Abstract
For the identification of the optimal column combinations, a comparative orthogonality study of single columns and columns coupled in series for the first dimension of a microscale two-dimensional liquid chromatographic approach was performed. In total, eight columns or column combinations were chosen. For the assessment of the optimal column combination, the orthogonality value as well as the peak distributions across the first and second dimension was used. In total, three different methods of orthogonality calculation, namely the Convex Hull, Bin Counting, and Asterisk methods, were compared. Unfortunately, the first two methods do not provide any information of peak distribution. The third method provides this important information, but is not optimal when only a limited number of components are used for method development. Therefore, a new concept for peak distribution assessment across the separation space of two-dimensional chromatographic systems and clustering detection was developed. It could be shown that the Bin Counting method in combination with additionally calculated histograms for the respective dimensions is well suited for the evaluation of orthogonality and peak clustering. The newly developed method could be used generally in the assessment of 2D separations.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The technological progress to more sensitive detection techniques such as mass spectrometry reveals the complexity of environmental samples including wastewater samples from communal wastewater treatment plants [1, 2]. Such samples might contain several thousands of different compounds [3, 4]. Analytical methods based on one-dimensional liquid chromatography (1D-LC) have limitations in terms of peak capacity [5]. Therefore, alternative separation techniques with higher peak capacity are deemed necessary to resolve as many compounds as possible.
One very promising approach is online two-dimensional liquid chromatography (2D-LC) due to the possibility to re-analyze the effluent from the first dimension (D1) on a second dimension (D2) column with a different selectivity. 2D-LC systems are well established in several analytical fields including proteomic [6, 7] and genomic [8] research and may also be a powerful tool for the analysis of complex food [9] and environmental [2, 10] samples.
Two-dimensional liquid chromatographic systems can be operated in different modes as, e.g., “heart-cut-mode” (LC-LC) [11] or “selective-mode” (sLCxLC) [12, 13], where only one or a few selected fractions from the first dimension effluent are re-analyzed on the second dimension. A more complex approach is the “comprehensive-mode” (LCxLC), where the whole effluent of D1 is transferred to the second dimension in small fractions [11, 14, 15]. The hardware configuration of online comprehensive LCxLC systems is very complex and has some disadvantages, one of which is the dilution introduced by the modulation between the first and second dimension, resulting in lower sensitivity. A further disadvantage is the very fast cycle time needed on the second dimension separation. Cycle times of less than 1 min are preferred to fulfill the Murphy–Schure–Foley (M-S-F) criterion, which implies that every D1 signal must be sampled at about three to four times to conserve the D1 separation [16, 17]. To achieve cycle times of less than 1 min, very high flow rates up to 5 mL min−1 are necessary when columns with an inner diameter (i.d.) of 2.1 mm are used [18–20]. Unfortunately, this is not the optimal flow rate for electrospray ionization (ESI) mass spectrometry. To minimize the solvent load introduced into the ESI source, flow splitters are frequently used [21] with drawbacks in terms of reproducibility and loss in sensitivity.
For a splitless coupling to mass spectrometry, a microscale online LCxLC system was developed in a previous work [1]. The system is based on nano-LC for the first and micro-LC for the second dimension with a maximum flow rate of 50 μL min−1. To counteract the previously mentioned dilution effect caused by the modulation and to increase the sensitivity, large volume injection of about 1.6 μL was applied on the first dimension nano-LC column with an i.d. of 0.1 mm and a length of 50 mm. The optimal injection volume (smaller than 10 % of the column void volume) of columns with this dimension is <25 nL. To achieve such an extraordinarily large volume injection without significant peak broadening and loss of signal intensity, a stationary phase material based on porous graphitic carbon (PGC) was used. The high retentivity even for polar compounds allows focusing on the head of the column [22]. Unfortunately, non-polar compounds may be adsorbed permanently or eluted as very broad bands when the plateau of the gradient is reached [22]. In this respect, the length of the column significantly contributes to the retention of non-polar compounds. In a recent study [23], we used a short PGC column of 10 mm length to overcome the problem of band broadening at the end of the gradient while maximizing the injection volume for polar compounds. Li et al. [24] recently demonstrated that a serial column coupling of two stationary phases for the first dimension has a pronounced influence on the overall selectivity of the two-dimensional system, which resembles the concept of phase optimized liquid chromatography. Therefore, the aim of this study was to further increase the selectivity of the two-dimensional system. A column screening was performed using stationary phases on the basis of pentafluorophenyl (PFP), cyano (CN), hydrophilic interaction chromatography (HILIC), and porous graphitic carbon. All columns were used as single stationary phases and also serially coupled to a short PGC pre-column. For the evaluation of the optimal column combination, the orthogonality value was used and calculated on the basis of three well-established concepts. In addition, the peak distribution across the respective dimensions was evaluated. As a result, a new concept for peak distribution assessment across the separation space of two-dimensional chromatographic systems and clustering detection was developed.
Experimental section
Solvents and additives
Acetonitrile, methanol, and water were all of LC-MS grade and purchased from Th. Geyer (Chemsolute; Th. Geyer, Renningen, Germany). The eluents were acidified by adding 0.1 % formic acid (FA) by volume (Puriss p. a. 98 %; Sigma-Aldrich, Schnelldorf, Germany).
Multi-component reference standard solution
In order to obtain valid information about the orthogonality of the column combinations, a multi-component reference standard solution with a total of 42 different compounds was prepared. The selected compounds represent a subset of so-called emerging contaminants in the field of water analysis. A detailed compound list is provided in the Electronic Supplementary Material (ESM) Table S1. The resulting multi-component reference standard contained 10 μg mL−1 of each compound. Based on the log D value, the polarity of the compounds could be estimated. They cover a very broad log D range between −3.16 and 5.46 (predicted by ChemAxon, chemicalize.org). For the detailed discussion, the compounds were classified into three groups, polar (log D = −3.16 to 0.29), semi-polar (log D = 0.30 to 2.59), and non-polar (log D = 2.60 to 5.46) compounds.
2D-HPLC instrument
For column screening, the Eksigent NanoLC 425 system (SCIEX, Darmstadt, Germany) with a fully integrated autosampler was used. This system contained two binary-gradient pneumatic pumps, which can be operated separately at flow rates between 0.1 and 50 μL min−1 at a maximum backpressure of ∼690 bar (10 kpsi). The integrated autosampler contained a two-position six-port valve, used for the injection of the sample onto the first dimension, and an additional two-position ten-port valve used for the modulation of the first dimension effluent. For the modulation, the two-loop technique with symmetrical flow paths (see ESM Fig. S1) was applied. For the first dimension, an external column oven μOV HTG200-17 (AμMass, Leverkusen, Germany) was used and adjusted to 30 °C. The integrated column oven of the NanoLC 425 was used for temperature control of the second dimension column and adjusted to 50 °C. The 2D-LC system was controlled by the Eksigent Control Software (version 4.1, build 130717-1043).
In order to operate the NanoLC 425 system in comprehensive mode, some further system modifications are required. Since the modulation valve is part of the autosampler and not the pump module, the modulation steps need to be programmed into the autosampler methods. A detailed description of the programmed autosampler methods is given in Table S2 and Table S3 in the ESM.
Furthermore, the gradient delay volume had to be reduced in order to speed up the cycle time of the second dimension and the total analysis time of a single 2D-LC run. In order to achieve very low gradient delay volumes, the gradient mixers of the pumps were positioned closer (∼20 cm) to the modulation and injection valve. For the complete flow path of the first dimension, fused silica capillaries with an i.d. of 25 μm were used. For the second dimension and the modulation loops, capillaries with an i.d. of 50 μm were installed.
For the separation on the first dimension, the following stationary phases are used: Hypersil GOLD PFP (PFP, 5 μm), Bio Basic CN (Cyano, 5 μm), and Hypercarb (PGC, 5 μm) all purchased from Thermo Fisher Scientific (Dreieich, Germany) and SeQuant ZIC-HILIC (HILIC, 3.5 μm; Merck, Darmstadt, Germany). For the selectivity study in the first dimension, different columns and column combinations listed in Table 1 were used.
Acidified (0.1 % FA) water (solvent A) and methanol (solvent B) were chosen for the mobile phases of the first dimension, and the flow rate was adjusted to 300 nL min−1. The injection volume was always 450 nL. A solvent gradient was applied according to the following programs: (1) used for PGC, PFP, and Cyano columns—gradient start at 3 % B, in 18 min 3–97 % B, 16 min hold at 97 % B, in 0.5 min 97–3 % B, re-equilibration for 16.5 min; (2) used for HILIC column—isocratic at 99 % B, hold at 99 % B for 51 min.
For the separation on the second dimension, superficially porous 2.6 μm SunShell RP-AQUA C28 particles (ChromaNik Technologies, Osaka, Japan) packed into 0.3 × 50 mm i.d. hardware were used. The flow rate was adjusted to 35 μL min−1. Acidified (0.1 % FA) water (solvent A) and acetonitrile (solvent B) were chosen for the second dimension mobile phases. A solvent gradient was applied according to the following program: gradient start at 3 % B, in 0.5 min 3–97 % B, 0.08 min hold at 97 % B, in 0.04 min 97–3 % B, re-equilibration for 0.13 min. The total gradient cycle time of 0.75 min was repeated until the end of the gradient of the first dimension. In total, 68 D2 cycles were performed. The transfer volume onto the second dimension column was 225 nL. For transfer loops, fused silica capillaries with an i.d. of 50 μm and a length of 15.6 cm were used.
MS instrument
For the mass spectrometric detection, a 3200 QTRAP MS/MS system with a Turbo V ion source and a TurboIonSpray probe for electrospray ionization was used (SCIEX, Darmstadt, Germany). To avoid band broadening, an emitter tip optimized for micro-LC flow rates and an i.d. of 50 μm replaced the standard emitter tip with an i.d. of 130 μm. The mass spectrometer was controlled by SCIEX Analyst 1.6.2 software, which was also used for data evaluation. The mass spectrometer was operated in multiple reaction monitoring (MRM) mode to detect two transitions of each target during the complete 2D-LC run. The detailed MS conditions are listed in ESM Tables S4 and S5.
Theory and calculation
The orthogonality value is a well-suited parameter for the evaluation of the different column combinations. It describes the distribution of the targets over the two-dimensional chromatographic separation area. In general, a homogeneous distribution of all target compounds should be achieved to fully utilize the separation space and to minimize the number of co-eluting analytes. The distribution of target analytes across the separation space depends on the overall selectivity of the first and second dimension and is not related to efficiency or peak capacity.
In order to achieve the best peak distribution, the respective separation mechanisms need to be independent. Such configuration is called orthogonal and represents a theoretical optimum for a two-dimensional separation system. In practice, this cannot be achieved because each separation mechanism is a combination of several interactions. Therefore, the same interactions will occur on both dimensions.
Examples of a low and a high orthogonal system are shown in ESM Fig. S2. The dots represent the peak maxima of each compound and do not provide information about peak area, width, or height. If the separation mechanisms of the first and the second dimension are identical, the distribution is lowest and the targets are located around the bisecting line.
Three different methods are mainly used for the calculation of orthogonality. One of these methods describes the effective area by using a vector cross product calculation [25–27]. In this method, the orthogonality determination is based on the location of the outer analytes, defining the convex hull area. The compound eluting first from the first and second dimension is defined as the origin corner point. For calculating orthogonality, the convex hull area is divided into triangles defined by vectors between the origin corner point and the outermost analytes (see ESM Fig. S3).
Unfortunately, cluster detection is not possible when applying this method. Moreover, the unused separation area within the hull is not considered. Also, compounds which are located in one of the corner points will significantly affect the orthogonality value, although the majority of compounds might elute in a very small fraction of the separation space inside the convex hull.
Another approach was introduced by Camenzuli and Schoenmakers [28] and is termed Asterisk method. This method describes the orthogonality by calculation of spread (\( {S}_{Z_{-}} \), \( {S}_{Z_{+}} \), \( {S}_{Z_1} \), and \( {S}_{Z_2} \)) and standard deviation (σ) of peaks to four lines (Z −, Z +, Z 1, and Z 2) crossing the separation space. Z −, Z + are affected by both dimensions, with Z 1 being related to the spread of components in the first dimension while the spread around the Z 2 line is only related to the second dimension (see ESM Fig. S4).
The authors noted that this approach is more suited to samples that contain more than 50 components. If less than 50 compounds are used for method development, the orthogonality value dropped considerably and the standard deviation increased significantly for different simulation scenarios (up to 14 %). In terms of method development, it would be beneficial if the orthogonality value could also be calculated with a lower standard deviation when fewer compounds are used. The reason is that the availability of reference standards is often related to high costs. Moreover, using fewer reference compounds for method development would also decrease the overall complexity of method development and concomitant data analysis.
The third method is the Bin Counting method introduced by Gilar et al. in 2005 [29]. This method defines the effective area by dividing the separation space into rectangular bins of a defined size, which depends on the total number of compounds. By definition, the maximum distribution of 63 % could be achieved if a Poisson distribution is assumed. Therefore, 100 % orthogonality is equal to 63 % coverage of the separation space. In a non-orthogonal system, the compounds are located across the bisecting line of the normalized separation space. Therefore, 0 % orthogonality corresponds to the number of bins covering the bisector line.
At first, the size of the bins (BS) is calculated according to Eq. 1, where NC total is the total number of compounds.
In total, 42 compounds were used for method development in our study. The calculated bin size value is 6.48 and not an integer. Therefore, it was rounded down to 6 for a practicable calculation of the orthogonality.
Next, the normalized 2D plots were divided into 6 × 6 bins, which represent the maximum bin number B max. For the calculation of the orthogonality value O, the bins filled with at least one compound need to be summed (see ESM Fig. S5), and the calculation is performed according to Eq. 2.
In order to be able to compare the column combinations with all methods described above, the fraction numbers of the first dimension and the retention times of the second dimension were normalized by Eq. 3.
RT min and RT max represent the fraction numbers or the retention times of the least and the most retained compound, respectively. The normalized values RT i(norm) ranged between 0 and 1. The normalization allows a comparison of different chromatographic data in a uniform two-dimensional distribution space.
Since the Convex Hull and the Bin Counting methods do not provide any information of peak distribution across the respective dimensions, additional histograms for the first and second dimension are proposed in this study. For the calculation, the elution window of each dimension is divided into equally broad segments S x as shown in Fig. 1. The width of each segment is calculated according to Eq. 1 and therefore equal to the bin size of the Bin Counting method.
The percentage distribution (D x ) of segment x is calculated according to Eq. 4, where NC total is the total number of compounds and NC area, x the number of compounds eluting inside the respective segment of the chosen dimension.
A fully orthogonal system would provide the same D x values, which are equal to D x(orthogonal) for all respective segments of the first and second dimension as shown in Fig. 1. A D x value significantly higher than D x(orthogonal) indicates a clustering inside the respective segment, whereas a D x value significantly lower than D x (orthogonal) indicates a void space.
In general, the square root of the sum of the squares of the differences between the respective D x values and D x (orthogonal) can reveal the clustering or anti-clustering across the first (SD first) and second (SD second) dimensions as well as the coverage of the effective separation space (SD total), according to Eq. 6. For the calculation of SD total, D x values for both dimensions should be used.
Assuming a homogeneous distribution of all compounds across the respective two-dimensional separation space, an SD value of 0 should be calculated. With a higher clustering, the SD value will increase. Therefore, a column combination with the lowest possible value should be chosen. However, it must be noted that this concept should be used in combination with the orthogonality value. The reason is that an even distribution is not necessarily equivalent to a high orthogonality. This has been demonstrated for simulated examples of peak spreading and is included in the ESM (Figs. S6a–k and S7a–i, and Tables S6 and S7).
Results and discussion
Overview of the normalized dot-plots and histogram distributions
For the comparative orthogonality study of single columns and columns coupled in series for the first dimension, a multi-component reference mix was analyzed using the microscale online comprehensive miniaturized 2D-LC system. The normalized dot-plots are shown in Fig. 2.
Normalized dot-plots for single columns and serial column combinations used in the first dimension and the RP-C28 column in the second dimension. Stationary phases for the first dimension: a PGC phase, b 10 mm PGC pre-column, c PFP phase, d 10 mm PGC pre-column coupled to PFP phase, e Cyano phase, f 10 mm PGC pre-column coupled to Cyano phase, g HILIC phase, h 10 mm PGC pre-column coupled to HILIC phase
The histograms shown in Fig. 2 show the compound distribution across the respective dimensions. Since the column used for the second dimension remained unchanged, the small differences in D x values are a result of slight retention time shifts. The polarity distribution is also given by the color of dots. As expected, the polar compounds elute at the beginning of the gradient on the RP-C28 phase used for the second dimension, followed by the semi-polar and non-polar compound groups. However, a retention time prediction based only on the specific log D value is not possible as some of the semi-polar compounds elute before some of the polar compounds and some of the non-polar compounds elute before some of the semi-polar compounds. In fact, this holds true for both dimensions.
For further evaluation, the orthogonality values have been calculated and all three methods are used and compared.
Orthogonality comparison calculated by Convex Hull method
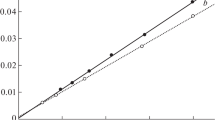
The advantage of the Convex Hull method is the low dependence on the number of analytes [26, 27]. The orthogonality values calculated according to the Convex Hull method are summarized in Fig. 3a.
The 50 mm PGC column yields an orthogonality value of 46.2 %. However, the disadvantages that were mentioned earlier exclude the further use of this column dimension. Compared to the 50 mm PGC column and based solely on the orthogonality values, it can be concluded that the HILIC phase in the first and RP-C28 phase in the second dimension is the best column combination for the separation of the reference mixture.
A detailed view on the distribution histograms shown in Fig. 4 clearly demonstrates that 71.4 % of all compounds elute in a single fraction on the HILIC phase in the first dimension. Therefore, despite the high orthogonality value, it appears that this column combination is not appropriate for the separations of the target analytes of this study.
The reason is that the calculated orthogonality value does not correlate with the distribution of the compounds inside the hull and only a few outlier compounds significantly increase the orthogonality value as can be seen in Fig. 4. The elimination of only one point (D1/D2, 1.0:0.3) reduces the orthogonality value from 63.1 to 51.0 % for the HILIC × RP-C28 combination. This effect has also been demonstrated for other 2D scenarios [30]. Therefore, this method is not suitable for the orthogonality study of this work, despite the fact that the missing information concerning clusters and compound distribution within the hull can be shown by the additionally calculated histograms.
Orthogonality comparison calculated by Asterisk method
The biggest advantage of the Asterisk method is the cluster detection based on Z x parameters. The orthogonality values calculated according to the Asterisk method are given in Fig. 3b. The 50 mm PGC column yields an orthogonality value of 51.8 %. The single HILIC phase and the combination of the 10 mm PGC pre-column with the HILIC phase for the first dimension leads to high orthogonality values of 69 and 66.1 %. It appears that both approaches could be an alternative to the use of the 50 mm long PGC phase for the first dimension. However, as already mentioned and shown in Fig. 4, this column or column combination is not suitable for the separation of the chosen reference compounds as demonstrated by the Z x parameters listed in Table 2. The higher the Z x parameter value, the better the distribution around the Z x line. Therefore, a fully orthogonal system would provide the value of 100 % for all four parameters. The smaller the Z x parameter value, the higher the clustering around the Z x line.
Since the column used for the second dimension remained unchanged, the mean Z 2 value is indicative of the retention time stability for the second dimension separation. The mean Z 2 value of 88 % and the standard deviation of only 1.3 % show the very low clustering for the second dimension and therefore a good distribution is obtained. A comparison of the Z 1 value for the 50 mm PGC column of 98 % and the HILIC column of 95 % indicates that only negligible clustering and an equally good separation is obtained. However, as discussed before, 71.4 % (see Fig. 4) of all compounds elute in the first fraction when using the HILIC phase. Based on the given Z x values for the HILIC phase, it is not possible to detect this clustering. This could be a result of a low number of compounds used for the reference mix as has been noted by Camenzuli et al., which underlines that the Asterisk method is not applicable for the orthogonality study of this work.
Orthogonality comparison calculated by Bin Counting method
In comparison to the Convex Hull method, the Bin Counting method considers the separation space that is not filled with peaks. Single outer-most compounds have only a negligible influence on the orthogonality value, which is the main advantage of this method. The orthogonality values of the normalized 2D plots are shown in Fig. 3c which demonstrates that the HILIC phase and 10 mm PGC/HILIC combination have a much lower orthogonality value of only 30.9 %, when compared to the Convex Hull and Asterisk methods. The Bin Counting method confirms that the expected low orthogonality value of the HILIC phase is caused by the clustering in the first segment, although it is not possible to locate the cluster. Therefore, this concept seems to be suitable for method development and the missing information about clusters is obtained by the additional calculation of the histograms. The calculated SD first, SD second, and the SD total values are listed in Table 3.
As can be seen, the use of the single 10 mm PGC pre-column results also in a moderate orthogonality value of 48.5 % with an acceptable SD first value of 24. This result demonstrates that the short pre-column could be used in the first dimension without an additional main column. However, as the data reveals, an even higher orthogonality value and therefore better selectivity is obtained if the PGC pre-column is coupled to a Cyano phase.
The orthogonality values obtained with single PFP, Cyano, and HILIC columns for the first dimension are significantly lower than the value reached by the 50 mm PGC and RP-C28 column combination. Therefore, the single columns are not suitable as an alternative for the first dimension. However, it could be shown that the 10 mm PGC pre-column concept increases the system orthogonality, if used in combination with PFP or Cyano main columns for the first dimension. In particular, the combination of 10 mm PGC pre- and Cyano main column resulted in a higher orthogonality of 61.7 % with the lowest SD first value of 15 and therefore the best distribution across the first dimension. A detailed view on the histograms for the first dimension given in Figure S8 (see ESM) shows a slight clustering on the 50 mm PGC phase located within the first and fourth segments. The peak distribution when using the 10 mm PGC/Cyano phase for the first dimension was equal and only a negligible clustering could be observed within segments three and four. The peak distribution for the second dimension was almost constant and varied only slightly due to minor variations in the second dimension retention times. The histograms for the second dimension show a moderate clustering in the second and third segment.
For the analysis of complex samples on the basis of a suspected or non-target screening approach, the retention time is a very important identification criterion that should not be ignored. Especially for a suspected target screening, the accurate mass in combination with the retention time can be used for compound identification. A high variation in retention time could lead to false-positive or false-negative results. Therefore, the intraday retention time stability was evaluated on the basis of the standard reference mixture, analyzed on three consecutive days using the optimal column combination. The detailed list containing the elution fraction numbers for the first and the retention times for the second dimension is given in Table S8 in the ESM.
The data evaluation provides a mean standard deviation for the elution fraction number of 2.1 % and for the retention time for the second dimension of 1.5 %. The results fulfill the maximum acceptable standard deviation of 2.5 % and demonstrate the stability of the miniaturized 2D-LC system.
Conclusion
Existing procedures for determining the orthogonality and therefore the separation performance of two-dimensional systems have drawbacks since they provide no or only partial information about the peak distribution of the compounds across the separation space. However, this information is important because it is indicative of whether the separation space is equally filled. These disadvantages have been successfully overcome with the introduction of the additional histogram information presented in this work. The advantage of this concept is that the retention times of the selected target analytes have to be measured only once on each column. The optimal column combination can then be deduced according to the respective SD first and SD second values.
Independent of the method used for the determination of the orthogonality, the concept described for the identification of clusters reveals the possibility of getting information for the optimal column combination for both the first and second dimensions through the use of one single investigation of each column in any desirable combination. In this respect, the approach might be not only applicable for LCxLC, but also for other 2D separation methods such as GCxGC.
References
Haun J, Leonhardt J, Portner C, Hetzel T, Tuerk J, Teutenberg T, et al. Online and splitless NanoLC × CapillaryLC with quadrupole/time-of-flight mass spectrometric detection for comprehensive screening analysis of complex samples. Anal Chem. 2013;85(21):10083–90.
Leonhardt J, Teutenberg T, Tuerk J, Schlüsener MP, Ternes TA, Schmidt TC. A comparison of one-dimensional and microscale two-dimensional liquid chromatographic approaches coupled to high resolution mass spectrometry for the analysis of complex samples. Anal Methods. 2015;7(18):7697–706.
Krauss M, Singer H, Hollender J. LC-high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal Bioanal Chem. 2010;397(3):943–51.
Zedda M, Zwiener C. Is nontarget screening of emerging contaminants by LC-HRMS successful? A plea for compound libraries and computer tools. Anal Bioanal Chem. 2012;403(9):2493–502.
Neue UD. Peak capacity in unidimensional chromatography. J Chromatogr A. 2008;1184(1–2):107–30.
Nägele E, Vollmer M, Hörth P. Improved 2D nano-LC/MS for proteomics applications. A comparative analysis using yeast proteome. J Biomol Tech. 2004;15(2):134–43.
Ralston-Hooper KJ, Turner ME, Soderblom EJ, Villeneuve D, Ankley GT, Moseley MA, et al. Application of a label-free, gel-free quantitative proteomics method for ecotoxicological studies of small fish species. Environ Sci Technol. 2013;47(2):1091–100.
Zhao L, Liu L, Leng W, Wei C, Jin Q. A proteogenomic analysis of Shigella flexneri using 2D LC-MALDI TOF/TOF. BMC Genomics. 2011;12:528.
Willemse CM, Stander MA, Vestner J, Tredoux AG, de Villiers A. Comprehensive two-dimensional hydrophilic interaction chromatography (HILIC) × reversed-phase liquid chromatography coupled to high- resolution mass spectrometry (RP-LC-UV-MS) analysis of anthocyanins and derived pigments in red wine. Anal Chem. 2015;87(24):12006–15.
Ouyang X, Leonards PEG, Tousova Z, Slobodnik J, de Boer J, Lamoree MH. Rapid screening of acetylcholinesterase inhibitors by effect-directed analysis using LC × LC fractionation, a high throughput in vitro assay, and parallel identification by time of flight mass spectrometry. Anal Chem. 2016;88(4):2353–60.
Mondello L, Lewis AC, Bartle KD. Multidimensional chromatography. Chichester: Wiley; 2001.
Groskreutz SR, Swenson MM, Secor LB, Stoll DR. Selective comprehensive multi-dimensional separation for resolution enhancement in high performance liquid chromatography. Part I: principles and instrumentation. J Chromatogr A. 2012;1228:31–40.
Wang S, Qiao L, Shi X, Hu C, Kong H, Xu G. On-line stop-flow two-dimensional liquid chromatography-mass spectrometry method for the separation and identification of triterpenoid saponins from ginseng extract. Anal Bioanal Chem. 2015;407(1):331–41.
Li D, Jakob C, Schmitz O. Practical considerations in comprehensive two-dimensional liquid chromatography systems (LCxLC) with reversed-phases in both dimensions. Anal Bioanal Chem. 2015;407(1):153–67.
Li D, Schmitz OJ. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass QTOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal Bioanal Chem. 2015;407(1):231–40.
Murphy RE, Schure MR, Foley JP. Effect of sampling rate on resolution in comprehensive two-dimensional liquid chromatography. Anal Chem. 1998;70(8):1585–94.
Stoll DR, Cohen JD, Carr PW. Fast, comprehensive online two-dimensional high performance liquid chromatography through the use of high temperature ultra-fast gradient elution reversed-phase liquid chromatography. J Chromatogr A. 2006;1122(1):123–37.
Dugo P, Cacciola F, Donato P, Mondello L. Comprehensive two-dimensional liquid chromatography applications. In: Mondello L, editor. Comprehensive chromatography in combination with mass spectrometry. Hoboken, NJ: Wiley; 2011. p. 391–427.
Dugo P, Cacciola F, Donato P, Mondello L. Comprehensive two-dimensional liquid chromatography combined with mass spectrometry. In: Mondello L, editor. Comprehensive chromatography in combination with mass spectrometry. Hoboken: Wiley; 2011. p. 331–90.
Stoll DR, Wang X, Carr PW. Comparison of the practical resolving power of one- and two-dimensional high-performance liquid chromatography analysis of metabolomic samples. Anal Chem. 2007;80(1):268–78.
Francois I, Sandra K, Sandra P. History, evolution, and optimization aspects of comprehensive two-dimensional liquid chromatography. In: Mondello L, editor. Comprehensive chromatography in combination with mass spectrometry. Hoboken: Wiley; 2011. p. 281–330.
West C, Elfakir C, Lafosse M. Porous graphitic carbon: a versatile stationary phase for liquid chromatography. J Chromatogr A. 2010;1217(19):3201–16.
Leonhardt J, Hetzel T, Teutenberg T, Schmidt TC. Large volume injection of aqueous samples in nano liquid chromatography using serially coupled columns. Chromatographia. 2015;78(1–2):31–8.
Li D, Dück R, Schmitz OJ. The advantage of mixed-mode separation in the first dimension of comprehensive two-dimensional liquid-chromatography. J Chromatogr A. 2014;1358:128–35.
Semard G, Peulon-Agasse V, Bruchet A, Bouillon J-P, Cardinaël P. Convex hull: a new method to determine the separation space used and to optimize operating conditions for comprehensive two-dimensional gas chromatography. J Chromatogr A. 2010;1217(33):5449–54.
Dück R, Sonderfeld H, Schmitz OJ. A simple method for the determination of peak distribution in comprehensive two-dimensional liquid chromatography. J Chromatogr A. 2012:69–75
Rutan SC, Davis JM, Carr PW. Fractional coverage metrics based on ecological home range for calculation of the effective peak capacity in comprehensive two-dimensional separations. J Chromatogr A. 2012;1255:267–76.
Camenzuli M, Schoenmakers PJ. A new measure of orthogonality for multi-dimensional chromatography. Anal Chim Acta. 2014;838:93–101.
Gilar M, Olivova P, Daly AE, Gebler JC. Orthogonality of separation in two-dimensional liquid chromatography. Anal Chem. 2005;77(19):6426–34.
Gilar M, Fridrich J, Schure MR, Jaworski A. Comparison of orthogonality estimation methods for the two-dimensional separations of peptides. Anal Chem. 2012;84(20):8722–32.
Acknowledgments
The authors would like to thank Harald Möller-Santner and Eike Logé from Sciex for the loan of the Eksigent NanoLC 425 system. Furthermore, we would like to thank Juergen Maier-Rosenkranz for organizing the packing of PGC columns. In addition the authors would like to thank Altmann Analytik GmbH & Co. KG and Merck KGaA for providing the HILIC column.
Notes
For the automated data evaluation, based on the in this work presented method, a Python 3.x based Script (2D-Distribution.py) was developed. The script as well as an introduction is a part of the supplementary material and is available at https://uni-duisburg-essen.sciebo.de/index.php/s/tWXvtNIluTCTZv8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research in this manuscript did not involve human participants and/or animals. All authors of this manuscript were informed and agreed for submission.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1206 kb)
Rights and permissions
About this article
Cite this article
Leonhardt, J., Teutenberg, T., Buschmann, G. et al. A new method for the determination of peak distribution across a two-dimensional separation space for the identification of optimal column combinations. Anal Bioanal Chem 408, 8079–8088 (2016). https://doi.org/10.1007/s00216-016-9911-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9911-3