Abstract
A method based on stop-flow two-dimensional liquid chromatography coupled with electrospray ionization mass spectrometry (2D LC-ESI MS) was established and applied to analyze triterpenoid saponins from the main root of ginseng. Due to the special structure of triterpenoid saponins (they contain polar sugar side chains and nonpolar aglycones), hydrophilic interaction chromatography (HILIC) and reversed-phase liquid chromatography (RPLC) were used for the two dimensions, respectively. A trap column was used to connect the two dimensions. The dilution effect, which is one of the main shortcomings of traditional comprehensive 2D LC methods, was largely avoided. The peak capacity of this system was 747 and the orthogonality was 56.6 %. Compared with one-dimensional HILIC or RP LC MS analysis, 257 and 185 % more mass spectral peaks (ions with intensities that were higher than 1,000) were obtained from the ginseng main root extracts, and 94 triterpenoid saponins were identified based on MSn information and summarized aglycone structures. Given its good linearity and repeatability, the established method was successfully applied to classify ginsengs of different ages (i.e., years of growth), and 19 triterpenoid saponins were found through statistical analysis to vary in concentration depending on the age of the ginseng.

Process of saponin's analysis using stop-flow 2D LC method
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panax ginseng is one of the most valuable traditional Chinese medicines (TCMs), and it plays important roles in, for example, the pharmaceutical industry and disease treatment [1, 2]. It grows mainly in eastern Asia, e.g., in northeastern China, Korea, and Japan, as well as in North America, where it is known as Panax quinquefolium. The roots (which contain ginsenosides, organic acids, vitamins, sterols, etc.) are the most active part of the plant from a medical perspective, and have been used in TCMs for thousands of years. Triterpenoid saponins, the main components of secondary metabolites in many TCMs (including Panax ginseng), are considered to be bioactive molecules with many pharmacological functions, such as antimicrobial, anticancer, and antioxidant properties [2–4]. Triterpenoid saponins are glycosides with triterpenoid aglycones and sugar moieties that are mainly composed of hexose, deoxyhexose, and pentose.
Favorable separation aids the identification and preparation of active components. Many separation methods have been applied to triterpenoid saponins, such as thin-layer chromatography (TLC), capillary electrophoresis (CE), gas chromatography (GC), and liquid chromatography (LC) [5–7]. In practice, reversed-phase LC (RPLC) is the favored option for separating triterpenoid saponins due to its good reproducibility [1], while hydrophilic interaction chromatography (HILIC) is also employed to separate triterpenoid saponins because of their strongly polar sugar side chains [8]. However, satisfactory separation is rarely obtained using one-dimensional (1D) LC analysis because of the tremendous number of different triterpenoid saponins present in a sample and the extremely wide concentration distribution of those substances.
Two-dimensional liquid chromatography (2D LC) has gained increasing attention in relation to the separation of complex mixtures because of its high peak capacity and substantially enhanced resolution [9–14]. In recent years, several 2D LC methods for separating triterpenoid saponins have been established. Bankefors reported an off-line 2D LC method employing RP columns in both dimensions for analyzing the saponins from Q. saponaria Molina [15]. Xing et al. chose more orthogonal columns for the two dimensions and developed an off-line HILIC × RPLC method for triterpenoid saponin separation [16]. Twenty-one fractions were collected and concentrated to dryness in order to avoid the dilution effect. Finally, 224 saponins were found in Panax notoginseng. However, off-line approaches are time-consuming and labor-intensive. Recently, on-line 2D LC modes utilizing HILIC × HILIC and RPLC × RPLC have been used to separate saponins from Quillaja saponaria and Radix platycodi, respectively [17, 18], and these techniques showed greatly improved peak capacities. However, the orthogonality of these on-line comprehensive 2D LC methods needs to be improved, as similar separation mechanisms are used in the two dimensions. Furthermore, the dilution effect is an important and unavoidable drawback of comprehensive on-line 2D LC methods.
In the work reported in the present paper, we used an on-line stop-flow 2D LC method employing HILIC and RPLC columns in the two dimensions for triterpenoid saponin separation. The dilution effect was largely avoided in this comprehensive 2D LC method by using a trap column. The peak capacity, orthogonality, linearity, and repeatability of the method were investigated. A total of 94 triterpenoid saponins were identified based on MSn and aglycone structural information. Finally, the method was used to compare ginseng samples (in which saponins are regarded as the main bioactive components) of different ages.
Experimental
Reagents
HPLC-grade acetonitrile (ACN) and methanol were purchased from Merck (Darmstadt, Germany). Water was purified using a Milli-Q system (Millipore, Billerica, MA, USA). Acetic acid was obtained from TEDIA (Fairfield, OH, USA). Digoxin (United States Pharmacopeia Reference Standard), which was used as the internal standard, was purchased from Sigma–Aldrich (Munich, Germany) and dissolved in pyridine at a concentration of 1.8 mg/mL as a stock solution. Saponin standards of Rd, Rb1, Rg1, Rg2, Rg3, Re, Rc, R1, and Rh2 were produced by Cogan-chem Co., Ltd (Sichuan, China) and were used to prepare a 20-μg/mL standard mixture. This standard mixture was then diluted stepwise to 10,000, 5,000, 1,000, 500, 100, 20, 5, and 1 ng/mL.
Sample preparation
Roots of Panax ginseng with different ages (i.e., number of years of growth) were obtained from Shengan Co., Ltd. (Jilin, China). The main roots of Panax ginseng were first ground for 1 min at 25 Hz using a mixer mill instrument (MM400, Retsch, Haan, Germany). Quality-control (QC) samples were prepared by mixing together all of the ginseng main root samples. Briefly, 30 mg of powdered ginseng (from either an individual sample or a QC sample) were weighed accurately into an Eppendorf tube, and then triterpenoid saponins were extracted using 1.5 mL methanol/water (4/1, v/v) with a 3-μg/mL internal standard. After vortex-mixing for 5 min, ultrasonic extraction was carried out for an hour at room temperature and the resulting mixture was centrifuged at 14,000 rpm and 15 °C for 10 min. Then, 200 μL supernatant were transferred and freeze-dried under vacuum conditions. The dried residues were stored at −80 °C until use and redissolved in 1 mL methanol/water (4/1, v/v) for injection.
Instrumentation and conditions
Procedure for on-line stop-flow 2D LC analysis
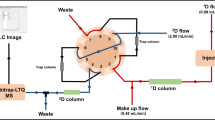
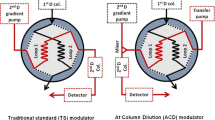
An on-line stop-flow 2D LC approach (as described in our previous work [19]) was employed to analyze triterpenoid saponins. The specific scheme is presented in Fig. 1. First, valves were switched to the trap position. The sample was injected into the first-dimension LC system and separated into different fractions. The fractions were then captured by the trap column in this position. Meanwhile, a make-up flow of water with 0.05 % acetic acid was introduced and mixed with the first-dimension outflow via a two-way eight-port valve (Valco Instruments Co. Inc., Houston, TX, USA) and a 100-μL mixer (Shimadzu, Kyoto, Japan) in order to reduce the strong elution of the first-dimension mobile phase. After gathering the components into one fraction, the valves were switched to the analysis position and the trapped compounds were transferred to the second-dimension RP LC system for further separation. During this procedure, the first-dimension LC was stopped and kept stable in an airtight system. After the second-dimension separation was finished, the valves were switched back to trap the next fraction. The abovementioned processes were repeated until all of the fractions had been completely separated.
Liquid chromatography conditions
Due to their good separation characteristics and—most importantly—their different separation mechanisms, a TSK-GEL Amide-80 column (2.0 × 150 mm, 3.0 μm, Tosoh, Tokyo, Japan) and an Acquity BEH C18 (2.1 × 100 mm, 1.7 μm, Waters Corp.) column were used in the two dimensions, respectively. An Acquity BEH C18 (2.1 × 5 mm, 1.7 μm, Waters Corp.) was used as the trap column. The first-dimension LC system consisted of a Shimadzu Prominence system containing a CBM-20A controller, a DGU-20A5 degasser, and two LC-20 AD pumps, as well as a SIL-30 AD autosampler. Water and ACN without any additive were used as the mobile phases A and B. The first dimension was operated at room temperature. The second-dimension LC system included two LC-30 AD pumps and a CTO-30A column oven. The flow rate of this dimension was 0.25 mL/min and the column oven temperature was maintained at 35 °C. Mobile phases C and D were H2O and ACN, both of which contained 0.05 % acetic acid, based on a reported study [20]. The components used in dilution flow were the same as those of mobile phase C. The make-up flow rate was 1 mL/min. The two separation systems were connected by a two-way ten-port valve (Valco Instrument Co. Inc.). Specific LC gradient conditions of the stop-flow 2D LC method are listed in Table 1.
One-dimensional HILIC and RP LC analyses were performed on a Shimadzu LC system containing a CBM-20A controller, a DGU-20A5 degasser, two LC-20 AD pumps, a SIL-30 AD autosampler, and a CTO-30A column oven. The columns were the same as those used in the stop-flow 2D LC. One-dimensional HILIC analysis was performed at ambient temperature. The flow rate was 0.15 mL/min. The gradient was as follows: 0–1 min, 10 % C; 1–23 min, from 10 to 50 % C; 23–25 min, equilibrate at 50 % C; 25–26 min, from 50 % back to 10 % C and re-equilibrate for 4 min. One-dimensional RP LC analysis was performed at 35 °C. The flow rate was 0.25 mL/min. The gradient was as follows: 0–1 min, 5 % D; 1–23 min, from 5 to 100 % D; 23–24 min, equilibrate at 100 % D; 24–24.5 min, from 100 % back to 5 % D, and re-equilibrate for 5.5 min.
Mass spectrometry conditions
An LTQ-Orbitrap XL mass spectrometer (Thermo Fisher Scientific, Rockford, IL, USA) was used as the detector. The MSn experiment was conducted in the ESI-positive (ESI +) mode with the following parameters: spray voltage 4.5 kV, capillary temperature 325 °C, capillary voltage 49 V, and collision energy 32 eV. The MSn procedure was performed in the linear ion trap. Precursor ions were trapped for collision-induced dissociation (CID) and then detected by a linear trap quadrupole (LTQ). Analysis of the standards and ginseng samples was performed in ESI-negative (ESI−) mode under the following conditions: spray voltage −4 kV, capillary temperature 325 °C, capillary voltage −40 V, resolution 15,000, and mass range for full-scan analysis m/z 450–1,500.
Statistical analysis
All of the samples with different ages (i.e., years of growth) were randomly arranged in a sequence for injection. Peak integration for the saponins identified in all of the samples was conducted using Xcalibur (Thermo Fisher Scientific) and the peak table was then exported. Next, the peak areas were normalized based on the internal standard and the new dataset was imported into the SIMCA-P 11.0 software (Umetrics, Umea, Sweden) for further analysis. Partial least squares discriminant analysis (PLS-DA) was first applied to generalize and visualize the dataset. Then, a Mann–Whitney U (nonparametric) test was carried out using the Statistical Package for the Social Sciences (SPSS, v.16.0, SPSS Inc., Chicago, IL, USA). The variables could be used to distringuish among classes if the variable importance plot (VIP) value in PLS-DA was greater than 1.0 and the p value was less than 0.05, and the corresponding triterpenoid saponins were then regarded as “differential metabolites” (i.e., these metabolites could be used to differentiate between samples of different ages).
Results and discussion
Separation of triterpenoid saponins using the stop-flow 2D LC method
Considering the strong polarity caused by sugar chains of triterpenoid saponins, HILIC was used in the first dimension of the stop-flow 2D LC system. Figure 2a shows that triterpenoid saponins were separated with good resolution on the Amide-80 column. During the stop-flow 2D LC analysis, the injected samples were divided into nine fractions according to their order of elution in the first-dimension analysis. Since the strongly organic phase in the effluent was incompatible with the C18 trap column that was used to gather saponins, an extra make-up flow was mixed with the first-dimension effluent to effectively reduce the organic-phase concentration. In this study, the highest percentage of organic phase in the first-dimension gradient was 90 % ACN. When the percentage of ACN was diluted to 11.7 %, all nine saponin standards were completely trapped and no saponin peaks were observed when the effluent of trap column was directly detected by MS. Therefore, the make-up flow rate was selected as 1.0 mL/min.
After one fraction from the first dimension was collected, the first-dimension analysis was stopped and the valves were switched to the analysis position (Fig. 1) in order to transfer the newly trapped component into the second-dimension column for further separation. Peak broadening in the second dimension and the dilution effect were avoided without decreasing sensitivity since the first-dimension solvent was eliminated when saponins were trapped in the trap column. The conventional column and the flow rate adopted in the second dimension also helped to reduce the dilution effect as compared with the comprehensive 2D LC method. Moreover, separation was significantly improved because a long (100 mm) C18 column packed with sub-2-μm particles was used in the second dimension. When the second-dimension analysis of the trapped fraction was finished, the first-dimension separation was restarted to produce the next fraction, and this process was performed until all nine fractions were completely analyzed. The TIC chromatogram was eventually acquired, as shown in Fig. 2b.
The same QC sample was analyzed by stop-flow 2D LC and a 1D LC mode. Compared with 1D HILIC or 1D RP LC analysis, 257 and 185 % more mass spectral peaks (ions with intensities that were higher than 1,000) were detected, respectively. The resolution of the stop-flow 2D LC was clearly better than that of the 1D LC. Furthermore, strongly polar interfering components were removed with the trap column, reducing ion suppression and enhancing the sensitivity of the stop-flow 2D LC method.
Peak capacity and orthogonality of the stop-flow 2D LC method
The attractiveness of the 2D LC method is that the high peak capacity and resolution enable a substantial improvement in the separation power for complex samples. The peak capacity of this stop-flow 2D LC method was calculated using a previous reported method [19]. Generally speaking, the total peak capacity (n c,t) equals the sum of the peak capacity of each fraction in the second dimension. The n c,t value of this stop-flow 2D LC method was found to be about 747.
The separation power of a 2D LC system is closely related to its orthogonality. In this study, before evaluating the orthogonality of the stop-flow 2D LC method, the retention time (t Ri) was normalized to 0–1 using Eq. 1 according to Gilar [21] (regardless of the absolute retention time and void spaces where no components of interest were eluted):
where t Rmax and t Rmin are the retention times of the most strongly and weakly retained saponins, respectively. The retention times in the second dimension were accumulated in fraction order in this study, and the parameter t Ri 2D equals the retention time of an identified saponin (t R) minus the corresponding valve time (t vf) when the first dimension is stopped and the second-dimension analysis continues; i.e.,
The orthogonality of the 2D LC method was then validated according to the geometric approach [22]. To create a grid in the 2D plot, the number of fractions (N f) was used as the grid number on the horizontal axis, and the grid number on the vertical axis was calculated with the following equation:
where N p is the number of peaks identified with the stop-flow 2D LC method. Finally, the fraction number of each identified peak was plotted on the horizontal axis and the normalized retention time of that peak (t Ri(norm) 2D), as calculated using Eqs. 1 and 2, was plotted on the vertical axis (see Fig. 3a). The orthogonality (O%) was then calculated via
where Σbins and Σbins(blank) are the number of bins occupied by the identified saponins when using this stop-flow 2D LC system and the nonorthogonal system, respectively, and P max is the number of bins that account for the total peak capacity of the 2D LC system. In order to obtain the parameter Σbins(blank), a nonorthogonal system with a C18 column in both dimensions was simulated as follows. First, all identified saponins were rearranged in order of t Ri(norm) 2D regardless of their actual fractions. The rearranged order of t Ri(norm) 2D was the same as the order of elution in 1D C18 column analysis because the same second-dimension gradient was used in all fractions in our stop-flow method. Then, these peaks were divided into nine segments according to the same time ratio used in the stop-flow 2D LC method and the nonorthogonal RP-RP 2D LC system was simulated. A scatter plot diagram was constructed with the same axis information and geometric approach employed as for the stop-flow 2D LC system (Fig. 3b). The orthogonality was calculated as 56.6 %, indicating that our stop-flow 2D LC method is appropriate for analyzing triterpenoid saponins.
Identification of triterpenoid saponins
The structures and molecular weights of the reported aglycones ([aglycone + Na]+) are summarized in Fig. 4, and these aglycones can be classified into three types based on the locations of the sugar moieties: type I is substituted at C-3 and C-20; type II is substituted at C-6 and C-20; type III is substituted at C-3 and C-6. The sugars commonly found in ginseng are hexose (glucose, galactose, etc.), pentose (arabinose, xylose, etc.), and deoxyhexose (rhamnose).
Nine triterpenoid saponin standards were used to optimize the MS parameters and explore the fragmentation behavior beforehand. Adduct molecular ions [M + Na]+ were often detected in ESI+ mode, whereas [M – H]− as well as [M + CH3COO]− ions were seen in ESI− mode. In the MSn experiment, the main fragment ions were formed upon the cleavage of the glycosidic bond. Because fragment ions were more abundant in the ESI+ mode than in the ESI− mode, the ESI+ mode was used for triterpenoid saponin identification.
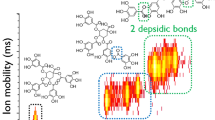
Three triterpenoid saponins of different types are used here to illustrate the fragmentation rules, based on their MSn information. Compound 80 is a type I saponin with [M + Na]+ at m/z 1131.6, and its related MSn spectra are shown in Fig. 5a. The labels Y, Z, and C shown in the structure are defined by Perreault and Costello [23]. Since the saccharide chain at C-20 position is easier to cleave than those at the C-3 and C-6 positions, the Z0α ion (α denotes a sugar moiety at C-20) at m/z 789.5, which is possibly formed by the neutral loss of a glucose-glucose residue, is observed as a base peak in the MS2 spectrum. The presence of a glucose-glucose side chain at the C-20 position is further verified by the appearance of a C2α ion at m/z 365.1. From the MS3 spectrum of the Z0α ion at m/z 789.5, the base peaks of the C2β ion (β denotes a sugar chain at C-3 or C-6) at m/z 365.1 and the Y1β ion at m/z 627.1 indicate a glucose-glucose chain at the C-3 position. The molecular weight of the aglycone can then be calculated as 483.2 Da, which primarily indicates an aglycone structure of type I-1. Combining all of this information, compound 80 was identified as ginsenoside Rb1, and this was confirmed by using the standard.
The specific structure and MSn information for compound 26, which is a type II saponin that presents a molecular ion at m/z 823.5, are shown in Fig. 5b. The base peak of the typical Z0α ion at m/z 643.5 in the MS2 spectrum reveals that there is a glucose at C-20. Unlike type I saponins, Z0β ions of type II saponins can appear together with C1β ions in the MS3 spectrum. For compound 26, the two ions of Z0β and C1β at m/z 463.4 and 203.0 indicate the presence of a glucose at C-6. As a result, compound 26 was characterized as ginsenoside Rg1 by calculating the molecular weight of aglycone (499.3 Da) and comparing the MSn spectra and retention time with those of the corresponding standard.
Due to the lack of a sugar moiety at the C-20 position, type III saponins show different MS2 spectra to type I and II saponins. However, glycosidic bond cleavage at C-3 and C-6 leads to similar fragmentation behavior for both type I and type II: the creation of Z0β and C1β ions when the glycosidic bond is broken at C-6, or the production of the C2β ion when the glycosidic bond is broken at C-3.
Malonic esterified triterpenoid saponins are unstable and tend to form [M + Na - 44]+ ions (by losing CO2) and [M + Na - 86]+ ions (through the further loss of CH2=CO) [24]. An example—compound 47, with an [M + Na]+ ion at m/z 1217.6—is given in Fig. 5c. In addition to the base peak at m/z 875.5 in the MS2 spectrum, which indicates a glucose-glucose side chain at C-20, the other ions at m/z 1173.6, 1131.6, 831.6, and 789.7 were characterized as [M + Na - 44]+, [M + Na - 86]+, [M + Na - 342–44]+, and [M + Na - 342–86]+, respectively. The Z0α ion at m/z 831.6 in the MS3 spectra of 1173.6 and 875.5 points to a glucose-glucose chain at C-20. The C2β ions at m/z 407.2 and 451.4 as well as the Y1β ion at m/z 627.4 in the MS3 spectra of 875.5 and 831.6 indicate the presence of a malonyl-glucose-glucose moiety at C-3 and a type I aglycone structure. The molecular weight of the aglycone, 483.2 Da, further suggests that the aglycone structure is of type I-1. Finally, compound 47 was preliminarily identified as malonyl-Rb1.
Based on the strategy described above, 94 saponins were ultimately identified in the QC samples in ESI+ mode; those saponins are listed in Table S1 of the ESM.
Evaluation of the stop-flow 2D LC method
Method evaluation and ginseng sample analysis were carried out in the ESI− mode because of the better MS responses of the saponins in this mode than in the ESI+ mode.
Standard mixtures at different concentrations were first used to investigate the calibration curves. As shown in Table 2, the saponin standards present good linearities across a wide range of concentrations, covering at least three orders of magnitude.
QC samples were analyzed at particular intervals during one day to explore the intra-day repeatability. Among the 94 identified saponins, the 79 saponins that occupied 97 % of the total area had relative standard deviation (RSD) values of <30 % (Fig. 6a). Experiments were performed on three continuous days to investigate the inter-day repeatability. The RSD values of the 74 saponins that occupied 96 % of the total area were <30 % (Fig. 6b). All of these results suggest that this stop-flow 2D LC method has good reproducibility and is appropriate for performing a profiling analysis of triterpenoid saponins.
Intra-day (a) and inter-day (b) repeatabilities of QC sample analysis using the stop-flow 2D LC-MS method. Each column shows the number of saponins in a certain RSD range. Each point connected by lines to other points presents the accumulated percentage of the area that is smaller than the corresponding RSD value on the horizontal axis
Comparison of Panax ginsengs of different ages
It is well recognized that the number of years that a ginseng has grown is linked to its pharmacological efficacy: older ginsengs are commonly thought to be of greater pharmaceutical value. Therefore, it is important to determine the differences between the constituents of ginsengs of different ages. In this study, the main root extracts of 1-, 2-, 3-, 5-, and 6-year-old ginsengs were analyzed using the stop-flow 2D LC method.
The statistical results from PLS-DA show that all of these ginseng samples can be classified into three classes based on age: 1-year-old ginsengs; 2- and 3-year-old ginsengs; and 5- and 6-year-old ginsengs (Fig. 7). Saponins with VIP >1 were considered to play an important role in classification and were further studied by two-class Mann–Whitney U (nonparametric) tests between 1-year-old ginsengs and 2- and 3-year-old ginsengs, 1-year-old ginsengs and 5- and 6-year-old ginsengs, and 2- and 3-year-old ginsengs and 5- and 6-year-old ginseng samples. Finally, 19 saponins with p < 0.05 were found to vary in concentrations depending on the age of the ginseng (i.e., they were defined as “differential components”), as shown in Fig. S1 of the ESM. The relative concentrations of most of these differential saponins increased with ginseng age, suggesting that those saponins may be related to the differences observed between ginsengs of different ages in terms of pharmaceutical activity. Further experiments are needed to confirm the pharmacological action of these triterpenoid saponins.
Conclusions
In this study, a stop-flow 2D LC method of separating triterpenoid saponins from ginseng extracts was established. The serious dilution effect associated with comprehensive 2D LC was avoided by using a trap column. HILIC with an Amide-80 column and RPLC with a C18 column were used in the two dimensions. The orthogonality of this system was 56.6 %, as calculated using a geometric approach, and the peak capacity of this stop-flow 2D LC method was 747.
The established stop-flow 2D LC method was successfully used to separate triterpenoid saponins. Ninety-four triterpenoid saponins were identified in the main root of ginseng using the ESI+ mode. The satisfactory linearity and reproducibility of this method showed that it was appropriate for performing a profiling analysis of triterpenoid saponins. Ginsengs of different ages were classified into three classes, and 19 differential triterpenoid saponins (i.e., saponins that varied in concentration depending on the number of years of growth of the ginseng) were found through statistical analysis. The results of this study demonstrate that the stop-flow 2D LC method can be used in the analysis of complex plant extracts.
References
Hu C, Wei H, Kong H, Bouwman J, Gonzalez-Covarrubias V, van der Heijden R, Reijmers TH, Bao X, Verheij ER, Hankemeier T, Xu G, van der Greef J, Wang M (2011) Linking biological activity with herbal constituents by systems biology-based approaches: effects of Panax ginseng in type 2 diabetic Goto-Kakizaki rats. Mol BioSyst 7(11):3094–3103
Wang W, Zhao YQ, Rayburn ER, Hill DL, Wang H, Zhang RW (2007) In vitro anti-cancer activity and structure–activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol 59(5):589–601
Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, Jurzysta M (2006) Antimicrobial activity of saponins from Medicago sp.: structure–activity relationship. Phytother Res 20(6):454–457
Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong ZY, Zhao HB, Cui J, Xun SF, Huang XL, Zhou Z, Wang SQ (2009) Ginsenoside Rg1 protects rat cardiomyocyte from hypoxia/reoxygenation oxidative injury via antioxidant and intracellular calcium homeostasis. J Cell Biochem 108(1):117–124
Vanhaelen-Fastré RJ, Faes ML, Vanhaelen MH (2000) High-performance thin-layer chromatographic determination of six major ginsenosides in Panax ginseng. J Chromatogr A 868(2):269–276
Tian Y, Lu Y, Xie J, Cheng Y, Qi R, Wu Y, Zhang S (2009) Rapid determination of ginsenoside Rg1, Re and Rb1 in ginseng samples by capillary electrophoresis. Anal Methods 1(3):203–207
Cui J-F, Björkhem I, Eneroth P (1997) Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B 689(2):349–355
Guo X, Zhang X, Guo Z, Liu Y, Shen A, Jin G, Liang X (2014) Hydrophilic interaction chromatography for selective separation of isomeric saponins. J Chromatogr A 1325:121–128
Giddings JC (1995) Sample dimensionality: a predictor of order–disorder in component peak distribution in multidimensional separation. J Chromatogr A 703:3–15
Wang Y, Wang J, Yao M, Zhao X, Fritsche J, Schmitt-Kopplin P, Cai Z, Wan D, Lu X, Yang S, Gu J, Haring HU, Schleicher ED, Lehmann R, Xu G (2008) Metabonomics study on the effects of the ginsenoside Rg3 in a beta-cyclodextrin-based formulation on tumor-bearing rats by a fully automatic hydrophilic interaction/reversed-phase column-switching HPLC-ESI-MS approach. Anal Chem 80(12):4680–4688
Donato P, Cacciola F, Sommella E, Fanali C, Dugo L, Dacha M, Campiglia P, Novellino E, Dugo P, Mondello L (2011) Online comprehensive RPLC × RPLC with mass spectrometry detection for the analysis of proteome samples. Anal Chem 83(7):2485–2491
Li Q, Lynen F, Wang J, Li H, Xu G, Sandra P (2012) Comprehensive hydrophilic interaction and ion-pair reversed-phase liquid chromatography for analysis of di- to deca-oligonucleotides. J Chromatogr A 1255:237–243
Kalili KM, Vestner J, Stander MA, de Villiers A (2013) Toward unraveling grape tannin composition: application of online hydrophilic interaction chromatography × reversed-phase liquid chromatography-time-of-flight mass spectrometry for grape seed analysis. Anal Chem 85(19):9107–9115
Wang Y, Lehmann R, Lu X, Zhao X, Xu G (2008) Novel, fully automatic hydrophilic interaction/reversed-phase column-switching high-performance liquid chromatographic system for the complementary analysis of polar and apolar compounds in complex samples. J Chromatogr A 1204(1):28–34
Bankefors J, Nord LI, Kenne L (2010) Multidimensional profiling of components in complex mixtures of natural products for metabolic analysis, proof of concept: application to Quillaja saponins. J Chromatogr B 878(3–4):471–476
Xing Q, Liang T, Shen G, Wang X, Jin Y, Liang X (2012) Comprehensive HILIC × RPLC with mass spectrometry detection for the analysis of saponins in Panax notoginseng. Analyst 137(9):2239–2249
Jeong EK, Cha HJ, Ha YW, Kim YS, Ha IJ, Na YC (2010) Development and optimization of a method for the separation of platycosides in Platycodi radix by comprehensive two-dimensional liquid chromatography with mass spectrometric detection. J Chromatogr A 1217(26):4375–4382
Wang Y, Lu X, Xu G (2008) Development of a comprehensive two-dimensional hydrophilic interaction chromatography/quadrupole time-of-flight mass spectrometry system and its application in separation and identification of saponins from Quillaja saponaria. J Chromatogr A 1181(1–2):51–59
Wang S, Li J, Shi X, Qiao L, Lu X, Xu G (2013) A novel stop-flow two-dimensional liquid chromatography–mass spectrometry method for lipid analysis. J Chromatogr A 1321:65–72
Liang Y, Guan T, Zhou Y, Liu Y, Xing L, Zheng X, Dai C, Du P, Rao T, Zhou L, Yu X, Hao K, Xie L, Wang G (2013) Effect of mobile phase additives on qualitative and quantitative analysis of ginsenosides by liquid chromatography hybrid quadrupole–time of flight mass spectrometry. J Chromatogr A 1297:29–36
Gilar M, Olivova P, Daly AE, Gebler JC (2005) Orthogonality of separation in two-dimensional liquid chromatography. Anal Chem 77(19):6426–6434
Liu Y, Xue X, Guo Z, Xu Q, Zhang F, Liang X (2008) Novel two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography, an excellent orthogonal system for practical analysis. J Chromatogr A 1208(1–2):133–140
Perreault H, Costello CE (1994) Liquid secondary ionization, tandem and matrix-assisted laser desorption/ionization time-of-flight mass spectrometric characterization of glycosphingolipid derivatives. Org Mass Spectrom 29(12):720–735
Kite GC, Howes MJ, Leon CJ, Simmonds MS (2003) Liquid chromatography/mass spectrometry of malonyl-ginsenosides in the authentication of ginseng. Rapid Commun Mass Spectrom 17(3):238–244
Acknowledgments
The study was supported by the National Basic Research Program (nos. 2012CB720801, 2012CB517506) from the State Ministry of Science and Technology of China, the Foundations (nos. 21175132 and 21275141) and the Creative Research Group Project (no. 21321064) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in the topical collection Multidimensional Chromatography with guest editors Torsten C. Schmidt, Oliver J. Schmitz, and Thorsten Teutenberg.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 321 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Qiao, L., Shi, X. et al. On-line stop-flow two-dimensional liquid chromatography–mass spectrometry method for the separation and identification of triterpenoid saponins from ginseng extract. Anal Bioanal Chem 407, 331–341 (2015). https://doi.org/10.1007/s00216-014-8219-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8219-4