Abstract
Lutein and zeaxanthin, two xanthophylls supposed to delay formation of age-related macular degeneration (AMD), are found in numerous new dietary supplements appearing on the international market. Usually, the lutein concentration ranges from 0.25 to 20 mg/serving size. The lutein contents of 14 products with lutein highlighted on the label were evaluated. Oily formulations were dissolved, and powdery capsule contents were extracted with solvents before high-performance liquid chromatography (HPLC) analysis (diode-array detector, 450 nm) using a C30 column. If lutein diesters from marigold (Tagetes erecta) were present, the extracts were saponified with methanolic KOH. To unequivocally identify carotenoids, HPLC-(atmospheric pressure chemical ionization)mass spectrometry was applied. In this study only all-trans-lutein was quantified, whereas cis isomers (approximately 1–5 area% of total lutein) were not taken into account. The lutein concentration of half of the products investigated was found to be below the amount stated, varying here from 11 to 93%. With the exception of one product, all dietary supplements contained zeaxanthin in amounts typical for the use of marigold oleoresin (6.0±1.4 area% of all-trans-lutein). The high discrepancy found between the amounts labeled and determined in half of the products may be attributed to degradation reactions or to improper storage conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the main cause of irreversible loss of vision in Western countries. Since the macula selectively accumulates lutein and zeaxanthin, it is anticipated that an increased intake of food or dietary supplements containing both xanthophylls may protect against AMD and age-related cataract formation [1–4]. Lutein and zeaxanthin are supposed to act by their ability to absorb blue light damaging the retina and by enhancing the antioxidative status of the environmental tissue. These findings led to an expanding international market for dietary xanthophyll supplements, especially for eye health formulations, which typically supply lutein, zeaxanthin, antioxidants, minerals, and the trace elements zinc, copper, and selenium. Additionally, dietary supplements comprising bioactive nutraceuticals lie within the common trend of the market. However, only a few dietary supplement studies have measured macular pigment density up to now. For example, one study documented a monthly increase of 4–5% in pigment density after giving 10 mg lutein daily for 4 months to eight volunteers [5].
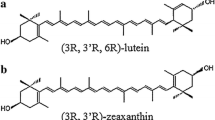
Lutein and zeaxanthin are structurally closely related. The only difference is the position of the double bond in one ionone endring: lutein exhibits one allylic double bond, whereas zeaxanthin features two β-ionone rings with conjugated double bonds (Fig. 1). Lutein is usually produced as a bulk compound from marigold oleoresin (Tagetes erecta), which has been used in traditional folk medicine for a long time [6]. Since lutein is diesterified in marigold with fatty acids, utilization of the native oleoresin causes the appearance of typical peaks in the apolar region of high-performance liquid chromatography (HPLC) chromatograms. If the oleoresin was saponified prior to the formulation, free lutein appears as the predominant xanthophyll. Because zeaxanthin esters typically occur as minor compounds in marigold oleoresin, about 5% zeaxanthin is present in the respective chromatograms [7]. Further natural sources of lutein are spinach (Spinacia oleraceae) and broccoli sprouts (Brassica oleracea italica), which both contain lutein and minor amounts of zeaxanthin in their free forms. In recent years, biotechnological processes have become an attractive alternative to “naturally grown” xanthophylls: for example, the green microalga Chlorella protothecoides was investigated as a potential lutein source [8]. As far as we know, no synthetic lutein is available as a bulk product on the international market. Additionally, oleoresins containing zeaxanthin as sole or even major xanthophyll are not available.
Material and methods
Samples
All products were obtained from local supermarkets, drugstores, and pharmacies in Germany and in the USA. All analyses were performed before expiry. Table 1 lists the time period before expiry at the date of analysis. According to the labeled instructions, most of the samples (G1, G3–G6, U5–U7; Table 1) had to be “stored in a cool and dry place” or had to be “protected from heat (below 25 °C) and sunlight”. In three cases the storage temperature was specified (controlled room temperature between 15 and 30 °C; samples U1, U2, U4). No information about storage conditions was designated for samples G2, G7, and U3. Before analyses, all samples were stored in a cool (20 °C) and dark room. In local shops all products were maintained at room temperature; none were sold refrigerated.
Chemicals
Acetone, diethyl ether, ethanol, ethyl acetate, light petroleum (boiling fraction 40–60 °C), and methanol were purchased from Merck (Darmstadt, Germany; p.a. each), tert-butyl methyl ether (TBME; HPLC grade) and papain (from papaya, 3.0 U/mg) were obtained from Sigma-Aldrich (Taufkirchen, Germany). All solvents were purified by batch column distillation (diameter of glass beads, 5 mm). High-purity water was prepared with a Milli-Q 185 Plus water purification system (Millipore, Eschborn, Germany). Marigold oleoresin was a gift from Euram Food (Stuttgart, Germany).
Extraction of carotenoids
Oil-filled capsules
Two capsules were opened in a beaker beneath the surface of a binary solvent mixture (TBME/methanol, 1:1, v/v, 20 ml) with a scalpel. If a lutein concentration of 20 mg/capsule was labeled, only one capsule was used. To improve solubilization, the beaker was sonicated for 1 min and the suspension was quantitatively transferred to a volumetric flask (100–250 ml, depending on the xanthophyll content). After filling up to the marked level, an aliquot was membrane-filtered using a Chromafil-PET-45/25 filter (Macherey-Nagel, Düren, Germany) with 0.45-μm pore size and directly subjected to HPLC/diode-array detector (DAD) analysis. To prevent the analytes from photoisomerization all procedures were performed in dim light.
Tablets and capsules with powdery content
Two tablets were ground using a mortar and pestle. The resulting powder was quantitatively transferred into an Erlenmeyer flask, suspended in water (50 ml), heated in a water bath (50 °C; 2 min), and sonicated (1 min) to disintegrate carotenoid formulations. Care was taken to completely dissolve microencapsulated carotenoids. To accelerate solubilization, the addition of acetone (2 ml) as a modifier was helpful. The resulting suspension was transferred to a separating funnel and extracted immediately with a ternary solvent mixture (methanol/ethyl acetate/light petroleum, 1:1:1, v/v/v; 50 ml each, four times). The upper layers from multiple extractions were combined, transferred to a round-bottom flask, and evaporated in vacuum at 30 °C using a rotary evaporator. To remove traces of water, ethanol (2 ml) was added and the extract was evaporated to dryness. The residue was dissolved in TBME/methanol (1:1, v/v; 100–250 ml, depending on the concentration anticipated). Prior to HPLC/DAD analysis, an aliquot was membrane-filtered.
Saponification
Saponification of xanthophyll esters was performed as described by Weller and Breithaupt [9]. In brief, an aliquot (5 ml) of the final extract was evaporated to dryness and the residue was redissolved in diethyl ether (50 ml). After addition of methanolic KOH (30%, w/v; 5 ml), the flask was stored overnight. The reaction mixture was transferred into a separating funnel and washed with water (3×100 ml). The organic layer was evaporated to dryness using a rotary evaporator (water bath temperature 30 °C) and treated as described previously (final volume 5 ml).
Recovery studies
For recovery experiments, a zeaxanthin solution (c=250 mg/l in TBME/methanol, 1:1, v/v), obtained from a saponified wolfberry (Lycium barbarum) extract, was used [9]. One tablet (sample G4; see Table 1) was ground and suspended in water (50 ml). After addition of an aliquot of the zeaxanthin solution (1 ml), heating, sonication, and extraction was performed as described previously (final volume 4 ml; cF). An additional aliquot of the zeaxanthin solution (1 ml) was evaporated, redissolved in TBME/methanol (1:1, v/v; 4 ml), and analyzed by HPLC/DAD (cS). Recoveries were calculated on the basis of AOAC methods [10] as follows: \( {\text{percentage recovery}} = {\left[ {{c_{{\text{F}}} - c_{{\text{U}}} } \mathord{\left/ {\vphantom {{c_{{\text{F}}} - c_{{\text{U}}} } {c_{{\text{S}}} }}} \right. \kern-\nulldelimiterspace} {c_{{\text{S}}} }} \right]} \times 100, \)where cF is the concentration of zeaxanthin measured in the fortified sample, cU is the concentration of zeaxanthin measured in the unfortified sample, and cS is the concentration of zeaxanthin added to the fortified sample. Quantitative amounts were calculated using the lutein calibration curve.
Calibration
Pure lutein was isolated from a saponified marigold (T. erecta) extract [11]. For preparing the calibration curve, appropriate volumes of the stock solution were diluted with TBME/methanol (1:1, v/v; 0.5–15 mg/l) and subjected to HPLC/DAD (450 nm) analysis. Resulting peak areas were plotted against the concentrations.
Liquid chromatography [HPLC-DAD and HPLC-(atmospheric pressure chemical ionization)mass spectrometry]
The HPLC system consisted of an HP1050 modular system (Hewlett-Packard, Waldbronn, Germany) with a DAD, set to 450 nm (band width ±50 nm). For separation, a YMC analytical column (YMC Europe, Schermbeck, Germany) with 5- μm C30-reversed-phase material (250×4.6-mm inner diameter, i.d.) including a precolumn (10×4.0-mm i.d.) was used and kept at 35 °C. For gradient elution, two mobile phases were employed: methanol/TBME/water 81/15/4 (v/v/v; A) and 6/90/4 (v/v/v; B). The following gradient was used (min/%A): 0/99; 39/44; 45/0; 50/99; 55/99 (flow rate 1 ml/min, injection volume 20μl). HPLC-(atmospheric pressure chemical ionization, APcI)mass spectrometry, MS, was run on an HP1100 HPLC system, coupled to a Micromass (Manchester, UK) VG platform II quadrupole mass spectrometer, operated in the positive mode. Further MS parameters have been detailed by Breithaupt et al. [12].
Results and discussion
Two main types of dietary supplements were available on the international market: (1) hard or soft gel capsules containing an oily paste and (2) tablets containing a powdery carotenoid formulation. In a few cases, powders enclosed in soft-gel capsules were provided. Oily pastes were directly dissolved in a solvent mixture composed of TBME and methanol, resulting in a clear solution ready for HPLC analysis. Powdery formulations were extracted with a ternary solvent mixture containing methanol, ethyl acetate, and light petroleum. Special care was taken to dissolve microencapsulated carotenoids by applying multiple heating and sonication steps. If marigold oleoresin was labeled or typical lutein diester peaks appeared in the respective HPLC chromatogram, saponification of aliquots of the final extract was performed. The resulting chromatograms allowed for unequivocal identification and quantification of all-trans-lutein. Fig. 2A shows the characteristic pattern of lutein diesters (3–8) present in marigold oleoresin (T. erecta), identified by HPLC-(APcI)MS in detail in a previous study [12]. After saponification, free lutein (1) accompanied by a minute amount of free zeaxanthin (2) was formed, exhibiting a typical marigold xanthophyll pattern [7]. Several peaks with retention times close to lutein have been identified as lutein mono- or di-cis isomers [7, 13, 14] and were not studied in detail. The applicability of HPLC-(APcI)MS analysis for unequivocal identification of carotenoids present in dietary supplements is demonstrated in Fig. 3. To scan for lutein diesters, m/z 533.4 (upper trace) was used since this mass represents the backbone of lutein after loss of two fatty acids from the respective quasimolecular ion ([M+H]+). The peak pattern of m/z 533.4 exactly reflected that of native marigold oleoresin detected at 450 nm by DAD (lower trace). Since extracts of the alga Dunaliella salina and tomato were labeled on the specific supplement, m/z 537.4 ([M+H]+) was used to scan for β-carotene and lycopene, which possess the same molecular weight. The respective signals clearly indicated the presence of both carotenes (9/10). Lycopene eluted typically within the retention time window of lutein diesters (middle trace).
Typical high-performance liquid chromatography (HPLC) chromatogram (diode-array detector, DAD, 450 nm) of a supplement containing marigold (Tagetes erecta) oleoresin before (A) and after (B) saponification. Peak assignment: 1 all-trans-lutein; 2 all-trans-zeaxanthin; 3 C12:0/C14:0-lutein; 4 C14:0/C14:0-lutein; 5 C14:0/C16:0-lutein; 6 C16:0/C16:0-lutein; 7 C16:0/C18:0-lutein; 8 C18:0/C18:0-lutein
Typical HPLC chromatogram (DAD, 450 nm; lower trace) of a supplement containing marigold (T. erecta) oleoresin, β-carotene (Dunaliella salina), and lycopene (tomato) extracts. Mass traces: m/z 537.4 for detection of β-carotene and lycopene (middle trace); m/z 533.4 for detection of lutein diesters (upper trace). Peak assignment: 9 all-trans-β-carotene; 10 all-trans-lycopene
Method performance
To evaluate the applicability of the extraction process performed for powdery formulations, recovery studies were accomplished. Zeaxanthin was used as a model xanthophyll. Since zeaxanthin is structurally related to lutein (Fig. 1), similar behavior during workup was anticipated. For matrix simulation, a supplement which contained a low amount of zeaxanthin was used. The recovery determined (105.4±4.4%; n=3) demonstrated the applicability of the method. Furthermore, chromatograms of spiked samples showed no zeaxanthin isomers, proving that the workup process, especially short heating and sonication, did not cause xanthophyll isomerization.
Quantitative analysis of commercial dietary supplements
Table 1 lists the lutein contents determined in 14 commercial dietary supplements. Additionally, the concentrations labeled on each product were given and compared with the amounts detected (expressed as ratio Q). If no additional comment about the carotenoid source is given in Table 1, just “lutein” was labeled. The lutein concentration of half of the supplements was found to be below the amount stated (Q<100%), varying here from 11 (G7) to 93% (U1) (Table 1). The highest lutein excess was found in G3 (Q=136%), a supplement with comparatively low lutein concentration (0.250 mg/T). To test if gelatine capsules adsorbed lutein, capsules which were still colored after solvent extraction were investigated additionally after an enzymatic papain treatment (100 mg/5 ml water; 1 h at 37 °C). In a few cases coloring additives (e.g., annatto or synthetic dyes) were present. Free or esterified lutein was detected in none of the capsules. The chromatograms of two supplements (U4, U6) showed no typical lutein diester peaks although “marigold” was given as a lutein source: saponified marigold oleoresin could have been used to formulate them. Remarkably, only one of the German supplements contained free lutein, whereas products from the USA were made with free lutein in either case. It is understood that only 14 dietary supplements were investigated and that other dietary supplements may contain esterified or free lutein instead of the forms used.
After separation by HPLC, the quantification was based on all-trans-lutein only. Photometric methods will detect total xanthophylls (all-trans and cis forms of lutein and zeaxanthin), resulting in higher xanthophyll amounts. The concentrations of cis-lutein isomers varied and accounted for 1–5 area% of total lutein, detected at 450 nm. Only two products showed unusually high cis-isomer concentrations: 11 (G6) and 22 area% (G2) of total lutein. Assuming identical molar extinction coefficients, the total lutein concentrations can be estimated to be in most cases 1–5% higher than that given in Table 1. According to Hadden et al. [7], lutein cis isomers are artifacts of the drying process, as they are absent in freshly harvested marigold flowers. On the basis of plasma response Gaziano et al. [15] suggested that cis isomers of β-carotene are less bioavailable than the all-trans form. Thus, enhanced lutein cis-isomer concentrations may reduce the health benefit of the supplement.
To test whether zeaxanthin generally was a typical component of dietary lutein supplements, UV/vis spectra of peaks eluting after all-trans-lutein were monitored: a sharp maximum at 452 nm clearly proved the presence of zeaxanthin (2, Fig. 2B). If the concentration was low, extracts were spiked with zeaxanthin obtained by saponification of a wolfberry (L. barbarum) extract [9]: co-chromatography proved the presence of zeaxanthin. With the exception of sample G7, all supplements contained zeaxanthin in amounts typical for marigold oleoresins (6.0±1.4 area% of all-trans-lutein, n=13). Consequently, persons consuming the respective supplements will also receive zeaxanthin in concentrations amounting to roughly 5–7% of that of lutein. Based on application of marigold oleoresin, the zeaxanthin concentration of G7 was unusually high (amount labeled, 3 mg/T). However, using the lutein calibration graph, the zeaxanthin concentration was determined to be 0.46±0.02 mg/T (Q=15%).
The high discrepancy found between the amounts labeled and determined in half of the supplements may be attributed to degradation reactions or to improper storage conditions. This is particularly important with respect to the long date of expiry of some supplements. Stability during storage under different conditions was not investigated further in this study. Concerning the declaration of lutein on dietary supplements, it is not clear whether manufacturers refer to all-trans-lutein or to the amount of total lutein isomers. Unequivocal labeling may forestall wrong information of consumers in the future.
References
Landrum JT, Bone RA (2001) Arch Biochem Biophys 385:28–40
Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE (2002) J Nutr 132:518S-524S
Beatty S, Boulton M, Henson D, Koh HH, Murray IJ (1999) Br J Ophthalmol 83:867–877
Somerberg O, Keunen JEE, Bird AC, van Kujik FJGM (1998) Br J Ophthalmol 82:907–910
Berendschot TT, Goldbohm RA, Klopping WA, van de Kraats J, van Norel J, van Norren D (2000) Invest Ophthalmol Vis Sci 41:3322–3326
Neher RT (1968) Econ Bot 22:317–324
Hadden WL, Watkins RH, Levy LW, Regalado E, Rivadeneira DM, van Breemen RB, Schwartz SJ (1999) J Agric Food Chem 47:4189–4194
Cisneros M, Benavides J, Brenes CH, Rito-Palomares M (2004) J Chromatogr B 807:105–110
Weller P, Breithaupt DE (2003) J Agric Food Chem 51:7044–7049
Horwitz W (ed) (2000) Official methods of analysis of the Association of Official Analytical Chemists International, 17th edn. Recovery studies. Byrd, Richmond, VA, p 20, item 27
Breithaupt DE (2004) Food Chem 86:449–456
Breithaupt DE, Wirt U, Bamedi A (2002) J Agric Food Chem 50:66–70
Delgado-Vargas F, Parades-López O (1998) J Agric Food Chem 45:1097–1102
Khachik F, Steck A, Pfander H (1999) J Agric Food Chem 47:455–461
Gaziano JM, Johnson EJ, Russel RM, Manson JE, Stampfer MJ, Ridker PM, Frei B, Hennekens CH, Krinsky NI (1995) Am J Clin Nutr 61:1248–1252
Acknowledgements
We thank M. Najduszynska and Z. Dogan for their preliminary experiments and R. Aman (Institute of Food Technology, University of Hohenheim) for valuable help with enzymatic hydrolysis of gelatine capsules.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breithaupt, D.E., Schlatterer, J. Lutein and zeaxanthin in new dietary supplements—analysis and quantification. Eur Food Res Technol 220, 648–652 (2005). https://doi.org/10.1007/s00217-004-1075-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-004-1075-2