Abstract
In the present work, optimization of pectinase-assisted and tri-solvent-mediated extraction of lycopene from waste tomato peels was carried out. The optimized parameters for enzymatic pre-treatment were 2% pectinase concentration, pH 5.5, 4-h incubation, 45 °C and 150 rpm. Maximum recovery of lycopene from tomato peels using optimized tri-solvent extraction was achieved at 45 °C, 120-min incubation and 200 rpm. The extracted lycopene was confirmed through functional and characteristic peaks in UV–Vis and FTIR spectra and with retention time in HPLC. The radical scavenging activity was 72.30 ± 2.70 and 43.40 ± 2.01 µg ascorbic acid equivalents (AAE)/ml for 1,1-diphenyl-2-picrylhydrzyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radicals, respectively. The optimized method resulted in 7.38, 4.65 and 1.59 times enhancement in lycopene extraction and recovery in correlation with single solvent, enzyme-treated and tri-solvent extraction methods, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lycopene, a red pigment produced and stored in the chromoplasts of some plant cells, is one of the naturally observed carotenoids and is highly soluble in fats and oils (Ciriminna et al. 2016). It is an acyclic tetraterpenic hydrocarbon with 13 carbon–carbon double bonds. 11 of these 13 are conjugated linearly in arrangement, giving the deep red color of ripe tomatoes (Papaioannou and Karabelas 2012). Utilization of lycopene in various sectors has seen tremendous escalation, there by receiving considerable attention for research insights. Widespread applications include food, cosmetic, pharmaceutical, and other industries as an antioxidant, natural dye, anti-aging and anti-cancer agent (Lenucci et al. 2015). Pertaining to its strong antioxidant property, lycopene supplementation has been reported in cardiovascular diseases and cancer (prostate); however, the underlying mechanism of action is yet to be completely understood (Palozza et al. 2012). Lycopene exhibits not only single oxygen quenching activity but also free radical scavenging activity, with a quenching constant that is two to ten times than that of β-carotene and α-tocopherol (Shi et al. 1999). Lycopene is ubiquitous in tomato viz. peel, the fibrous fraction of pulp and water-insoluble fraction. A considerable amount of lycopene (72–98%) is principally present in the skin and water-insoluble fraction of tomato peels (Ranveer et al. 2013).
Commercial production of lycopene requires specific cultivation of tomatoes for this sole purpose, thereby increasing the production costs enormously (Zuorro et al. 2011). To meet the increasing lycopene demand, there is a need for newer sources and cost effective advanced extraction techniques. During the post-harvest processing of tomatoes, thousands of tons of waste is generated in the form of deteriorated tomatoes containing seeds and peels viz. skin residues. Moreover, the tomato peels contain approximately 5 times higher lycopene than pulp which act as a vital source of lycopene (Sadler et al. 1990). The extraction of lycopene through conventional food grade organic solvents (e.g., ethanol, hexane, ethyl acetate, etc.) has lower extraction efficiency than enzyme-assisted extraction (Papaioannou and Karabelas 2012). The main hurdle in recovering lycopene from tomato peels arises due to peculiar localization of lycopene into the chromoplasts which prevent its release into the surrounding extraction solvent system (Lavecchia and Zuorro 2008).
To enhance the lycopene recovery, various extraction strategies are required. Supercritical fluid and enzyme-assisted extraction are the most commonly employed approaches for lycopene extraction. Amongst these extraction procedures, enzymatic methods have been widely applied for successful release of a various biological compounds from the intracellular constituents of a variety of plant materials (Lenucci et al. 2015). The enzymatic pre-treatment is typically followed by bi- or tri-solvent extraction using food grade solvents. Tri-solvent extraction is a simple bio-separation process that uses three solvents system to precisely separate out and recover lipophilic biological compounds and pigments from biological sources. Under optimized conditions, three separate phases are formed—lipids and pigments in the first, proteins in the second while sugars and carbohydrates in the third layer. The separation is primarily based on the solubility of molecules in specific solvents (Mulchandani et al. 2015).
Hydrolytic enzymes such as pectinase and cellulase have been successfully used for recovery of carotenoids from chilli pepper (Santamaria et al. 2000), flavonoids from Ginkgo biloba (Chen et al. 2011), lutein from marigold flowers (Barzana et al. 2002), lycopene from tomato tissues (Choudhari and Ananthanarayan 2007; Cuccolini et al. 2013; Papaioannou and Karabelas 2012; Zuorro et al. 2011), and oleoresin from ginger (Zingiber officinale) rhizome (Varakumar et al. 2017). Recently, Lenucci et al. (2015), Cuccolini et al. (2013), Ranveer et al. (2013), and Konwarh et al. (2012) have investigated the combined effect of a multi-enzymatic system (e.g., cellulases, pectinase, proteases, xylanase, etc.) to augment the extraction of tomato lycopene. Considering the economic feasibility of industrial processes, pectinase alone has been found to be more effective for lycopene release from tomatoes, peels of tomato and fruit pulp waste than cellulase (Choudhari and Ananthanarayan 2007; Lavecchia and Zuorro 2008; Ranveer et al. 2013).
The present study involves optimization of process parameters for single enzyme-(i.e., pectinase alone) assisted hydrolysis (enzyme concentration, pH, temperature, incubation time and agitation speed) followed by optimization of parameters for tri-solvent-mediated extraction conditions (temperature, extraction time and agitation speed) for release and recovery of lycopene from tomato peels. Confirmatory analysis included spectroscopic (UV–Vis and FTIR) and HPLC followed by antioxidant activity (DPPH and ABTS assay) determination of confirmed extract.
Materials and methods
Materials
Fully ripe but undeteriorated tomatoes of ‘Abhinav’ variety mainly considered as market waste were obtained from a local super market in Jalgaon, Maharashtra (India). Pectinase enzyme from Aspergillus niger with the claimed activity of 8000 U/g was procured from Hi-Media Laboratories Pvt. Ltd. Mumbai (India). Ethanol, acetone and hexane with 99.5, 99.0 and 99.0% purities, respectively, were purchased from S. D. Fine Chemicals, Mumbai, India. Butylated hydroxy toluene (BHT), DPPH and ABTS used were obtained from Sigma-Aldrich, Mumbai, India. All other chemicals used were of analytical grade with highest purity.
Preparation of tomato peel powder
The waste tomatoes obtained were subjected to hot water (70–75 °C) wash for two min followed by cold water treatment (8–10 °C) for three min in order to facilitate peeling. The removed peels were stored and refrigerated at −4 °C for 24 h for better retention of lycopene. The peels were dried in a hot air oven at 45 ± 2 °C for 24 h. The dried peels were ground, sieved, packed in an air tight container and then stored in air tight high density polythene bags under refrigerated conditions for further use (Ranveer et al. 2013).
Determination of lycopene content
Lycopene content in tomato peel powder (before and after enzyme treatment) was determined spectrophotometrically (Ranveer et al. 2013) with some modifications. The sample was extracted in acetone (containing 0.05% w/v BHT), ethanol and hexane in 1:1:2 (v:v:v) ratio followed by continuous shaking at 100 rpm. Deionized water was added to this mixture with continuous shaking and mixture was allowed to return to room temperature and achieve phase separation. The upper solvent phase was collected, diluted with hexane in 1:50 ratio (v:v) and the absorbance was measured at 503 nm using UV–Vis Double beam spectrophotometer (2205 Systronics India, Ltd.). The lycopene content was calculated as μg/g and then converted to mg/100 g.
Pectinase-assisted release of lycopene
Effect of pectinase concentration
Enzymatic pre-treatment of prepared peel powder was carried out by procedure suggested by Ranveer et al. (2013) with slight modifications. The prepared tomato peel powder (1 g) was treated in 10 ml of 100 mM citrate buffer (pH 5.0) containing varying concentrations of pectinase (1, 1.5, 2, 2.5 and 3% w/v). The reaction mixtures were incubated for 90 min at 100 rpm and 25 ± 2 °C on an orbital shaking incubator and lycopene content was determined.
Determination of optimum pH
The optimum pH for pectinase activity to release the maximum lycopene was measured by mixing tomato peel powder using optimized amount of pectinase in 100 mM buffer (pH 4.5, 5, 5.5, 6, 6.5 and 7) and incubating at 25 ± 2 °C and 100 rpm for 90 min.
Impact of incubation temperature and time
The effect of temperature on pectinase treatment was assessed by incubating the tomato peel powder in 100 mM citrate buffer with optimized pectinase concentrations and pH at different temperature (30–70 °C) for 1 h. The impact of incubation time was further estimated by reacting tomato peel powder with optimized enzyme concentration, pH and temperature for varying time (1, 2, 3, 4 and 5 h).
Optimization of agitation speed
The agitation speed for enzyme mediated release of lycopene from tomato tissue was optimized by using a rotary shaker. Tomato peel powder was treated with pectinase enzyme (2%) under optimized pH, temperature and time at different agitation speeds (50–250 rpm).
Tri-solvent extraction based recovery of lycopene
Lycopene was recovered after enzymatic treatment by using tri-solvent extraction system (acetone, ethanol and hexane) based on the method explained by Fish et al. (2002) with slight modifications. Under optimized conditions, pectinase-treated tomato peel powder was mixed with tri-solvent extraction system consisting acetone (12 ml containing 0.05% BHT), ethanol (12 ml) and hexane (24 ml) along with deionized water (12 ml). The reaction mixture was incubated at 100 rpm and 25 ± 2 °C for 90 min. The lycopene content was determined by diluting the upper layer in hexane (1:50) as mentioned previously.
Optimization of tri-solvent extraction parameters
The optimum temperature for lycopene recovery using tri-solvent extraction was determined by incubating reaction mixture (pectinase-treated tomato peel powder in tri-solvent system) at different temperature (25–50 °C) and 100 rpm for 90 min. The extraction time was further optimized by incubating reaction mixture at optimized temperature and 100 rpm for various contact time (0.5–3 h). The optimum agitation speed was determined by rotating the reaction mixture at different agitation speeds (50–250 rpm) at optimized temperature and time.
Spectroscopic analysis of lycopene
Lycopene was analyzed spectroscopically by measuring λ max on a UV–Vis spectrometer and functional characteristic peaks were determined by an infrared spectrophotometer (FT-IR, Model Miracle 10, Shimadzu) in the range 4000–400 cm−1.
HPLC analysis of lycopene
Lycopene was confirmed by determining its retention time using HPLC method (Olives Barba et al. 2006). The sample was prepared and injected in a C-18 column with methanol/acetonitrile (90:10 v/v) + 9 µM tri-ethyl amine as a mobile phase and was analyzed at 475 nm using UV–Vis detector on HPLC (model 1260 Infinity, Agilent Technologies).
Determination of lycopene antioxidant activity
The antioxidant activity of lycopene was estimated using DPPH and ABTS assay (Sonawane and Arya 2014). The antioxidant capacity to scavenge free radicals of DPPH and ABTS was expressed as ascorbic acid equivalents, i.e., µg AAE/ml.
Statistical analysis
The data were analyzed by Microsoft Excel (2010) and SPSS (Version-16). The mean differences for all treatments were tested with one-way ANOVA and statistical significance differences between the mean values were established at p ≤ 0.05 and Duncan’s new multiple range test. The results were expressed as mean ± SD.
Results and discussion
Lycopene content of market waste tomato (Abhinav variety) peel powder
The total average lycopene content of market waste tomato peel powder was 95.4 ± 2.3 mg/100 g on dry weight basis and the results were in correlation with Konwarh et al. (2012) and Lopez-Cervantes et al. (2014). Cuccolini et al. (2013) stated that lycopene content is predominantly higher in the chromoplasts region of the tomato peel tissues which is due to transformation of chloroplast into chromoplast during ripening. The results indicate that market waste of Abhinav variety tomatoes are good source of lycopene and therefore was used for the said work.
Optimization of pectinase-assisted extraction
Pectinase concentration
The preliminary step in devising an eco-friendly technique for lycopene extraction from tomato peel waste is the opening of tomato peel cells, which are extremely resistant (Cuccolini et al. 2013). Pre-treatment of tomato peels with pectinase breaks down pectin, i.e., loosens the cellular structures and helps to release lycopene into the surrounding extraction solvent system (Papaioannou and Karabelas 2012). From an economic point of view it is necessary to determine an optimum enzyme concentration required for extraction of lycopene from tomato peels. The optimum concentration of enzyme was quantified by incubating pectinase at different concentrations ranging from 1 to 3%. Results indicated that the lycopene content gradually increased with increasing pectinase concentration and eventually reduced with further increase in pectinase concentration (Fig. 1). Significantly higher lycopene release (40.7 ± 1.3 mg/100 g) was observed at 2% pectinase concentration.
Tomato peels consist of pectin network which is mainly present in the middle lamella of primary cell walls. The pectin network is primarily responsible for lower recovery of lycopene from tomato peels and hence enzymes at optimized concentrations are utilized for better recovery (Choudhari and Ananthanarayan 2007). At lower enzyme concentration, pectinase is unable to penetrate into the middle lamella to hydrolyze the pectin network and hence may be unable to release lycopene at greater extent. Higher enzyme concentration resulted in rapid hydrolysis leading to more release of lycopene in the reaction mixture which lead to increased oxidation of the end product as the reaction was carried for same duration of time. Similar findings were observed by Cinar (2005) and Ranveer et al. (2013).
pH, time, and temperature
Optimum reaction conditions for enzyme hydrolysis viz. pH, incubation time and temperature are required to be determined to achieve good product yield. Significantly higher pectinase activity with superior release of lycopene was observed at pH 5.5 (47.8 ± 1.0 mg/100 g), 4 h incubation (90.3 ± 5.0 mg/100 g) and 45 °C (71 ± 2.6 mg/100 g), respectively. It was found that the release of lycopene was lower at either sides of optimum pH (Fig. 2a) which might be due to better stability of pectinase at moderately acidic environment than towards highly acidic and neutral conditions.
To extract maximum lycopene from the tomato peels powder, there should be a good exposure time between powder and pectinase added. The release of lycopene from tomato peels increased with incubation period till 4 h and then reduced significantly (Fig. 2b). Pectinase hydrolyses the cell wall pectin very rapidly during first four hours of incubation leading to maximum lycopene recovery as these molecules can further be easily extracted in the solvent mixture (Ranveer et al. 2013). Prolonged incubation time causes rapid oxidation of released lycopene from protective cell chromoplast due to oxidative degradation in the external environment (Choudhari and Ananthanarayan 2007; Xianquan et al. 2005).
It was depicted that the release of lycopene from tomato peel powder increased gradually with increasing temperature till 45 °C and steadily decreased with further increase in temperature (Fig. 2c). The reduction in lycopene release at higher temperatures might be due to enzymatic denaturation caused by breakdown of bonds in its active site leading to enzyme denaturation (Ladole et al. 2014; Sahoo et al. 2016). Another potential reason might be increased oxidation of released lycopene from the protective cell chromoplast at higher temperature (Ciriminna et al. 2016).
Agitation speed
Maximum recovery or release of lycopene from tomato peel powder requires a good contact with enzyme which is provided by adequate mixing. Agitation not only ensures sufficient contact but also promotes heat and mass transfer within the reaction vessel. In order to achieve higher lycopene release, the reaction mixture was agitated at varying speed (50–250 rpm) under optimum conditions (pH 5.5, 45 °C and 4 h). It was found that lycopene release increased steadily with increment in agitation speed up to 150 rpm and then slightly reduced on further increase (Fig. 2d). It was observed that considerably higher lycopene (89.5 ± 3.2 mg/100 g) was released at 150 rpm. At lower agitation speed, there might be less interaction of enzyme with substrate molecules which hampers lycopene release. However, high speed agitation the enzymes might get physically damaged reducing the conversion yield (Nadar et al. 2016).
Optimization of tri-solvent extraction-mediated recovery of lycopene
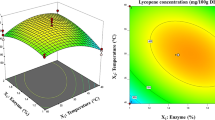
To extract and recover maximum lycopene, the pectinase-treated tomato peels were subjected to tri-solvent extraction using the mixture of three solvents namely acetone, ethanol and hexane in 1:1:2 proportions. Tri-solvent extraction is an easy bio-separation method that is successfully used to separate lipophilic biomolecules and pigments from biological sources. Mulchandani et al. (2015) and Varakumar et al. (2017) stated that under standardized parameters, three phases are developed, concentrating the lipids and pigments in the upper organic phase while precipitated proteins in the intermediate layer and sugars. The recovery of lycopene from enzyme-treated tomato peels using tri-solvent extraction was optimized for extraction temperature, time and agitation speed using one factor at a time. Significantly superior recovery of lycopene was observed at 45 °C (101.8 ± 3. 9 mg/100 g), 120 min incubation (126.6 ± 3.3 mg/100 g) and 200 rpm (142.2 ± 4.7 mg/100 g) respectively.
In order to recover significant amount of lycopene from the enzyme-treated tomato peels powder, a good contact between powder and extraction solvents should be achieved at a specified temperature and period. It was observed that the extraction and recovery of lycopene increased progressively with increasing temperature till 40 °C and then decreased gradually on further increments in temperature (Fig. 3a). Reduced recovery at lower temperatures might be due to comparatively low penetration and solubility of the solvent (Lavecchia and Zuorro 2008). Additionally, at higher temperatures the penetration and solubility of solvents is considerably higher but the target compounds may get degraded (Izadifar and Baik 2008; Mohamad et al. 2013). The recovery of lycopene from tomato peels was higher with increasing incubation time of extraction till 2 h and then decreased subsequently (Fig. 3b). Long-time exposure of lycopene to external surrounding medium is probably responsible for oxidation of lycopene leading to reduction in its content on prolonged incubation (Xianquan et al. 2005) and formation of cleavage products like apo-lycopenals/ones and apo-carotendials (Caris-Veyrat et al. 2003). It was depicted that with increase in agitation speed the removal of lycopene from tomato peel powder enhanced gradually till 200 rpm and further increase in agitation speed reduced lycopene recovery steadily (Fig. 3c). Agitation not only promotes adequate contact between solid particles and solvents but also maintains uniform heat and mass transfer within the reaction vessel. During extraction, the solute moves from inside of solid to outside surface of tomato peels through diffusion. As soon as lycopene is at the surface, the removal is limited by convective mass transfer. Higher agitation speed leads to improved mass transfer and enhances convective mass transfer thus augmenting the extraction yields (Mohamad et al. 2010). Additionally, higher agitation speed is not preferred for extraction due to physical limitations of the process, such as squish effect, vortex formation and geometry effect (Mohamad et al. 2013).
Spectroscopic studies of extracted lycopene
The λ max of lycopene was determined on UV–Vis spectrophotometer and functional characteristic groups on infrared spectrophotometer to further estimate the structure and purity of the extracted lycopene. The extracted lycopene in hexane showed three strong absorbance peaks at 446, 471 and 508 nm (Fig. 4a). Choudhary et al. (2009) also stated the use of UV–Vis spectrophotometry for rapid detection of lycopene extracted from watermelon and tomato puree. Similar absorbance peaks were observed by Kamil et al. (2011) for lycopene extracted from tomato and their products.
Moreover, the appearance of characteristic sharp peaks at 2957.08, 2924.81 and 2871.31 cm−1 in FTIR indicated the asymmetrical stretching of C-H groups. The presence of noticeable peaks at 1461.73 and 1378.76 cm−1 indicated C-H2 bending, some minor peaks between 1140 and 1210 also reveals the bending of CH2 groups. A typical peak at 1461.73 cm−1 confirms the V symmetry of the CH2 in lycopene molecule, the vibrational peak at 885.61 cm−1 indicate stretching of C–C-H2 and C–OH groups of lycopene. The obtained results of FTIR spectrum (Fig. 4b) were in correlation with the spectral values of standard lycopene and extracted lycopene from tomato products as explained by Kamil et al. (2011). Lopez-Cervantes et al. (2014) and Rubio-Diaz et al. (2011) also noticed similar FTIR spectral readings for lycopene extracted from over ripe tomatoes.
HPLC analysis of lycopene
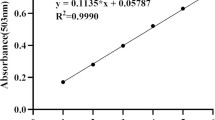
HPLC is one of the suitable analytical methods for the detection due to instability of lycopene to the surrounding environmental conditions leading to precise results. The retention time of the extracted lycopene from tomato peels was 5.2 min (Fig. 5) and the appearance of sharp peak confirmed the presence of lycopene. Similar method and reports were noticed by Cuccolini et al. (2013), Olives Barba et al. (2006) and Lopez-Cervantes et al. (2014) in determination and quantification of lycopene from tomato.
Antioxidant activity of extracted lycopene
The antioxidant capacity indicates the capability to inhibit oxidation process. The extracted lycopene showed 72.31 ± 2.70 and 43.40 ± 2.01 µg AAE/ml antioxidant capacities for DPPH and ABTS radicals, respectively. The decrease in radical scavenging activity of extracted lycopene might be due to oxidation occurred during drying, enzymatic treatment and also tri-solvent based recovery and extraction steps. The findings were in agreement with Kamil et al. (2011), Floegel et al. (2011) and Kim et al. (2014).
Comparative studies of different extraction techniques
The comparison of different extraction techniques for lycopene recovery namely single solvent (n-hexane), tri-solvent (acetone, ethanol and hexane), and enzyme (pectinase) followed by single solvent (n-hexane), and enzyme followed by tri-solvents extraction are shown in Fig. 6. Single solvent system yielded up to 19.26 ± 1.05 mg/100 g of lycopene from tomato peels. Significantly superior lycopene was extracted and recovered by using enzyme followed by tri-solvent extraction technique rather than tri-solvent or enzyme treatment individually. The recovery was 7.38-fold higher in contrast to single solvent system while 4.65 and 1.59 times higher than tri-solvent and enzyme-assisted extraction individually. These results indicated that in order to recover high amount of lycopene both enzyme followed by tri-solvents system is required. Use of single system was unable to recover maximum lycopene. Table 1 indicates the comparative studies of lycopene yield (mg/100 g) obtained from tomato peels by different extraction techniques used by several researchers.
Conclusion
A method for extraction and recovery of lycopene from market waste tomato peels was successfully optimized using a single enzyme, i.e., pectinase followed by the optimization of tri-solvent extraction parameters. The extracted lycopene confirmed by UV–Vis, FTIR, and HPLC possessed prominent antioxidant activity. This optimized process for lycopene extraction and recovery may help to reduce the production cost and facilitate to meet the market demands. Furthermore, the use of single enzyme system at optimum reaction conditions yielded maximum recovery of lycopene which is economically feasible for industrial application as compared to traditional solvent extraction. Further studies are required to increase the lycopene stability during pre-treatment and extraction needs to be carried out to overcome processing-related losses.
References
Barzana E, Rubio D, Santamaria RI, Garcia-Correa O, Garcia F, Sanz VER, Lopez-Munguia A (2002) Enzyme-mediated solvent extraction of carotenoids from marigold flower (Tagetes erecta). J Agric Food Chem 50(16):4491–4496. doi:10.1021/jf025550q
Caris-Veyrat C, Schmid A, Carail M, Bohm V (2003) Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J Agric Food Chem 51(25):7318–7325. doi:10.1021/jf034735
Chen S, Xing XH, Huang JJ, Xu MS (2011) Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzyme Microb Technol 48(1):100–105. doi:10.1016/j.enzmictec.2010.09.017
Choudhari SM, Ananthanarayan L (2007) Enzyme aided extraction of lycopene from tomato tissues. Food Chem 102(1):77–81. doi:10.1016/j.foodchem.2006.04.031
Choudhary R, Bowser TJ, Weckler P, Maness NO, McGlynn W (2009) Rapid estimation of lycopene concentration in watermelon and tomato puree by fiber optic visible reflectance spectroscopy. Postharvest Biol Technol 52(1):103–109. doi:10.1016/j.postharvbio.2008.10.002
Cinar I (2005) Effects of cellulase and pectinase concentrations on the colour yield of enzyme extracted plant carotenoids. Process Biochem 40(2):945–949. doi:10.1016/j.procbio.2004.02.022
Ciriminna R, Fidalgo A, Meneguzzo F, Ilharco LM, Pagliaro M (2016) Lycopene: emerging production methods and applications of a valued carotenoid. ACS Sustain Chem Eng 4:643–650. doi:10.1021/acssuschemeng.5b01516
Cuccolini S, Aldini A, Visai L, Daglia M, Ferrari D (2013) Environmentally friendly lycopene purification from tomato peel waste: enzymatic assisted aqueous extraction. J Agric Food Chem 61(8):1646–1651. doi:10.1021/jf3027815
Fish WW, Perkins-Veazie P, Collins JK (2002) A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J Food Compos Anal 15(3):309–317. doi:10.1006/jfca.2002.1069
Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 24(7):1043–1048. doi:10.1016/j.jfca.2011.01.008
Izadifar M, Baik OD (2008) An optimum ethanol-water solvent system for extraction of podophyllotoxin: experimental study, diffusivity determination and modeling. Sep Purif Technol 63(1):53–60. doi:10.1016/j.seppur.2008.03.041
Kamil MM, Mohamed GF, Shaheen MS (2011) Fourier transformer infrared spectroscopy for quality assurance of tomato products. J Am Sci 27(6):253–260
Kim CH, Park MK, Kim SK, Cho YH (2014) Antioxidant capacity and anti-inflammatory activity of lycopene in watermelon. Int J Food Sci Technol 49(9):2083–2091. doi:10.1111/ijfs.12517
Konwarh R, Pramanik S, Devi KSP, Saikia N, Boruah R, Maiti TK, Deka RC, Karak N (2012) Lycopene coupled ‘trifoliate’ polyaniline nanofibers as multi-functional biomaterial. J Mater Chem 22(30):15062–15070. doi:10.1039/c2jm32530f
Ladole MR, Muley AB, Patil ID, Talib MI, Parate VR (2014) Immobilization of tropizyme-P on amino-functionalized magnetic nanoparticles for fruit juice clarification. J Biochem Technol 5(4):838–845
Lavecchia R, Zuorro A (2008) Improved lycopene extraction from tomato peels using cell-wall degrading enzymes. Eur Food Res Technol 228(1):153–158. doi:10.1007/s00217-008-0897-8
Lenucci MS, De Caroli M, Marrese PP, Iurlaro A, Rescio L, Böhm V, Dalessandro G, Piro G (2015) Enzyme-aided extraction of lycopene from high-pigment tomato cultivars by supercritical carbon dioxide. Food Chem 170:193–202. doi:10.1016/j.foodchem.2014.08.081
Lopez-Cervantes J, Sanchez-Machado DI, Valenzuela-Sanchez KP, Nunez-Gastelum JA, Escarcega-Galaz AA, Rodríguez-Ramirez R (2014) Effect of solvents and methods of stirring in extraction of lycopene, oleoresin and fatty acids from over-ripe tomato. Int J Food Sci Nutr 65(2):187–193. doi:10.3109/09637486.2013.839630
Mohamad M, Ali MW, Ahmad A (2010) Modelling for extraction of major phytochemical components from Eurycoma longifolia. J Appl Sci 10(21):2572–2577
Mohamad M, Ali MW, Ripin A, Ahmad A (2013) Effect of extraction process parameters on the yield of bioactive compounds from the roots of Eurycoma longifolia. J Teknol 60:51–57. doi:10.11113/jt.v60.1441
Mulchandani K, Kar JR, Singhal RS (2015) Extraction of lipids from Chlorella saccharophila using high-pressure homogenization followed by three phase partitioning. Appl Biochem Biotechnol 176(6):1613–1626. doi:10.1007/s12010-015-1665-4
Nadar SS, Muley AB, Ladole MR, Joshi PU (2016) Macromolecular cross-linked enzyme aggregates (M-CLEAs) of α-amylase. Int J Biol Macromol 84:69–78. doi:10.1016/j.ijbiomac.2015.11.082
Olives Barba AI, Camara-Hurtado M, Sanchez-Mata MC, Fernandez Ruiz V, Lopez-Saenz De Tejada M (2006) Application of a UV-vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem 95(2):328–336. doi:10.1016/j.foodchem.2005.02.028
Palozza P, Catalano A, Simone RE, Mele MC, Cittadini A (2012) Effect of lycopene and tomato products on cholesterol metabolism. Ann Nutr Metab 61(2):126–134. doi:10.1159/000342077
Papaioannou EH, Karabelas AJ (2012) Lycopene recovery from tomato peel under mild conditions assisted by enzymatic pre-treatment and non-ionic surfactants. Acta Biochim Pol 59(1):71–74
Ranveer RC, Patil SN, Sahoo AK (2013) Effect of different parameters on enzyme-assisted extraction of lycopene from tomato processing waste. Food Bioprod Process 91(4):370–375. doi:10.1016/j.fbp.2013.01.006
Rubio-Diaz DE, Francis DM, Rodriguez-Saona LE (2011) External calibration models for the measurement of tomato carotenoids by infrared spectroscopy. J Food Compos Anal 24(1):121–126. doi:10.1016/j.jfca.2010.06.006
Sadler G, Davis J, Dezman D (1990) Rapid extraction of lycopene and β-carotene from reconstituted tomato paste and pink grapefruit homogenates. J Food Sci 55(5):1460–1461. doi:10.1111/j.1365-2621.1990.tb03958.x
Sahoo A, Badhe PS, Adivarekar R, Ladole MR, Pandit AB (2016) Synthesis of glycinamides using protease immobilized magnetic nanoparticles. Biotechnol Reports 12:13–25. doi:10.1016/j.btre.2016.07.002
Santamaria RI, Reyes-Duarte MD, Barzana E, Fernando D, Gama FM, Mota M, Lopez-Munguia A (2000) Selective enzyme-mediated extraction of capsaicinoids and carotenoids from chili guajillo puya (Capsicum annuum L.) using ethanol as solvent. J Agric Food Chem 48(7):3063–3067. doi:10.1021/jf991242p
Shi J, Maguer M, Le Kakuda Y, Liptay A, Niekamp F (1999) Lycopene degradation and isomerization in tomato dehydration. Food Res Int 32(1):15–21. doi:10.1016/S0963-9969(99)00059-9
Sonawane SK, Arya SS (2014) Effect of drying and storage on bioactive components of jambhul and wood apple. J Food Sci Technol 52(5):2833–2841. doi:10.1007/s13197-014-1321-y
Varakumar S, Umesh KV, Singhal RS (2017) Enhanced extraction of oleoresin from ginger (Zingiber officinale) rhizome powder using enzyme assisted three phase partitioning. Food Chem 2016:27–36. doi:10.1016/j.foodchem.2016.07.180
Xianquan S, Shi J, Kakuda Y, Yueming J (2005) Stability of lycopene during food processing and storage. J Med Food 8(4):413–422. doi:10.1089/jmf.2005.8.413
Zuorro A, Fidaleo M, Lavecchia R (2011) Enzyme-assisted extraction of lycopene from tomato processing waste. Enzyme Microb Technol 49(6–7):567–573. doi:10.1016/j.enzmictec.2011.04.020
Acknowledgements
The authors would like to thank the Department of Food Technology, University Institute of Chemical Technology, North Maharashtra University, Jalgaon, India for availing all the required facilities and financial support to carry out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest related to this work.
Rights and permissions
About this article
Cite this article
Munde, P.J., Muley, A.B., Ladole, M.R. et al. Optimization of pectinase-assisted and tri-solvent-mediated extraction and recovery of lycopene from waste tomato peels. 3 Biotech 7, 206 (2017). https://doi.org/10.1007/s13205-017-0825-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0825-3