of the food grade solvents ethanol and ethyl acetate by extracting lycopene, β-carotene, phytoene and phytofluene from tomato peel powder at varying heating intensities, and the influence of the solvent and heating intensity on carotenoids isomerization and degradation during extraction. The heat treatments assayed were 25, 35, 50 and 60 °C which were applied for periods of 5, 10, 20, 30 or 40 min. The carotenoid yield was higher in the extractions with ethanol than with ethyl acetate. In general, the temperature increase caused an increase in the carotenoid concentrations; however in the extractions performed with ethanol at 60 °C, the yield of (all-E)-lycopene and their (Z)-isomers was lower than at 50 °C. This could indicate that a great isomerization is produced in the high temperature extractions with ethanol but the oxidative degradation is the predominant reaction. On the contrary, the obtained results in the extractions with ethyl acetate indicate that the isomerization is the predominant reaction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is mounting evidence to support the belief that there are health benefits derived from lycopene and other carotenoids found in tomatoes. This belief is based on their apparent ability to provide protection against various cancers and many other chronic diseases due to, in most cases, their antioxidative properties. These antioxidant properties have been reviewed by Shi et al. [19].
Tomatoes are rich in lycopene and, in lower concentrations, also contain β-carotene, phytoene, phytofluene, lutein, γ-carotene, ζ-carotene and neurosporene [20]. Obtaining carotenoids from tomatoes can be performed using a variety of methods such as supercritical fluids and extraction with solvents. Extraction methods of carotenoids in vegetables has been recently revised by Quiros and Costas [9].
Supercritical fluid extraction has been proposed to obtain a high purity lycopen extract [7, 14, 22]. Gómez-Prieto et al. [6] reported a supercritical fluid method to obtain a high purity lycopene extracted from tomato peel. However, the use of solvents is less laborious than the supercritical fluids extraction.
Because tomato carotenoids are liposolubles, they are usually extracted with organic solvents such as chloroform, hexane, acetone, petroleum ether, etc. Since the samples can contain large amounts of water, water-miscible organic solvents such as ethanol, acetone, etc. are also used. A mixture of various solvents is usually used in carotenoid extraction. Lin and Chen [11] used five solvent systems and Sachindra and Mahendrakar [16] seven different vegetable oils for comparison of extraction efficiency in tomato juice and shrimp waste, respectively.
Extraction methods for lycopene in general tend to be time consuming procedures and prone to error due to oxidation and losses during extraction [18]. It is well known that light, heat, acids, etc. promote isomerization of (all-E) carotenoids, their usual configuration, to the (Z)- forms [1, 10]. Oxidative degradation, the principal cause of extensive losses of carotenoids, depends on the availability of oxygen and is stimulated by factors such as light [15].
The obtained carotenoid-rich extract is usually used in health foods, food additives, medicines and cosmetics. One of the problems is the elimination of the residual solvents to obtain a safe extract; this can be avoided using food grade solvents such as ethanol and ethyl acetate.
Boiling ethanol has been proposed for lycopene extraction from tomato [3]. Giori [5] proposed the use of water-saturated ethyl acetate and Xiaolin et al. [23] extracted lycopene with ethyl acetate. However, data concerning the influence of heat on the yield and on the isomerization and degradation of carotenoids during their extraction from tomato using ethanol or ethyl acetate have been not reported.
The present study was carried out to investigate the influence of the two food grade solvents (ethanol and ethyl acetate) and the intensity of heating on the extraction efficiency and on the isomerization and degradation of carotenoids from tomato peel powder.
Material and methods
Reagents
Lycopene 90–95% purity as obtained from Sigma Chemical (St. Louis, MO), (all-E)-β-carotene 97% purity and β-apo-8′-carotenal 98% purity and ethanol were purchased from Fluka Chemical (Buchs, Switzerland). All HPLC-grade solvents including methanol and methyl t-butyl ether (MTBE) were obtained from Labscan Ltd. (Dublin, Ireland) and food grade ethyl acetate was purchased from Panreac (Barcelona, Spain). Phytoene and phytofluene were obtained from tomato peel powder by supercritical fluid extraction following the method described by Gomez-Prieto et al. [6].
Sample preparation
Tomatoes bought in a local market, were carefully peeled and the skin was freeze-dried in a Virtis model FM12XL lyophiliser. The freeze-dried tomato skins were ground with a mill (0.05–0.250 mm particle size), flushed with nitrogen and stored in glass bottles protected from light with aluminium foil at −18 °C until extraction.
Extraction conditions
Initially 5 g of freeze-dried peel was moistened with 400 mL of the solvent kept at the desirable temperature (25, 35, 50 or 60 °C) for several seconds. Afterwards, it was stirred using a magnetic stirrer under darkness at the desirable temperature for varied time lengths (5, 10, 20, 30 or 40 min). After extraction, the bottle containing the sample was placed in ice-water for some seconds and was then filtered through Whatman No. 1 filter paper and the filtrate was evaporated to dryness and immediately analysed by HPLC.
The extraction in all the assayed conditions was performed three times and the obtained extracts were analysed by HPLC twice.
HPLC analysis
The RP–HPLC separation was performed on a Beckman System Gold binary delivery system (module 126) equipped with a UV-vis photodiode array detector model 168 (Beckman Instruments, Fullerton, CA). Analytical separation was carried out on a stainless steel (250×6.6 mm i.d.) Develosil UG C30 (5 μm particle size) column (Nomura Chemical, Sojo, Japan) with a guard cartridge (Phenomenex, Macclesfiel, U.K.) packed with ODS C18. Sample injection was performed by means of a valve (Rheodyne, Cotati, CA) with a 20 μL peek loop.
Elution conditions were based on the chromatographic method developed by Sander et al. [17]. Slight modifications in the water content and in the oven temperature were made in order to obtain adequate selectivity and sensitivity with the available HPLC system. The linear mobile phase gradient was methanol (4% H2O)/MTBE from 83:17 to 33:67 over 60 min at a flow rate of 1 mL min−1. The column was thermostated at 22 °C in a Shimazu CO-10AS (Columbia, MD) column oven. The Gold Nouveau software data system (Beckman Instruments) was used to evaluate peak areas.
Identification and quantification of carotenoids
The identification of (all-E)-carotenoids was carried out by comparing the retention times and absorption spectra with reference standards; in addition, were identified based on absorption spectrum characteristics and Q ratios as described in the literature [4, 21]. The (Z)-isomers of lycopene were identified based on absorption spectrum, all of them showed the characteristic spectrum of all-E-lycopene plus two maximum at 345 and 360 nm. Commercial standards and spectral data were used to assign carotenoid peaks.
The concentration of (all-E)-lycopene, (all-E)-β-carotene, phytoene and phytofluene were calculated using response factors relative to β-apo-8′-carotenal. Detection was performed at 472, 450, 347 and 285 nm to lycopene, β-carotene, phytofluene and phytoene, respectively. The (Z)-lycopene isomer's areas were summed and the response factor calculated to (all-E)-lycopene was used to quantified the (Z)-isomers.
The internal standard was dissolved in methanol/MTBE 25:75 (v:v). To prepare each sample, 200 μL of internal standard solution was added to 200 μL of carotenoids extract, the mixture was filtered through a 0.45 μm filter and a volume of 20 μL was injected.
A DU-70 spectrophotometer (Beckman, Instruments) was routinely used to check the concentration of the working standard solutions, these concentrations were calculated using the extinction 1% (1 cm) coefficient E 1% 1 cm [2]. This method was also used to determine the concentration of phytoene and phytofluene of the standard obtained by fluid supercritical extraction.
Determination of limits of detection and quantification
Both the limit of detection (LOD) and limit of quantification (LOQ) were determined following the method described by Lin and Chen [11].
Results and discussion
As can be observed in a chormatogram of an extract obtained from tomato peel powder treated with ethanol at 50 °C for 40 min, the proposed method allow the separation of (all-E-)-lycopene, (all-E-)-β-carotene, (all-E-)-phytofluene, (all-E-)-phytoene and four (Z)-lycopene isomers (Fig. 1).
Chromatogram of a a extract obtained form tomato peel powder treated with ethanol at 50 °C for 40 min measured at 472, 450, 347 and 285 nm. (1)- β-apo-8′-carotenal, (2)- (all-E-)-phytoene, (3)- (all-E-)-phytofluene, (4)- (all-E-)-β-carotene (5)- four peaks of (Z)-lycopene isomers and (6)-(all-E-)-lycopene
Ethanol extraction
The concentration of lipidic extract was slightly affected by the times and the temperatures of extraction assayed; for example, 3.06 g/100 g of peel powder and 3.36 g/100 g of peel powder were obtained in samples treated at 25 °C for 5 min and at 60 °C for 40 min, respectively.
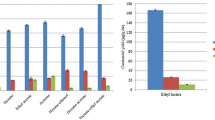
As expected, (all-E)-lycopene concentration increased with the increase in the extraction temperature from 25 to 50 °C (Fig. 2a); however, at 60 °C the obtained concentration was approximately half than at 50 °C at all the assayed times, this may be due to a lower stability of the (all-E)-lycopene at 60 °C than at 50 °C. The different stability could also explain that the extraction time has only a very slight influence on the (all-E)-lycopene yield obtained at 35, 50 and 60 °C. Time extraction influence was detected in those extraction performed at 25 °C increasing the concentration of (all-E)-lycopene with increasing extraction time; this can indicate low or zero degradation at this temperature.
Lycopene concentration obtained during the extraction of tomato peel powder with ethanol at 25 °C (square), 35 °C (circle), 50 °C (triangle) and 60 °C (rhombus) for different heating times. (a) Concentration of (all-E)-lycopene (mg/100 g of peel powder). (b) Concentration of (Z)- lycopene (mg/100 g of peel powder)
The low stability found in some assayed conditions indicates that the (all-E)-lycopene is undergoing isomerization and/or degradation during extraction. The concentration of total (Z)-lycopene isomers is shown in Fig. 2b; at 50 and 60 °C the concentration increased with the temperature and the extraction times. As can be seen in most cases all the obtained concentrations were lower at 60 °C. This can indicate that, although at that temperature the isomerization of the (all-E)-lycopene is higher than those found at other temperatures, the lycopene isomers are undergoing a higher auto-oxidation. Only slight changes were detected at 35 °C due to temperature and time and no detectable changes were found at 25 °C.
The highest β-carotene concentration was obtained at 25 °C, similar yields were obtained at 35 and 50 °C and the lowest values were found at 60 °C (Table 1). In general, the assayed times did not have a great influence on the β-carotene yield. The (Z)-isomers were not detected in quantifiable concentrations. The obtained results indicate that the increase in the extraction temperature favoured β-carotene auto-oxidation.
Phytofluene and phytoene were obtained under all the assayed conditions. In all the conditions phytoene concentration was higher than that for phytofluene. The influence of the temperature and time on the yield of these carotenoids was similar to that described for the β-carotene.
Ethyl acetate extraction
The concentration of lypidic extract obtained was influenced by the times and temperatures assayed thus increasing the concentration with the increase of the two parameters. At 25 or 35 °C the minimum and maximum concentrations were 1.0 and 1.52 g of lypidic extract/100 g of peel powder, respectively; whereas at 50 and 60 °C the obtained values were 1.64 and 2.91, respectively.
(All-E)-lycopene concentration was influenced by the extraction temperature, increasing the concentration with the increase in temperature (Fig. 3a). In those cases in which extraction time was maintained for 10 min the concentrations only decreased at 50 and 60 °C. (Z)-lycopene isomers concentration was higher at 60 and 50 °C than at 35 and 25 °C (Fig. 3b). The concentrations at the first two mentioned temperatures were similar; the (Z)-isomers were slightly higher at 35 than at 25 °C after 20 or higher extraction times. No noticeable variations were detected at any temperature in relation to the extraction time.
β-carotene was quantified in samples heated at 60 °C, no time influence was detected (Table 2); at lower temperatures only traces of this carotene was detected.
Traces of phytofluene were detected. Temperature and time influence on phytoene yield was detected in some of the assayed conditions (Table 2).
The obtained concentration of carotenoids in all the assayed conditions was noticeably lower than those found in our laboratory using other mechanical extraction methods such ultrasonication; however, magnetic stirring was finally chosen because this method allows to keep the sample at the desirable temperature for long periods of time.
The lower concentration of (all-E) and (Z)-lycopene found in the extracts obtained with ethanol at 60 °C rather than at 50 °C could indicate that at 60 °C there is both a higher isomerization than at 50 °C and also a higher oxidative degradation of both the formed (Z)-isomers and the (all-E)-lycopene. At the same temperatures the behaviour was different in samples extracted with ethyl acetate, where the obtained (all-E) and (Z)-lycopene was higher at higher temperatures which may indicate that the oxidative degradation in this solvent is not as influenced by the temperature as when ethanol is used.
The concentration of lypidic extract and the yield of each carotene were noticeably higher in the extractions performed with ethanol than with ethyl acetate. Taking into account that carotenoids are enclosed within cells, that the cell walls are of a complex composition, and that it is also known that ethanol can break cell walls, the best yield obtained with ethanol may be due to better accessibility of this solvent to the carotenoids. On the other hand, the higher polarity of ethanol than ethyl acetate can also influence the higher concentration obtained with ethanol.
Nevertheless, microscopic observation of peel after the treatments with either ethanol or ethyl acetates showed that most of the tomato-peel cell walls were not affected which could indicate that the treatments assayed, although performed under heat and using solvents that can break cell walls are not enough to breakdown the cells and subsequently not all the carotenoids are extracted.
Nguyen et al. [13] reported that heat treatment alters the bioavailability of carotenoids in tomato due to the thermal changes in the physical ultra-structure of the tomato tissue; Hart and Scott [8] found an increase in carotenoid concentration in heated vegetables which could possibly be due to an increase in its availability due to changes in the cell. However, Lee and Chen [10] and Mayer-Miebach et al. [12] found a decrease of lycopene concentration with increasing heating intensity.
We think that it could be of interest to perform studies regarding mechanical or enzymatic methods that allow the breakdown of cell walls while analysing the possible influence of this treatments on the yield of the carotenoids and on their possible isomerization and degradation. The use of high-heat treatments may not be indicated to avoid carotenoids degradation.
In conclusion, it is necessary to find a method that allows the extraction of the maximum yield of tomato carotenoids taking into account that the isomerization and degradation during extraction can be affected by either the solvent or both the solvent and the intensity of heat treatment.
References
Ax K, Mayer-Miebach E, Link B, Schuchmann H, Schubert H (2003) Eng Life Sci 3:199–201
Britton G (1995) Carotenoids. Spectroscopy, vol 1B. Birkhäuser Verlag, Berlin
Bortlik K, Mortezavi L, Saucy F (2001) Patent WO0138443
Chen BH, Peng HY, Chen HE (1995) J Agric Food Chem 43:1912–1918
Giori A (2005) Patent WO030079816
Gómez-Prieto MS, Caja MM, Santa-María G (2002) J Am Oil Chem Soc 79:897–902
Gómez-Prieto MS, Caja M, Herraiz M, Santa-María G (2003) J Agric Food Chem 51:3–7
Hart D, Scott KJ (1995) Food Chem 54:101–111
Quiros de ARB, Costas HS (2006) J Food Comp Anal 19:97–111
Lee M., Chen BH (2002) Food Chem 78:425–432
Lin CH, Chen BH (2003) J Chrom A 1012:103–109
Mayer-Miebach E, Behsnilian D, Regier M, Schuchmann HP (2005) Food Res Int 38:1103–1108
Nguyen M, Francis D, Schwartz S (2001) J Sci Food Agric 81:910–917
Pol J, Hyotylainen T, Ranta-Aho O, Riekkola ML (2004) J Chrom A 1502:25–31
Rodriguez-Amaya DB (2001) A guide of carotenoids analysis in foods. IlSI Press, Washington
Sachindra NM, Mahendrakar NS (2005) Bioresource Technol 96:1195–1200
Sander LC, Sharpless KE, Craft NE, Wise SA (1994) Anal Chem 66:1667–1674
Shi J, Le Maguer M (2000) Crit Rev Food Sci Nutr 40:1–42
Shi J, Qu Q, Kakùda Y, Yeung D, Jiang Y (2004) Crit Rev. Food Sci Nutr 44:559–573
Tonucci LH, Holden JM, Beecher GR, Khachik CS, Davis CS, Mulokozi G (1995) J Agric Food Chem 43:579–586
Tsukida K, Saiki T, Koyama Y (1982) J Chromatogr 245:359–364
Way CY, Chen BH (2006) Eurp Food Res Technol 222:347–353
Xiaolin D, Jianjun J, Pu X (2002) Patent. CN1358801
Acknowledgements
The authors acknowledge the technical support of María José Rodríguez. The research was supported by the MEC of Spain (project AGL2002-03615).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calvo, M.M., Dado, D. & Santa-María, G. Influence of extraction with ethanol or ethyl acetate on the yield of lycopene, β-carotene, phytoene and phytofluene from tomato peel powder. Eur Food Res Technol 224, 567–571 (2007). https://doi.org/10.1007/s00217-006-0335-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0335-8