Abstract
Deep eutectic solvents (DESs) were investigated as extracting solvent for headspace single-drop microextraction (HS-SDME). The extraction efficiency of 10 DESs mainly composed of tetrabutylammonium bromide (N4444Br) and long-chain alcohols was evaluated for the extraction of terpenes from six spices (cinnamon, cumin, fennel, clove, thyme, and nutmeg). The DES composed of N4444Br and dodecanol at a molar ratio of 1:2 showed the highest extraction efficiency and was selected to conduct the extractions of terpenes in the rest of the study. HS-SDME was optimized by design of experiments. Only two parameters from the four studied showed a significant influence on the efficiency of the method: the extraction time and the extraction temperature. The optimal extraction conditions were determined by response surface methodology. All extracts were analyzed by gas chromatography coupled to mass spectrometry (GC-MS). More than 40 terpenes were extracted and identified in nutmeg, the richest extract in terpenes in this study. Quantitative analysis based on 29 standards was conducted for each extract. Good linearity was obtained for all standards (R2 > 0.99) in the interval of 1 to 500 μg/g. Limits of quantification ranged from 0.47 μg/g (borneol) to 86.40 μg/g (α-farnesene) with more than half of the values under 2 μg/g. HS-SDME is simple, rapid, and cheap compared with conventional extraction methods. The use of DESs makes this extraction method “greener” and it was shown that DESs can be suitable solvents for the extraction of bioactive compounds from plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The search of natural products for drug discovery has become a keen interest among researchers in the past few decades [1]. Natural products and their bioactive compounds have been used from ancient times for the treatment of various diseases and their potential to substitute chemical drugs has been widely studied [2]. Natural bioactive components mostly come from secondary metabolites. Compared with primary metabolites essential to physiological processes of a living organism (growth, development, and reproduction), secondary metabolites are slightly less vital. Secondary metabolites are synthesized by the organism and can have different functions in the organism. They can serve as a defense against predatory agents or, on the contrary, attract species with beneficial effects (such as pollinators), or even allow communication between plants by sending warning signals [3].
There are three main categories of plant secondary metabolites: terpenes and terpenoids, alkaloids, and phenolic compounds [4]. With 70,000 known structures [5], terpenes represent the widest family of natural compounds. They are categorized by their isoprene (five-carbon) units. As such, monoterpenes are composed of two linked isoprene units, sesquiterpenes of three, diterpenes of four, sesterpene of five, and so on. Monoterpenes, as well as some sesquiterpenes, are highly volatiles compounds and the main constituents of essential oils [6]. They are broadly used in different fields such as fragrances in perfume industry or as flavor enhancers in food industry but their use as natural drugs has drawn the attention of many researchers and pharmaceutical industries [7]. Due to the diversity of their chemical structures, monoterpenes have shown a wide variety of biological activities such as antioxidant [8], anti-inflammatory [9], antibacterial [10], anticonvulsant [11], and antinociceptive [12]. The biological activity of a product is closely linked to its concentration. Thus, to evaluate precisely the concentration of the potential bioactive compound in an extract, the extraction and analytical steps should not be neglected.
The conventional methods used for the extraction of terpenes in natural products include maceration, Soxhlet extraction, percolation, and solvent extraction [7]. Usually these processes involve long and complicated extraction period, low yield, and large volume of hazardous organic solvents. In the search of making sample preparation “greener,” microextraction techniques have emerged. These methods have high sensitivity, require low volumes of organic solvents, and can even be solventless. Moreover, they are simple to use, low cost, and amenable to automation. Different microextraction techniques have been applied for the determination of volatile chemicals in plants such as solid-phase microextraction (SPME), stir bar sorptive extraction (SBSE), single-drop microextraction (SDME), hollow fiber liquid-phase microextraction (HF-LPME), and dispersive liquid-liquid microextraction (DLLME) [13]. To enhance the sensitivity towards volatile compounds, headspace microextraction techniques can be implemented without any sample pretreatment process. Headspace single-drop microextraction (HS-SDME) was first introduced by Theis et al. in 2001 [14]. In HS-SDME, a solvent drop of few microliters is suspended at the tip of a microsyringe needle and exposed to the headspace of a sample. The sample is heated, the target compounds volatilized and adsorbed on the solvent drop. After extraction, the suspended drop is retracted back into the microsyringe and analyzed most often by gas chromatography. This method is fast, simple, and inexpensive and requires only microliters of solvents. One of the most important parameters of HS-SDME is the choice of the extracting solvent. The later should answer to essential criteria to ensure the stability of the drop: low volatility, low vapor pressure, thermal stability, and enough viscosity. The common solvents used for SDME such as toluene, hexane, isooctane, decane, and n-octyl alcohol are toxic for the environment and often have non-negligible volatility which can cause the evaporation of the drop [15]. First ionic liquids (ILs) have emerged as an alternative to organic solvents in HS-SDME due to their negligible vapor pressure [16]. However, concerns about the application of ILs for the extraction of bioactive compounds have arisen due to the toxicity of these solvents, their potential effects on health and environment, and the high cost associated with their synthesis and purification requirements [17].
To overcome the limitations of ILs, deep eutectic solvents (DESs) have emerged. A DES is usually composed of a mixture consisting of a hydrogen bond acceptor (HBA) with a hydrogen bond donor (HBD). Those two compounds are mixed at a precise molar ratio called a eutectic point at which, simply by heating, they form a new solvent liquid at room temperature. The first DES was introduced by Abbot et al. and was made of choline chloride and urea at a molar ratio of 1:2 [18]. DESs have similar solvent characteristics to ILs but are cheaper to produce due to the low costs of raw materials, less toxicity, and often biodegradability. In addition to being eco-friendly, physicochemical properties of DESs are easily tunable by changing one of the two components of the system. An unlimited number of combinations exist to form DESs allowing them to have a wide range of applications. They have been used as dissolution solvents, as catalysis solvents, in organic synthesis, in electrochemistry, in the preparation of nanoparticles, and as extraction solvents [19]. DESs have been used for the extraction of bioactive compounds by different extraction techniques such as microwave-assisted extraction, ultrasonic-assisted extraction, heating-stirring extraction, and liquid-liquid extraction [17]. However, DESs have rarely been used for HS-SDME [20] or for the extraction of terpenes [21,22,23] and only once for the extraction of terpenoids by HS-SDME to our knowledge [24].
Based on the discussion above, the aim of the present study was to develop a robust and efficient extraction method for terpenes by coupling novel green solvents (DESs) to a well-known extraction method sensitive to volatiles compounds (HS-SDME). To conduct this study, the development of the extraction method was done for spices, model of plant rich in terpenes. DES-HS-SDME was first optimized by design of experiments and then applied to six spices (cinnamon, cumin, fennel, clove, thyme, and nutmeg) to evaluate the efficiency of the method for the extraction of terpenes from plants. Qualitative and quantitative analyses based on 29 standards were conducted for each extract.
Experimental
Chemicals and materials
Tetrabutylammonium bromide (N4444-Br, ≥ 99%), decanol (≥ 99%), β-citronellol (≥ 95%), anethole (≥ 98%), and α-terpineol (≥ 97%) were purchased from Fluka (Buchs, Switzerland). Butanol (≥ 99.5%) and ethanol (≥ 99.8%) were purchased from Fisher Scientific (Illkirch-Graffenstaden, France). Methanol (99.9%) was obtained from Carlo Erba (Val-de-Reuil, France). Methyltrioctylammonium chloride (N8881-Cl, ≥ 97%), octanol (99.3%), dodecanol (≥ 98%), hexanoic acid (99–100%), lactic acid (≥ 85%), choline chloride (≥ 98%), urea (≥ 99.5%), ⍺-pinene (99%), β-pinene (99%), camphene (95%), p-cymene (99%), 3-carene (≥ 90%), linalool (97%), limonene (97%), pulegone (97%), 4-terpineol (≥ 95%), caryophyllene (≥ 98.5%), menthone (97%), camphor (96%), menthol (99%), borneol (≥ 99%), estragole (98%), α-humulene (96%), farnesene (mixture of isomers), eucalyptol (99%), cuminaldehyde (98%), eugenol (99%), carvacrol (98%), menthyl acetate (97%), and thymol (98%) were purchased from Sigma-Aldrich (Steinheim, Germany). Geraniol (98%) was purchased from Carl Roth GmbH (Karlsruhe, Germany).
Cinnamon (Cinnamomum zeylanicum, Chamsyl), thyme (Thymus vulgaris, Chamsyl), cumin (Cuminum cyminum, Conquête des saveurs), fennel (Foeniculum vulgare, Ducros), clove (Syzygium aromaticum, Ducros), and nutmeg (Myristica fragrans, Ducros) were all bought from a local shop. Spices were obtained as fine-grained powders, except for fennel which was seeds, and thyme which was cut in small pieces. All food samples were used as bought; no additional grinding was done.
Preparation of deep eutectic solvents
The synthesis of deep eutectic solvents (DESs) was adapted from Tang et al. [25]. Briefly, two components, a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA), were weighed according to their appropriate molar ratio and put in a closed glass vessel. To form the DES, the mixture of HBD and HBA was heated at 80 °C under constant stirring until a homogeneous liquid was formed (approximatively 2 h). With 3 different HBAs and 7 HBDs, ten combinations of DESs (Fig. 1) were prepared.
Headspace single-drop microextraction procedure
Fifty milligrams of sample was weighed in a 20-mL headspace vial (23 × 75 mm) which was closed with PTFE-lined silicon septa and metallic screw caps. The needle of a 10-μL GC microsyringe (10R, SGE Analytical Science Pty Ltd, Australia) containing the DES was introduced in the headspace of the sample vial through the septum. The volume of DES was then pushed down the microsyringe to form a 1.5-μL drop at the tip of the needle. The vial with the microsyringe was placed in an incubator at 80 °C during 90 min allowing the absorption of the volatile compounds on the DES drop. Once the extraction process was completed, the drop was withdrawn into the microsyringe, disposed in a 250-μL insert (29 × 5.7 mm) placed in a 2-mL vial and weighed. To prevent the analytical instruments from damages, the drop was diluted in ethanol and spiked with an internal standard prior to injection in GC-MS. The microsyringe was washed 4 times with ethanol and 2 times with the extraction DES before each extraction.
Optimization of DES-HS-SDME conditions by design of experiments
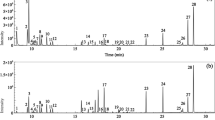
A design of experiments approach was used to optimize the different parameters of DES-HS-SDME. This approach allows to identify which parameters have a significant influence on the response, if there are interactions between the parameters and to find the optimal extraction conditions. The response was defined as the area of the peak of the corresponding compound: one peak corresponds to one response. To optimize the extraction conditions for a maximum of compounds, 27 different characteristic terpenes in nutmeg (Fig. 2) were used as responses. This way, the influence of the extraction parameters on 27 different compounds will be analyzed by the design of experiments approach. The 27 compounds all have different chemical properties, such as polarities and boiling points. The aim is to find optimal conditions which are a compromise of the optimal conditions for each individual terpene found in nutmeg. For data manipulation, JMP® Statistical Discovery™ 8 (SAS Institute) was used.
Total ion chromatogram obtained for nutmeg extracts by DES-HS-SDME. Selected compounds for the optimization by design of experiments. 1, ⍺ Pinene; 2, β Pinene; 3, Sabinene; 4, 3 Carene; 5, ⍺ Phellandrene; 6, 4 Carene; 7, Limonene; 8, γ Terpinene; 9, p Cymene; 10, Terpinolene; 11, trans-Sabinene hydrate; 12, Copaene; 13, Linalool; 14, 1-Terpineol; 15, Bornyl acetate; 16, 4 Terpineol; 17, 4-Terpineol acetate; 18, β-Terpineol; 19, (E)-β-Farnesene; 20, ⍺ Terpineol; 21, trans-Piperitol; 22, Safrole; 23, Methyl eugenol; 24, Eugenol; 25, Isoeugenol methyl ether; 26, Elemicin; 27, Myristicine
First, for the screening of the influential parameters, a 24 full factorial design was built. The number of experiments required for this design was equal to 19 (24 + 3 central points). The data obtained from those experiments were fitted according to the following equation (1) corresponding to a second-order model [26]:
where y is the response (the area of a selected peak); xi are the studied parameters; β0 is the constant; βi are the coefficients of the parameters; βij are the coefficients of the interaction parameters; and ε is the experimental error.
The aim of this first design is to calculate the significance of the coefficient of each factor on the response.
Then, choosing only the significant parameters, a 22 face-centered design was used to determine the optimal extraction conditions for each response. The number of experiments required for this design was equal to 11: 22 = 4 points corresponding to the full factorial design + 3 central points + 4 points on each face of the experimental domain corresponding to a square for a 22 design. The data obtained from those experiments were fitted according to the following equation (2), adding quadratic terms to the previous equation for the determination of the optimum conditions [26]:
where βii represents the coefficients of the quadratic parameters.
With this design, for each response, optimal extraction conditions were obtained, i.e., 27 slightly different optimal extraction conditions were determined. To find optimal conditions which are a compromise for all 27 responses, the desirability function approach was used [27]. This method consists of first drawing desirability functions (d) for each response. The desirability is defined as such: d = 0, lowest desirability obtained for the lowest peak area; d = 1, highest desirability obtained for the highest peak area. Then, an overall desirability function (D) is drawn from the partial desirability functions obtained for each compound. Optimal extraction conditions are found when the overall desirability is maximized, the aim being maximizing the peak area of each compound (corresponding to maximizing the extraction efficiency).
The experimental data was fitted by least squares. To validate the adequacy of the model’s design to fit the experimental data, three values were evaluated. Model’s explained variations R2 ≥ 0.8 and predicted variations Q2 ≥ 0.5 showed an acceptable fitting of the data [28]. The values of Q2 are not needed for the screening design because the aim of this design is not to predict the responses. They are calculated for the face-centered design for which the aim is to predict the optimal conditions for the responses. The lack of fit (LoF) of the model was calculated by comparing the model error with the experimental error by an F-test. The statistical significance of the coefficients of the extraction parameters (βi, βij, and βii) were estimated using an analysis of variance (ANOVA) with a 95% confidence level.
Gas chromatography-mass spectrometer conditions
Gas chromatography-mass spectrometer (GC-MS) analyses were conducted on a 450-GC/240-MS system (Varian, Les Ulis, France). Two microliters of the extract was injected in a split/splitless injector at 210 °C. The compounds were then carried on a DB-WAX capillary column (60 m × 0.25 mm × 0.15 μm) (Agilent Technologies, Les Ulis, France) by helium (purity 99.9999%) at 1 mL/min. They were separated along the column according to the following heating program: 1 min at 40 °C, increased to 100 °C at 10 °C/min, heated to 130 °C at 5 °C/min, heated to 150 °C at 10 °C/min, heated to 180 °C at 5 °C/min, heated to 230 °C at 10 °C/min, and then held isothermal at 230 °C for 5 min. For the MS parameters, the transfer line was set at 200 °C and the ion source at 150 °C. The mass spectrometer was operated in electron impact (EI) mode and the ionising electron energy was set to 70 eV. The mass spectra were recorded in a full scan mode in the range of 50–200 m/z. Peaks were identified by referring mass spectra to the NIST mass spectral database considering a match factor higher than 800 a good match. The identification was then confirmed by calculating the retention index (RI) of each compound and comparing it with the literature for DB-WAX type columns using the Twistaroma database (calculated with n-alkanes series). Furthermore, the RI of the compounds were validated using the 30 standards of this study as a homologue series.
Quantification of terpenes in spices
Quantification of terpenes in the extracts was carried out by using 29 standards (Fig. 3). Those compounds were chosen based on their difference in terms of physicochemical properties, namely polarity, volatility, and molecular mass, to cover the widest possible range of terpenes. For each compound, calibration curves were drawn with 10 points in two concentration ranges: from 1 to 10 μg/g and from 10 to 500 μg/g. Solutions were prepared in methanol. Each concentration was extracted in triplicates. For the extraction, 20 μL of the standards solution was mixed with 50 mg of inert Fontainebleau sand (previously heated at 600 °C for 4 h) and placed in a 20-mL headspace vial (23 × 75 mm) which was closed with PTFE-lined silicon septa and metallic screw caps. The extraction was then carried out according to “Headspace single-drop microextraction procedure.” Each standard was quantified according to the area of the compound’s selected ion which was extracted from the TIC analysis (usually the main ion of compound’s mass spectra). The limits of detection (LOD) and limits of quantification (LOQ), defined as the lowest concentrations detected at a signal-to-noise ratio of 3 or 10 respectively, were calculated for each standard. The calibration curves were drawn above the LOQ for all standards.
Results and discussion
Screening of DESs
The choice of the extracting solvent is a crucial parameter in the HS-SDME. In HS mode, to ensure drop stability, the solvent should have low volatility, low vapor pressure, and enough viscosity [15]. DESs have high thermal stability and negligible volatility [29]. With hundreds of combinations possible to obtain a DES, one can easily be tailored made to meet the physicochemical properties (such as the viscosity, for example) needed for HS-SDME and its polarity can conveniently be tuned to the one of the studied compounds.
Ten different DESs were tested for the extraction of terpenes in nutmeg. In this study, nutmeg was selected as a model as it is a plant rich in a wide variety of terpenes [30]. The effect of the extracting solvent on the extraction efficiency is shown in Fig. 4. This figure presents not only the number of identified compounds in the extracts obtained with the various DESs but also the quantity extracted (relative to the peak area) for 27 characteristic terpenes found in nutmeg. Those compounds have different polarities and boiling points; the aim is to find a DES able to extract the widest range of terpenes.
DESs are composed of two components, a HBA and a HBD; changing one of them can change the physicochemical properties of the DES. To find the most efficient DES for the extraction of terpenes, different combinations of DESs were tested. The aim was to compare new apolar solvents to more commonly used DESs based on choline chloride. First, the influence of an increase of the alkyl chain from 4 carbons (butanol) to 12 carbons (dodecanol) of the HBD on the extraction efficiency was studied (Fig. 4a). The corresponding HBA used was N4444Br. For most compounds, increasing the carbon chain from 4 carbons to 8 carbons increases the extraction efficiency. Beyond 8 carbons, the amount of terpenes extracted does not increase but using a carbon chain of 12 carbons (dodecanol) allows to extract more compounds (42) than the other HBDs studied (39, 34, and 32 compounds for butanol, octanol, and decanol, respectively). Even though the physicochemical properties of DESs are difficult to evaluate, an increase of the carbon chain decreases probably the polarity of the solvent which is more suitable for the studied compounds according to the results. From those results, N4444Br/DoDec was selected and compared with other N4444Br-based DESs by changing the chemical nature of the HBD.
Two other HBDs were studied: hexanoic acid and lactic acid. Using hexanoic acid as the HBD of the DES allowed the extraction of 36 compounds from nutmeg while with lactic acid as the HBD, only 30 compounds were identified in the extract (Fig. 4b). As lactic acid is likely to be more polar than hexanoic acid, N4444Br/LactA is less adequate for the extraction of terpenes than the other two DESs tested. For almost all studied compounds, N4444Br/hexanoic acid extracts with the same extraction efficiency as N4444Br/dodecanol. However, some compounds have more affinity with dodecanol than with hexanoic acid as 36 compounds are extracted by N4444Br/HexA compared with 42 by N4444Br/DoDec.
N4444Br/DoDec was again selected after those observations and compared with another DES by changing its HBA to N8881Cl. N8881Cl seems to be more apolar than N4444Br; however, the latter extracts more compounds (42) than the first one (32) (Fig. 4c). The extraction efficiency of N4444Br/DoDec was also compared with the one of other DESs and in particular to the most used DES [31]: choline chloride/urea (1:2). ChCl/Urea showed a very weak extraction efficiency for the studied compounds as well as ChCl/LacA: only 7 compounds were extracted by ChCl from nutmeg and 11 by ChCl/LacA (Fig. 4d). ChCl has an alkyl chain shorter than the other two HBA studied which results in a higher polarity. ChCl-based DESs are thus not well adapted for the extraction of terpenes. N4444Br/DoDec (1:2) showed higher extraction efficiency than the other 9 DESs studied in this word and was selected for the optimization of the DES-HS-SDME parameters.
Optimization of DES-HS-SDME conditions by design of experiments
Screening of the significant extraction conditions: 24 full factorial design
The optimization of the extraction parameters is an essential step of developing a robust and repeatable extraction method. When dealing with solid/gas and gas/liquid equilibria, like for HS-SDME, it is necessary to have extraction parameters at which the equilibrium state is reached. In most cases, the optimization of the HS-SDME parameters is done by optimizing one-variable-at-a-time (OVAT) while holding the others fixed [32,33,34,35]. Though this approach can lead to the best extraction conditions, it does not consider the interactions between the variables. With the design of experiments approach, the optimal conditions are found with a minimal number of experiments necessary while determining the influential parameters and their potential interactions.
The first step is to screen the different interaction parameters and find the influential ones. The parameters studied and their respective levels are reported in Table 1. Four parameters (extraction temperature T, extraction time text, drop volume V, and sample mass M) were tested at three levels (− 1; 0; + 1). Three experiments at the central point of each parameter have been carried out. A 24 full factorial design was used. The model used to fit the data of the experiments was considered well adapted (Table 2): R2 > 0.8 for all responses and no lack of fit was observed for 96% of the responses. T and text had a statistical positive influence on most responses (78% of the responses for T and 96% for text). That means that an increase of those parameters results in an increase of the responses. The data also showed a strong correlation between T and text as the coefficient of their interaction was statistically significant for 96% of the responses. This demonstrates that those two variables should not be studied separately from one another. No statistically significant interactions between the other factors were observed.
The increase of the drop volume from 0.5 to 2.5 μL resulted in an increase of one response (α-pinene) and a decrease of 34% of the responses, while the mass sample was statistically significant for only one response. As those two factors were not significant for almost all responses, they were fixed for the rest of the study. The volume of the drop was fixed at 1.5 μL. The sample mass was fixed at 50 mg, the lower value of the interval studied, in order to work with the lowest quantity of raw material possible. In fact, raw material can be rare or difficult to obtain, an extraction method needing few raw materials is therefore a great advantage.
Finding the optimum: 22 face-centered design
For the determination of the optimal extraction conditions, a 22 face-centered design was built with only the parameters which had a statistically influence on the responses (“Screening of the significant extraction conditions: 24 full factorial design”): the extraction temperature T and the extraction time text. As observed from the previous design (“Screening of the significant extraction conditions: 24 full factorial design”), an increase of T from 60 to 80 °C and text from 5 to 30 min leads to a significative increase of the responses. The studied intervals were therefore increased (from 70 to 90 °C for T and from 60 to 120 min for text) to find the optimum settings. Table 3 resumes the levels chosen for each factor. The model used to fit the data obtained from the experiments was considered well adapted: values of R2 > 0.8 were obtained for all responses but one (4-terpineol acetate), values of Q2 > 0.5 were obtained for 93% of the responses, and no lack of fit was observed for any of the responses (Table 4). text was found not statistically significant in this interval as an increase of text from 60 to 120 min had a significative impact on less than half the responses (41%).
A known phenomenon was observed regarding the results obtained for T. An increase of this parameter led to an increase of 29% of the responses but led to a decrease of 59% of the responses. This observation is strongly linked to the physicochemical properties of the studied compounds. In Table 4, the terpenes are ordered by their retention indexes which are directly related to their boiling point and to their polarity, i.e., α-pinene has the lowest boiling point (156 °C) while myristicine has the highest one (277 °C). The 29% of the responses (from α-terpineol to myristicine) which were increased by an increase of the temperature are the ones with high boiling point; those compounds are volatile at higher temperatures. On the contrary, the 59% of the responses (from α-pinene to 4-terpineol) which were decreased by an increase of the temperature are the ones with lowest boiling points; those compounds are volatile at lower temperatures. When an increase of temperature occurs, the gas phase will be enriched in molecules with higher boiling points in addition with the ones with low boiling points; thus, more high boiling point components will absorb in the DES drop resulting in an increase of their responses. Furthermore, at high temperatures, compounds with low boiling points might have more affinity with the gas phase than with the DES drop, which leads to the decrease of their responses. This phenomenon can be associated with the back-extraction of the compounds in the headspace [36].
The optimization approach used in this study shows the necessity to consider the greatest number of responses (i.e., the greatest number of analytes) when investigating the extraction parameters. Considering only the sum of peaks or number of peaks as done in most optimization by design of experiments cases [37] is not enough to fully understand the extraction process. The second step to optimize the extraction conditions by this approach is to find an optimum which is a compromise between all the optimums for each response, i.e., for each compound studied. The use of the desirability function allows finding such an optimum. The aims were to maximize the individual desirability functions for each response and to plot an overall desirability function. The contour plot of this function is shown in Fig. 5. The maximum overall desirability (D = 0.556) is reached at the following extraction conditions: 80 °C for T and 90 min for text. The overall desirability was not equal to 1 as it is a compromise between the desirabilities of the different compounds. If all 27 responses had the same optimums, the overall desirability would have been equal to 1.
The optimal extraction conditions selected for DES-HS-SDME were as follows: 50 mg sample mass, 1.5 μL drop volume, 80 °C extraction temperature, and 90 min extraction time.
Calibration
After determination of the optimal extraction parameters, calibration by DES-HS-SDME coupled to GC-MS was conducted for 29 terpenes. Table 5 summarizes the results obtained for the calibration of each terpene. The values of the correlation coefficient (R2) were above 0.99 for all studied compounds, which indicates good linearity in the concentration ranges studied of the extraction method. To analyze the repeatability of the calibration, the relative standard deviation (RSD) was calculated at 10 μg/g for each compound (n = 3). Most compounds showed acceptable repeatability (RSD < 20%), only three compounds (limonene, 4-terpineol, and α-farnesene) had higher RSDs.
LODs and LOQs were determined for each compound. LOQs were in the ranges of 0.47 to 86.40 μg/g. This result shows the importance of conducting a full qualitative analysis as semi-quantitative analysis is not reliable enough. Each compound, even compounds from the same chemical family, has its own reactivity not only with the extraction method but also with the analytical method. The sensibility of the process is related to the compound’s response for the analytical method, i.e., low LOQs show high response thus high sensibility, on the contrary, high LOQs show low response thus low sensibility. More than half of the studied compounds had LOQs lower than 2 μg/g, showing that DES-HS-SDME is well adapted for the extraction of terpenes. Furthermore, the LOQs calculated in this study were 10-fold lower than the ones found in previous work using DES-HS-SDME [24].
Application to the extraction of terpenes from spices
The optimized DES-HS-SDME method was applied to the extraction of terpenes from six spices, namely from cinnamon, cumin, fennel seeds, clove, thyme, and nutmeg. Chromatograms of each extract with their main identified components are shown in the Electronic Supplementary Material (ESM). The main constituents identified in the extracts (regarding the % peak area) are consistent with previous works on spice: cinnamaldehyde in cinnamon [38], cuminaldehyde in cumin [39], eugenol in clove [40], thymol in thyme [41], and myristicine in nutmeg [42]. The main component of fennel extract obtained by DES-HS-DES was estragole; however, anethole is known to be the main compound in fennel [43]. Anethole might lack affinity with the DES used for HS-SDME. Furthermore, the boiling point of estragole (216 °C) is lower than the one of anethole (234 °C) which could explain the difference of sensibility of the extraction method between the two compounds (LOQAnethole = 1.70 μg/g and LOQEstragole = 0.75 μg/g).
The choice of the extraction method is an important step of analytical chemistry as the content of an extract depends heavily on the extraction method. Those first results show that the DES-HS-SDME method is well adapted for the extraction of terpenes from natural materials. Compared with other headspace extraction techniques such as HS-SPME, HSSE, or HS-HF-LPME, HS-SDME has numerous advantages. This extraction method is often quicker than the others and is cheaper and it allows to target a wider range of compounds with different physicochemical properties as a wider range of absorption phases is available [13]. Full qualitative and quantitative analyses of the different extracts are summarized in Table 6. As expected, the plant containing the most terpenes was nutmeg. Forty-two compounds were identified using their mass spectra and retention indexes in nutmeg extract, 32 in thyme, 20 in cumin, 16 in cinnamon and in clove, and only 4 in fennel seeds. Not all identified compounds (by their mass spectra and RIs) were quantified, only the ones corresponding to the 29 standards used for the calibration (“Calibration”). If only a semi-qualitative analysis is conducted (relative to the percentage area of each compound), the concentration might be over or under evaluated. When comparing the relative concentration of two compounds in a same extract, the percentage areas of those compounds might not relate directly to one compound being more abundant than the other. Each compound has its own sensibility towards the extraction method; low sensibility does not necessarily mean low abundance of the compound. This is well illustrated in the spice extracts (Table 6). In the cumin extract, (E)-β-farnesene and carvacrol have almost the same abundance regarding the percentage of peak area (approximately 0.5%) but their quantities calculated by the calibration differ by a factor of almost 20 (13,640 ± 2062 μg/g for (E)-β-farnesene and 758 ± 123 μg/g for carvacrol). (E)-β-Farnesene is almost 20 times more abundant in cumin than in carvacrol. If a semi-quantitative analysis based on the relative percentage abundance had been done, the conclusion would have been that those two components are found in cumin at approximatively the same concentration. Conducting semi-quantitative analysis of different compounds using only one internal standard, relative concentrations of analytes are compared with the one of the internal standard, can also lead to false conclusions. α-Pinene and β-pinene are isomers; their chemical structures are similar. Those two compounds were found in the nutmeg extract at approximatively the same concentration (212,672 ± 28,137 μg/g for α-pinene and 231,250 ± 42,013 μg/g for β-pinene). However, if looking at only percentage peak area, β-pinene is 6 times more abundant in nutmeg (2.7%) than α-pinene (0.43%). If a compound with a chemical structure close to the ones of α-pinene and β-pinene, such as camphene, for example (Fig. 3), had been used as a standard to evaluate the relative concentration of β-pinene, it would have been over evaluated by a factor of 6. The DES-HS-SDME method allows the production of extract concentrated in a wide range of terpenes and terpenoids. The quantitative analysis used in this study provides a well understanding of the extraction and analytical method.
Conclusion
Optimal extraction conditions of DES-HS-SDME were easily determined by the use of chemometric. Full quantitative analysis of the extracts allowed a better understanding of the extraction technique and of the extracts. This study showed that DESs can be a possible alternative to organic solvent in HS-SDME for the extraction of volatile compounds in natural samples. DES-HS-SDME is simple, cheap, rapid, made eco-friendly by the use of DESs, and efficient for the extraction of terpenes from spices. DESs have an important potential in green extraction and analytical chemistry.
References
Newman DJ, Cragg GM, Kingston DGI. Natural products as pharmaceuticals and sources for lead structures. In: The practice of medicinal chemistry, Second Edi. Elsevier Inc., 2003; pp 91–109.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–77.
Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol. 2006;9:1–8.
Croteau R, Kutchan TM, Lewis NL. Natural products (secondary metabolites). In: Biochemistry and molecular biology of plants. 2000;, pp 1250–1318.
Vavitsas K, Fabris M, Vickers CE. Terpenoid metabolic engineering in photosynthetic microorganisms. Genes (Basel). 2018;9:1–19.
De Almeida RN, De Fátima AM, Maior FNS, De Sousa DP. Essential oils and their constituents: anticonvulsant activity. Molecules. 2011;16:2726–42.
Bart H-J. Extraction of natural products from plants – an introduction. In: Industrial scale natural products extraction. 2011;, pp 1–25.
Kozioł A, Stryjewska A, Librowski T, Sałat K, Gaweł M, Moniczewski A, et al. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Reviews Med Chem. 2014;14:1156–68.
Fonsêca DV, Salgado PRR, de Aragão Neto HC, Golzio AMFO, Caldas Filho MRD, Melo CGF, et al. Ortho-eugenol exhibits anti-nociceptive and anti-inflammatory activities. Int Immunopharmacol. 2016;38:402–8.
Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother. 2005;49:2474–8.
Salakhutdinov NF, Volcho KP, Yarovaya OI. Monoterpenes as a renewable source of biologically active compounds. Pure Appl Chem. 2017;89:1105–17.
Yano S, Suzuki Y, Yuzurihara M, Kase Y, Takeda S, Watanabe S, et al. Antinociceptive effect of methyleugenol on formalin-induced hyperalgesia in mice. Eur J Pharmacol. 2006;553:99–103.
Yang C, Wang J, Li D. Microextraction techniques for the determination of volatile and semivolatile organic compounds from plants: a review. Anal Chim Acta. 2013;799:8–22.
Theis AL, Waldack AJ, Hansen SM, Jeannot MA. Headspace solvent microextraction. Anal Chem. 2001;73:5651–4.
Wardencki W, Curyło J, Namieśnik J. Trends in solventless sample preparation techniques for environmental analysis. J Biochem Biophys Methods. 2007;70:275–88.
Clark KD, Emaus MN, Varona M, Bowers AN, Anderson JL. Ionic liquids: solvents and sorbents in sample preparation. J Sep Sci. 2018;41:1–416.
Zainal-Abidin MH, Hayyan M, Hayyan A, Jayakumar NS. New horizons in the extraction of bioactive compounds using deep eutectic solvents: a review. Anal Chim Acta. 2017;979:1–23.
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Novel solvent properties of choline chloride urea mixtures. Chem Commun. 2003:70–1.
Zhang Q, De Oliveira VK, Royer S, François J. Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev. 2012;41:7108–46.
Yousefi SM, Shemirani F, Ghorbanian SA. Enhanced headspace single drop microextraction method using deep eutectic solvent based magnetic bucky gels: application to the determination of volatile aromatic hydrocarbons in water and urine samples. J Sep Sci. 2018;41:966–74.
Jeong KM, Jin Y, Yoo DE, Han SY, Kim EM, Lee J. One-step sample preparation for convenient examination of volatile monoterpenes and phenolic compounds in peppermint leaves using deep eutectic solvents. Food Chem. 2018;251:69–76.
Su E, Yang M, Cao J, Lu C, Wang J, Cao F. Deep eutectic solvents as green media for efficient extraction of terpene trilactones from Ginkgo biloba leaves. J Liq Chromatogr Relat Technol. 2017;40:1–7.
Cao J, Chen L, Li M, Cao F, Zhao L, Su E. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018;20:1879–86.
Tang B, Bi W, Zhang H, Ho RK. Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia. 2014;77:373–7.
Tang W, Dai Y, Row KH. Evaluation of fatty acid/alcohol-based hydrophobic deep eutectic solvents as media for extracting antibiotics from environmental water. Anal Bioanal Chem. 2018;410:7325–36.
Candioti LV, De Zan MM, Cámara MS, Goicoechea HC. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta. 2014;124:123–38.
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–77.
Lundstedt T, Seifert E, Abramo L, Thelin B, Åsa N, Pettersen J, et al. Experimental design and optimization. Chemom Intell Lab Syst. 1998;42:3–40.
Espino M, de los Ángeles Fernández M, Gomez FJV, Silva MF. Natural designer solvents for greening analytical chemistry. TrAC - Trends Anal Chem. 2016;76:126–36.
Simpson GIC, Jackson YA. Comparison of the chemical composition of East Indian, Jamaican and other West Indian essential oils of Myristical fragrans Houtt. J Essent Oil Res. 2002;14:6–9.
Tang B, Row KH. Recent developments in deep eutectic solvents in chemical sciences. Monatsh Chem. 2013;144:1427–54.
Tang B, Tian M, Row KH. Determination of terpenoids in Chamaecyparis obtusa leaves by headspace single-drop microextraction with gas chromatography detection. Anal Lett. 2014;47:48–57.
Sawaddipanich V, Chanthai S. Headspace-single drop microextraction followed by gas chromatographic determination of key aroma compounds in tomato fruits and their sample products. Orient J Chem. 2016;32:1271–82.
Moradi M, Kaykhaii M, Ghiasvand AR, Shadabi S, Salehinia A. Comparison of headspace solid-phase microextraction, headspace single-drop microextraction and hydrodistillation for chemical screening of volatiles in Myrtus communis L. Phytochem Anal. 2011;23:379–86.
Jiang C, Wei S, Li X, Zhao Y, Shao M, Zhang H, et al. Ultrasonic nebulization headspace ionic liquid-based single drop microextraction of flavour compounds in fruit juices. Talanta. 2013;106:237–42.
Jalali Heravi M, Sereshti H. Determination of essential oil components of Artemisia haussknechtii Boiss. Using simultaneous hydrodistillation-static headspace liquid phase microextraction-gas chromatography mass spectrometry. J Chromatogr A. 2007;1160:81–9.
Sadeghian F, Ebrahimi P, Shakeri A, Jamali MR. Extraction of Citrus paradisi volatile components by headspace single-drop microextraction and statistical modeling. J Chromatogr Sci. 2016;54:1263–9.
Marongiu B, Piras A, Porcedda S, Tuveri E, Sanjust E, Meli M, et al. Supercritical CO2 extract of Cinnamomum zeylanicum: chemical characterization and antityrosinase activity. J Agric Food Chem. 2007;55:10022–7.
Yang J, Wei H, Yu C, Shi Y, Zhang H. Extraction of the volatile and semivolatile compounds in seeds of Cuminum cyminum L. using hydrodistillation followed by headspace-ionic liquid-based single-drop microextraction. Chromatographia. 2012;75:1435–43.
Santana De Oliveira M, Almeida Da Costa W, Santiago Pereira D, Santos Botelho R, Oliveira De Alencar Menezes T, Helena De Aguiar Andrade E, et al. Chemical composition and phytotoxic activity of clove (Syzygium aromaticum) essential oil obtained with supercritical CO2. J Supercrit Fluids. 2016;118:185–93.
Dawidowicz AL, Rado E, Wianowska D, Mardarowicz M, Gawdzik J. Application of PLE for the determination of essential oil components from Thymus vulgaris L. Talanta. 2008;76:878–84.
Piras A, Rosa A, Marongiu B, Atzeri A, Dessì MA, Falconieri D, et al. Extraction and separation of volatile and fixed oils from seeds of myristica fragrans by supercritical CO2: chemical composition and cytotoxic activity on caco-2 cancer cells. J Food Sci. 2012;77:1–6.
Diao W-R, Hu Q-P, Zhang H, Xu J-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control. 2014;35:109–16.
Funding
This work has been financially supported by “Association Nationale Recherche Technologie” with the CIFRE Contract No. 2016/0447.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 222 kb)
Rights and permissions
About this article
Cite this article
Triaux, Z., Petitjean, H., Marchioni, E. et al. Deep eutectic solvent–based headspace single-drop microextraction for the quantification of terpenes in spices. Anal Bioanal Chem 412, 933–948 (2020). https://doi.org/10.1007/s00216-019-02317-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02317-9