Abstract
With the worldwide use of penicillin antibiotics comes the need for tighter controls. Bacterial resistance is a genuine problem and governmental and international bodies, for example the European Medicines Agency (EMA) and the World Health Organization (WHO), have designed strategies to overcome this unfortunate consequence of antibiotic use. Foodstuffs are monitored to ensure they contain very low quantities of antibiotics, so they are not prejudicial to health and the environment. Detection is based on chromatographic methods. However, screening can be performed by use of simpler, rapid methods of detection, e.g. microbial inhibition test, lateral flow assays, immunoassays, and use of biosensors, to reduce the final number of samples to be analyzed by chromatography. In this review, we have gathered information regarding all such screening methods for the penicillins and have critically assessed their capability and specificity for detection of penicillins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The antibiotic penicillin has been used to cure a wide range of microbial infections since its discovery by Alexander Fleming in 1928. Use of penicillins has become imperative in the fight against many infections, but, as broadcast in many advertisements (i.e. yoghurts), not all bacteria are “bad”. The overuse of antibiotics has become a genuine problem and there is increasing demand for reduction of their use.
Antibiotics have been used in veterinary medicine since shortly after their use in humans [1]. The main diseases treated today by antibiotics are mastitis, lung infections, and skin and organ abscesses. The most commonly used antibiotics are the β-lactams, for example penicillins or cephalosporins, tetracyclines, fluoroquinolones, aminoglycosides, macrolides, and sulfonamides. Antibiotics may be administered by injection (intravenous, subcutaneous, or intramuscular), orally in food and water, topically, and by intramammary or intrauterine infusions [2]. All these routes of administration may result in the appearance of these antibiotic residues in food, for example meat, eggs, and milk. To protect public health, pharmacologically active substances have been classified, on the basis of scientific assessment of their safety, in four Annexes to Council Regulation (EEC), no. 2377/90 of 26 June 1990, which stipulates a Community procedure for establishment of maximum residue limits (MRL) of veterinary medicinal products in foodstuffs of animal origin.
In most countries β-lactams are widely applied in mastitis therapy and are therefore the major reason for failures to satisfy dairy control requirements, at least, for inhibitory substances. Many studies show the accumulation of antibiotic residues in animal milk [3, 4]. Farm management is important in helping to minimize the use of antimicrobials. However, with increasing density of livestock there is a need for better disease control, which leads to heavy use of antibiotics, often only as a precaution, which is an illegal practice in the EU.
The presence of penicillin has been beneficial in some areas of food production. Penicillium moulds are found in Blue cheese (e.g. Penicillium camemberti and Penicillium roqueforti). Penicillium nalgiovense is used to improve the taste of sausages and hams and to prevent colonization by other moulds and bacteria. However, milk containing penicillin may affect yoghurt and cheese production. Yoghurt is produced by fermentation of milk by lactic acid bacteria (e.g. Lactobacillus bulgaris or Streptococcus thermophilus). It is known that penicillins affect this lactic acid production, a requirement in the production of dairy byproducts [5]. According to the European Medicines Agency, concentrations as low as 6 μg kg−1 penicillin in milk substantially inhibit starter cultures and delay acid production [6]. In dairy products of acceptable quality the concentration must not exceed 3 μg kg−1.
Penicillins administered to animals may be released into the environment via faeces and urine or via the run-off from topical medications. The Water Framework Directive (2000/60/EEC) [7] of the European Union does not include the penicillins in their list of substances to be detected but there is little doubt that their presence exists with so many being administered. Some recent papers have included the penicillins in their lists of possible contaminants in water [8, 9].
Although it is difficult to monitor water for every chemical substance that may contaminate it, it should be noted that antimicrobial agents are a frequent contaminant of surface waters, thus adding to the problem of bacterial resistance. Bacterial resistance is discussed later with regard to the penicillins but ultimately the indiscriminate use of antibacterials has led to the emergence of bacteria which have modified some aspect of their being to avoid death from these antibacterials. Many reports have included antimicrobials in their list of possible contaminants [10, 11]. Adverse drug reactions are undesirable reactions to drug treatment. The penicillin antibiotics are regarded as quite non-toxic, because they do not have the severe side-effects of other antibiotics (e.g. vancomycin may cause hearing loss and kidney damage). The most common side effects are nausea, vomiting, bad breath, and sometimes diarrhoea, but treatment may continue. However, it must be noted that another serious consequence of the presence of penicillins in milk is the risk of an allergic reaction. Immunological reactions of this type are classified as Type I hypersensitivity reactions and occur in people who have previously been exposed to the penicillin to which specific IgEs have been produced. When the person is re-exposed there is an abnormal immunological response and chemical mediators such as histamine, prostaglandins, leukotrienes, and kinins are released. Symptoms include rashes, hives, and shortness of breath. In serious cases anaphylactic shock may occur [12].
The information gathered in this review highlights the availability of a variety of tests for detection of penicillins. We also critically assess the performance of such tests. The principal idea is to enable the reader to see where future developments may lie while collecting information already known about penicillin detection. Although we wanted to discuss the detection methods available, we also find it imperative to give an overview of the types of penicillin and their uses, mechanisms of bacterial resistance, legislation regarding these antibiotics, etc., thus giving the reader a wide range of information on this topic.
Penicillins
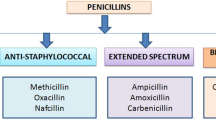
Penicillins are composed of a β-lactam ring attached to a thiazolidine ring, giving rise to 6-aminopenicillanic acid (6-APA). All penicillins have this core structure (Fig. 1). The penicillins can be classified into four main groups, on the basis of their ability to kill different types of bacteria—natural, penicillanase-resistant, aminopenicillins, and extended spectrum penicillins.
-
1.
The only natural penicillin is penicillin G, but modification of its structure has produced phenoxymethylpenicillins (e.g. penicillin V, phenethicillin, and propicillin). They are only administered intramuscularly.
-
2.
Penicillinase is an enzyme produced by some strains of bacteria which inactivates penicillin. Penicillinase-resistant penicillins are insensitive to the activity of this enzyme, which enables using them to fight against infectious caused by this type of resistant bacteria. They include oxacillin, nafcillin, cloxacillin, and dicloxacillin.
-
3.
Addition of an amine group to the 6-APA core gave rise to the aminopenicillins. They are acid-resistant and can be administered orally. Aminopenicillins include ampicillin and amoxicillin.
-
4.
Finally, the extended spectrum penicillins have the β-lactam backbone but feature a carboxylic acid or ester group in a variable side chain. They have similar activity to the aminopenicillins but have greater activity against Gram-negative bacteria. They are not included in legislation relating to veterinary products.
Mechanism of action
The peptidoglycan layer of Gram-positive bacteria is important for cell wall structural integrity. The final transpeptidation step in the synthesis of peptidoglycan is facilitated by transpeptidases known as penicillin-binding proteins (PBPs). Penicillins are analogues of d-alanyl-d-alanine. This structural similarity facilitates their binding to the active site of the PBPs. The β-lactam binds irreversibly to the active site. This prevents the final cross-linking of the peptidoglycan layer and disrupts cell wall synthesis (Fig. 2). Gram-positive bacteria have a simple structure which is permeable to polar molecules, for example the β-lactams. Gram-negative and mycobacteria contain pores through which penicillins may gain access. Components with a negative charge have more difficulty passing through. For penicillins to be bactericidal, it is necessary that the bacteria are growing and dividing. In this condition, loss of transpeptidation and the normal activity of wall hydrolases make the cell wall weaker and the bacteria are destroyed by osmotic lysis [13].
Bacterial resistance
Some bacteria have evolved to become resistant to the penicillins. The inappropriate use of antibiotics in human medicine, plus the residual presence of penicillins in foodstuffs resulting from abuse of these antibiotics to treat animals have led to a greater than expected bacterial resistance. The mechanisms of resistance of bacteria to β-lactams can be divided into three main mechanisms—production of β-lactamases, modification of PBP sites. and blockage of entry.
The production of β-lactamases
Production of β-lactamase enzymes is the most important mechanism of resistance in bacteria. The β-lactamases are enzymes that hydrolyse the β-lactam ring of the antibiotics and render them inactive (Fig. 3a). Penicillinase was the first β-lactamase to be identified, being isolated by Abraham and Chain in 1940 from Gram-negative E. coli even before penicillin began to be used clinically. Penicillinase expression quickly spread to bacteria that previously did not produce it or only produced it rarely. To fight these bacteria, penicillinase-resistant β-lactams, for example methicillin, were developed, but widespread resistance to this antibiotic was also observed before long.
Modification of penicillin-binding protein (PBP) sites
A common mechanism of resistance of Gram-positive bacteria is production of PBPs with less affinity for β-lactams (Fig. 3b). The most characteristic resistance by this mechanism is seen in Staphylococcus aureus toward methicillin. Methicillin has great affinity for PBP2 resulting in lysis of the cell. S. aureus strains may produce a new form of the protein called PBP2a which has lower affinity for methicillin, so these bacteria become resistant to penicillins. MRSA (methicillin resistant Staphylococcus aureus) is a quite prevalent health-care-associated infection (HAI) and, despite measures to reduce its occurrence, remains an important public health problem. More must be done to further reduce the risks of developing these infections The gene for PBP2a is only present in resistant strains of S. aureus, suggesting that the gene was acquired from another species of bacteria. The Enterococci have natural resistance to penicillins because one of their PBPs (PBP5) has lower affinity and, by producing more of this PBP5, they stop the binding of penicillins. The Pneumococci also have mutants which are resistant to β-lactams. They have altered up to three of their PBPs and thus the β-lactams have lower affinity for the proteins. The changes have been so numerous that it is believed that the strains have acquired a foreign gene. Similar activity occurs with Haemophilus and Neisseria.
Blocking of entry
The penicillins must gain access to the PBP by crossing the cytoplasmic membrane. This is easily achieved in Gram-positive bacteria. In Gram-negative bacteria, the pores may be used. Thus, by losing these pores or by modifying them, the Gram-negative bacteria have created a resistance mechanism. This form of resistance is seen in enterobacteria, for example Salmonella, Enterobacter, and Pseudomonas, and may be reversible.
Production and uses of penicillins
Natural penicillin—penicillin G—is produced by some strains of fungi, for example Penicillium chrysogenum (Fig. 4), Penicillium notatum, and Acremonium chrysogenum. Strains of these are grown in deep vats with precautions to prevent bacterial contamination. Phenylacetic acid is added and increases the yield of benzylpenicillin. The final product is a pure crystalline preparation. By the same method, i.e. by enriching the cultures with a specific chemical compound, other penicillanic acid derivatives can be prepared. It is also possible to modify benzylpenicillin after it has been synthesized by simple chemical reactions. Since 1959 it has been possible to synthesize many compounds by adding a variety of side chains to the penicillin nucleus—6-aminopenicillanic acid.
The β-lactam antibiotics account for over 65 % of the world antibiotic market [14]. The global anti-infectives market generated revenues of $79 billion in 2009. The future of the penicillins is uncertain because countries such as China and India are increasingly producing cheaper penicillins, whereas other countries have begun to lose interest. For example, GlaxoSmithKline has left the US market and has sold the rights of branded penicillin medications, for example Augmentin and Amoxil, to Dr Reddy’s Laboratories, an Indian generic drug producer. The global leader of penicillin products is DSM—Bright Science. Brighter Living.
The penicillins can be used against a wide range of infections by Gram-positive and, to a lesser extent, Gram-negative bacteria. They are usually the drug of choice for minor infections because they have little or no toxicity except for allergic reactions in some patients. With increasing bacterial resistance their use is being limited. The uses are many and vary among countries. The following are some common uses of amoxicillin in humans. Amoxicillin is used in the treatment and prevention of recurrent acute otitis media (AOM). It is used for treatment of skin infections caused by Streptococcus, Staphylococcus, or Escherichia coli and for treatment of pharyngitis and tonsillitis caused by Streptococcus pyogenes. Lower respiratory infections caused by susceptible Streptococcus (S. pneumonia), Staphylococcus, or Haemophilus influenzae, and urinary tract infections (UTIs) caused by Enterococcus faecalis, E. coli, or Proteus mirabilis are also treated with penicillins. Amoxicillin is highly recommended for pregnant women suffering Chlamydia trachomatis infection. It is an alternative treatment for gastroenteritis caused by non-typhoidal Salmonella. Amoxicillin is also used for treatment of Helicobacter pylori infection or duodenal ulcer disease. Antibiotics are not recommended for healthy individuals with uncomplicated gastroenteritis but when the disease is severe and the patient is at increased risk because of underlying conditions (e.g. HIV sufferers, severe atherosclerosis, vascular disease) treatment is recommendable and it can be administered.
Legislation regarding penicillins
As mentioned in the Introduction, use and misuse of antimicrobials in human medicine and animal husbandry over the past 70 years has led to a relentless rise in the number and types of microorganisms resistant to these medicines—leading to death, increased suffering, and disability, and higher healthcare costs. Combating antimicrobial resistance thus requires intervention of two types:
-
1.
improving antimicrobial use; and
-
2.
blocking transmission of resistant organisms.
Urgent and coordinated action is required at local, national, and international levels to ensure adequate treatment of patients and preservation of the life-saving power of antimicrobials for future generations. In this respect, since the late 1990s and 2000, the WHO has convened a series of consultative groups, expert workshops, and consensus meetings to assess the growing public health threat of antimicrobial resistance, to evaluate the effect of containment intervention, and to develop a series of recommendations for action. The culmination of this work was the publication in 2001 of the WHO Global Strategy for Containment of Antimicrobials in which distinct priorities are identified and recommendations addressed to distinct sectors, including patients and the general community, prescribers and dispensers, hospitals, antimicrobials use in food-producing animals, national governments and health systems, industry and research groups, and international organizations. Governmental agencies were to ensure a high level of human health protection. A comprehensive body of EU legislation has been put in place to achieve this objective. All of this legislation is publicly available and can be accessed via the European Commission’s EurLex website: http://eur-lex.europa.eu/en/index.htm. With regard to the safety of food, specific EU legislation is in place to prevent exposure of the population to residues of veterinary medicines, pesticides, and contaminants in food of animal origin The principal objective of the legislation is to detect illegal use of substances in animal production and the misuse of authorized veterinary medicinal products, and to ensure the implementation of appropriate actions to minimize recurrence of all such residues in food of animal origin. In this respect, the European Medicines Agency (EMA) is the body responsible of the protection and promotion of public and animal health, by evaluation and supervision of medicines for human and veterinary use, according to the EU legislation. One of most important regulations is Council Regulation 2377/90/EEC which gives the MRL allowed in foodstuffs. Thus, any analytical method including immunoassays must be able to detect below these levels. Table 1 shows MRLs of β-lactams in foodstuffs of animal origin.
Council regulation 2377/90/EEC [15]
All pharmacologically active substances which are used within the Community in veterinary medicinal products intended for administration to food-producing animals should be evaluated in accordance with regulation 2377/90/EEC. According to the EU, “residues of veterinary products” signifies all pharmacologically active substance whether active principles, excipients, or degradation products and their metabolites which remain in foodstuffs obtained from animals. The “maximum residue limit” (MRL) is defined as “the maximum concentration of residue resulting from the use of a veterinary medicinal product (expressed in mg kg−1) which may be accepted by the Community to be legally permitted or recognized as acceptable in or on food”. The limit is that without any toxic effect on human health and is measured as acceptable daily intake (ADI).
Council directive 96/23/EC [16]
Council Directive 96/23/EC sets out guidelines on measures to monitor specific substances and residues thereof in live animals and in animal products. Each member state designates at least one national reference laboratory. When the residues exceed the levels stipulated or illegal substances are detected, action must be taken out to ensure the safety of public health. There are two groups of substances—A and B. The penicillins are in Group B, and all animal foodstuffs are included in this group (e.g. bovine, poultry, milk, honey, etc).
Commission decision 97/747/EC [17]
This decision was made to provide further information on the correct procedure for sample taking. It includes the sampling methods for milk, eggs, and honey which were not included in Regulation 96/23/EC. Regarding milk samples, the average number of samples is 1 per 15,000 tonnes of the annual production of milk, with a minimum of 300 samples. This is a substantial number of samples, thus increasing the need for faster and more cost-effective tests for their detection. Each sample is taken by official competent authorities in such a way that it is always possible to trace it back to the farm of origin.
Commission decision 2002/657/EC [18]
This decision was made for “implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results”. The analytical methods accepted by the EU are based on chromatographic and/or spectrometric techniques. However, the EU has stated that regulatory laboratories must find the best analytical techniques for the detection of pharmacological substances and therefore it is not unlikely that other methods will have a place in future, if their efficiency can be proven.
The maximum residue limits for different countries can be seen in the Table 2. The European Union is stricter than most countries (e.g. the MRL for amoxicillin in USA is 10 μg kg−1 compared with 4 μg kg−1 for the EU). Also, the EU legislation includes more penicillins. Therefore, tests capable of detecting the penicillins at EU stipulated levels could be used all over the world.
Current detection methods
Bacteria have developed effective ways of reducing the efficiency of the antibiotics but it must be stated that the penicillins are relatively stable in food samples. Therefore the following methods of detection will not be hindered by instability of the molecule. For example, heat inactivation of the β-lactams occurs, but only under extreme conditions, e.g. 120 °C for 20 min caused degradation of 46.7 % for amoxicillin and 84 % for ampicillin in milk [19].
As already mentioned, the EU depends on chromatographic methods for detection and quantification of penicillin antibiotics. We will not discuss these methods here; a thorough review is available [20].
Capillary electrophoresis involves separating ionic species on the basis of their charge and frictional forces. Whereas in conventional electrophoresis electrically charged substances move in a conducting liquid medium under the action of an electric field, during capillary electrophoresis substances are separated on the basis of their size to charge ratio in the interior of a small capillary filled with an electrolyte. Once separated, the substances can be detected by a variety of means. For the penicillins, methods using UV–visible detection for water samples [21] and milk samples [22], or mass-spectrometry for fish samples [23] have been developed.
Molecularly imprinted polymers (MIPs) are polymers produced from the monomers in the presence of a template molecule that is later extracted, leaving specific cavities behind. These polymers have affinity for the original molecule and can be used for their detection. Their affinity for the molecules is lower than that of antibodies but they are easy to prepare and are inexpensive. Quantification can be achieved by competing with labelled analytes. The signal is inversely proportional to the analyte in the sample. Urraca et al. developed one such method for the β-lactams using fluorescently labelled competitors [24], and Wan et al. developed a chemiluminescence assay [25]. Other researchers have only developed MIPs for extraction of the antibiotics and later perform liquid chromatography using polymer-coated columns with mass spectrometry for quantification of the antibiotic [26]. In some regards the MIP are similar to immunoassays or receptor-based assays in that the analyte competes for a receptor but, strictly speaking, are not “bio”analytical because detection is achieved by use of polymers.
Because these are not bioanalytical methods we feel no need to discuss them in detail in this review.
Rapid tests
A list of the rapid tests currently available within Europe can be seen in the Bulletin of the International Dairy Federation, in which commercial assays are discussed in detail [27]. The tests include microbial inhibition tests, receptor-based lateral flow assays, solid phase immunoassays, and radio-labelled assays. Only those applicable to β-lactam antibiotics will be discussed.
Microbial inhibition tests
In 1982 Messer et al. [28] developed a microbial inhibition test for detection of β-lactams. Nowadays, these tests are commonly used to screen for different antibiotics present in milk and other matrices. Spores of bacteria are kept in an agar gel matrix containing nutrients and a pH indicator. The milk is added and the gel is incubated at the appropriate temperature so the spores germinate and grow. The bacteria produce acid while growing and therefore there will be a colour change (Fig. 5a). The strain most often used is Bacillus stearothermophilus, but some companies, for example Valio, have developed their own strains (e.g. Streptococcus thermophilus T101 strain). The indicators used are bromocresol purple and brilliant black which change from purple/blue to yellow. For detection of penicillins in animal tissue there are also agar plate assays. In these, inhibition is seen on plates which have been coated with bacteria such as Bacillus subtilus, Bacillus stearothermophilus, and Micrococcus luteus. The control plate is made with a disc containing another antibacterial (e.g. neomycin). This is to ensure that the bacteria are capable of growing. Often the samples are added without pretreatment, just liquidising. Some tests can be performed for live animals by taking urine and serum samples.
These tests are regarded as rapid but take from 3 to 24 h to perform and require incubators. The strains used have to be constantly monitored to ensure that they have not become resistant to the antibacterials. Interpretation of the results is subjective and may lead to false negatives or positives. The existence of natural inhibitors in abnormal milk (mastitis, colostrums) can be the cause of false positive results. Tests like these would be welcome in slaughterhouses, because they are relatively inexpensive and many can be performed together depending on the availability of incubators. For milk, these tests should be performed at the farm and not in the factories. It would be much more costly to throw all of the bulked milk away rather than a single container of milk obtained on the farm. The European Medicines Agency summary report for penicillins in 2008 stated that the microbial inhibition test using Bacillus stearothermophilus and the four-plate method were suitable for screening of milk and edible tissues respectively [6]. Table 3 shows commercially available microbial inhibition assays.
Lateral flow assays
Lateral flow tests are a simple devices used to detect analytes in a sample. They are often produced in a dipstick format. Lateral flow tests are a form of immunoassay in which the test sample flows along a solid substrate by capillary action. After the sample has been applied to the test it encounters a coloured reagent which mixes with the sample and moves the substrate, encountering lines or zones which have been pretreated with an antibody or antigen. Depending on the analytes present in the sample the coloured reagent can become bound at a test line or zone. Many coloured particles exist, but latex (blue colour) or nanometre sized particles of gold (red colour) are commonly used. The gold particles are red in colour because of localised surface plasma resonance. Fluorescent or magnetically labelled particles can also be used but require electronic readers to assess the test result (Fig. 5b).
Alternatives to lateral flow-based assays are microplate assays in which the receptor protein is immobilised on a microplate. Lamar et al. developed one such assay. The penicillin binding protein PBP 2x* was used for detection of penicillins in milk, bovine and porcine muscle, juice, honey, and egg [29].
An advantage of these tests is that they require little time and experience. They are, however, less sensitive than other techniques. The tests are selective for the family of penicillins, but they are not specific, they do not differentiate between compounds. If a patient has had a reaction to a food-product it is important to know the particular compound that has caused the reaction. For quantitative results special readers are needed. Table 4 shows some commercially available screening tests for detection of penicillins in milk. A variety of other assays are available but we have placed here one from each of the largest manufacturers. They are some of the most popular (Unisensor and BetaStar) because they are rapid and can test for different analytes at the same time. They all require incubators and special readers for quantification.
Immunoassays
Immunoassays are tests used for detection of substances based on the principle that an antibody binds to the substance it has been raised against. Therefore a labelled antibody can be monitored to see if it detects its antigen. The signal comes from the labelled antibody or a secondary reagent specific for the antibody. The non-competitive assays are usually used for high-molecular-weight molecules (e.g. proteins). The sandwich assay is the most popular format, and two different specific antibodies are required, one of them containing the marker. An increase in signal is directly proportional to the amount of analyte present.
For low-molecular-weight analytes competitive immunoassays usually have to be developed. Two main formats are used, direct and indirect competitive assays. In the direct format, equilibrium is established between the antibody bound to the solid surface, the analyte, and the competitor tracer (analogue of the analyte linked to an enzyme) which are in solution. After the main incubation step, the unbound reagents are washed away and the amount of label bound to the solid phase by the antibody is measured. A decrease in the signal is directly proportional to the amount of analyte present. In the indirect format, the antigen is bound to the surface. The analyte is added with the antibody and the concentration is indirectly proportional to the number of antibodies attached to the antigen.
Immunoassays can also be divided on the basis of the label used. There are five types—radioimmunoassay, enzyme, fluorescent, luminescent, and magnetic immunoassay. In the scope of this review, neither magnetic immunoassays nor luminescent immunoassays have been developed for penicillins. Radioimmunoassays use antibodies or antigens labelled with a radioactive substance, often iodine, but this is not a commonly used technique nowadays, because of safety concerns. A review of radio-labelled immunoassays for antibiotics published by Broughton and Strong in 1976 [30]. It outlines the problems associated with penicillin assays. Charm Sciences have a test incorporating a liquid scintillation counter and luminometer for detection of the β-lactams. It is highly specific and rapid (10 min for milk, 60 min for tissue extracts) but there are concerns over safety. It can only be performed by laboratory personnel.
Fluorescent immunoassays (FIA)
Fluorescent immunoassays (Table 5) may use fluorescent compounds, for example cyanine dyes (Cy2, Cy3, Cy5), rhodamine, or phycobiliprotein from algae. Benito-Peña et al. [31] developed an assay for detection of ampicillin using a PAAP-Ab complex, by raising antibodies against the core 6-aminopenicillanic acid (6-APA) structure. They were able to reach an IC50 of 30 ng mL−1. Parallux (Medexx) developed a 4-min FIA for detection of the β-lactam antibiotics using a europium chelator (Eu3+). It has a detection limit of 2.3 ng mL−1, lower than the MRL. Nevertheless, these assays have many limitations. The choice of label is important. The quantum yield of the fluorophore must be high enough because it is not possible to amplify the signal. Sometimes the assays require expensive equipment and experienced operators.
Enzyme-linked immunosorbent assays (ELISA)
The labels used in ELISAs are enzymes which react with an appropriate substrate, producing a chromogen that absorbs in the visible range. The enzymes are coupled to the reactants as indicators or labels. The most commonly used enzymes are horseradish peroxidase (HRP), glucose oxidase (GO), alkaline phosphatase (AP), and β-galactosidase (βG). ELISAs have been developed for detection of penicillins in milk. Many are either not sufficiently sensitive or are unspecific. Table 5 gives an outline of the results obtained. In 2001, Cliquet et al. [32] described how ampicillin was coupled to different carrier proteins (BSA, ovalbumin, thyroglobin). Different coupling methods were used—two used cross-linkers, one used a carbodiimide-mediated coupling method, and one was without cross-linker or mediator. Monoclonal antibodies were produced. The specificity and affinity of these antibodies were demonstrated by inhibiting their binding with a 10 mmol L−1 solution of ampicillin. More than 10 mmol L−1 ampicillin was needed to obtain 50 % competition in the inhibition ELISA. Samsonova et al. [33] produced polyclonal antibodies against ampicillin-BSA immunogen and developed an indirect immunoassay for the detection of ampicillin in the range 10–1000 ng mL−1 in milk. The antibodies were specific for ampicillin and had low cross-reactivity with other penicillins. In 2010, Zhang et al. [34] developed an immunoassay for detection of benzylpenicilloic acid in milk. This compound is a degradation product of penicillin G that may be found in milk when it has been spiked with β-lactamases. This strategy avoids the direct detection of penicillin G. The same methodology was used by Kress et al. for analysis of a hydrolysed penicillin G derivative in goat milk. The limit of detection was 1–2 ng mL−1 [35]. This idea may have arisen from an earlier paper by Grubelnik et al. who, by producing antiserum against the hydrolysed form of the β-lactam antibiotics, detected penicillin G at a level of 0.05 ng mL−1. However, the ELISA required addition of penicillanase to the assay. As mentioned by Grubelnik, “specific and sensitive antibodies against β-lactam antibiotics are difficult to raise due to the chemical reactivity of the β-lactam ring” [36]. Strasser et al. [37] raised antibodies against an ampicillin–BSA conjugate, obtaining an EC50 of 2–4 ng mL−1. There was high cross-reactivity which was removed after hydrolysis of the antibiotics. Another example has been published by Fitzgerald et al. [38], who developed a direct competitive immunoassay that could detect 11 different β-lactams with limits of detection below European MRLs.
It is apparent all these enzyme-linked immunoassays have had to alter the structure of the β-lactams to produce antibodies with sufficient specificity. To conclude, there are no highly sensitive ELISAs for detection of penicillins, especially the aminopenicillins which are the most commonly used worldwide. It seems that penicillin immunogens are unstable, probably because of opening of the β-lactam ring. It is likely that in the next immunogen generation, haptens, will be designed to be sufficiently stable to resist degradation by host animals. This could be achieved by changing the nitrogen of the β-lactam ring for a carbon atom or any other heteroatom (phosphorus or silicon) except nitrogen. Nevertheless, for the moment it is apparent that it is difficult to develop immunoassays against penicillins.
Biosensors
Biosensors contain biological species that interact with the analyte in close contact with a physical transducer which senses the physicochemical change after the interaction. Thus, the biological response is converted into an electrical signal that is amplified, stored, and quantified by use of a specially designed processor. Biosensors may be classified according to the biological element (e.g. enzymatic or receptor–ligand) or according to the transducer used (e.g. amperometric, optical, piezoelectric, etc.). Biosensors are rapidly being developed for detection of a variety of analytes. Most have been set up solely as a proof of concept, and they have not been employed in further applications. Biosensing devices have been developed for detection of penicillin antibiotics by use of biosensors (Table 6). We have also included some sensors that detect penicillins but do not have a biological element [39–41], but rather use the fact that the penicillins oxidise Fe(III) to Fe(II) which can be measured by the sensor.
With regard to the biological element used on the biosensor, enzymes are highly specific and sensitive but may be difficult to use because their instability. Enzymes require co-factors, are difficult to purify, and are expensive. As an example, Setford et al. developed an enzymatic biosensor using glucose oxidase and an amperometic transducer for detection of β-lactam residues in milk [42]. Antibodies are the most common type of biological element used. They may be highly specific, are user-friendly, and do not require complicated steps. The main problem is that antibodies against penicillins (e.g. aminopenicillins) with very good sensitivity have not yet been prepared.
Optical biosensors
Optical biosensors work on the basis of surface plasmon resonance (SPR) and evanescent wave techniques. For example, a thin layer of gold on a high-refractive-index glass surface can absorb laser light, producing electron waves (surface plasmons) on the gold surface. This occurs only at a specific angle and wavelength of incident light and is highly dependent on the surface of the gold, such that binding of a target analyte to a receptor on the gold surface produces a measurable signal. Other optical biosensors are mainly based on changes in absorbance or fluorescence of an appropriate indicator compound and do not need total internal reflection geometry. A widely used research tool, the microarray, can also be regarded a biosensor. Microarrays are “lab-on-a-chip” tests in which the target analyte is coated to a solid surface (e.g. silicon or a glass slide) and can be detected by the receptor. Biacore has developed biosensors for detection of penicillins. They are based on the ability of microbial proteins to bind to the penicillins, which in turn inhibits carboxypeptidase activity. The advantage is that only intact β-lactam structures are detected. The interaction creates surface plasmon resonance that may be quantified. This was described by Gustavsson et al. [43], who achieved a limit of detection for penicillin G of 3.6 μg kg−1. Another type of biological element used is the antibody. Cliquet et al. produced monoclonal and polyclonal antibodies against ampicillin. By applying this to biosensors they were able to develop an optical biosensor whereby interaction of the immobilized ampicillin with the antibody could be quantified. The results were compared with ELISA and were shown to be more sensitive. The limit of detection was 10 ng mL−1 [44].
Gaudin et al. described a biosensor-based immunoassay for screening of milk for penicillin residues. However, this was used to screen for open-ringed structures, requiring enzymatic or chemical pretreatment of the samples. In that work, the biosensor did not reach the MRL [45]. A portable wavelength-interrogated optical system (WIOS) exploited class-selective bioreceptors for simultaneous screening of the most frequently used veterinary antibiotics (e.g., sulfonamides, fluoroquinolones, β-lactams and tetracyclines) [46]. The label-free sensor uses the evanescent-wave principle, by which changes in the refractive index close to the modified chip surface were detected by scanning the resonance condition at which a light wave is coupled in the waveguide through a conveniently designed grating. The bioreagents used were developed to detect a wide range of antibiotics below the MRL values established for milk samples. Finally, another example of an optical biosensor was produced by Jiang et al. [47]. Their paper reports a sensitive and selective immuno-nanogold resonance-scattering spectral assay developed for determination of trace hapten penicillin G, based on the resonance scattering (RS) effect of nanogold at 560 nm. Formation of the nanogold-labelled immunocomplex increased on addition of penicillin G. The enhanced RS intensity at 560 nm Delta I(RS) was linear for penicillin G concentrations in the range 7.5–1700 ng mL−1, with a detection limit of 0.78 ng mL−1. These results indicate that the immunonanogold-labelled RS spectral assay has high specificity and sensitivity for quantitative determination of penicillin G in raw milk samples.
Electrochemical biosensor
Electrochemical biosensors are often based on enzymatic catalysis of a reaction that produces or consumes electrons (redox enzymes). The sensor substrate usually contains three electrodes—a reference electrode, a working electrode, and a counter electrode. The analyte is involved in a reaction that occurs on an active electrode surface, and the reaction may cause either electron transfer across the double layer (producing a current) or contribute to the double layer potential (producing a voltage). The current (rate of flow of electrons is proportional to the analyte concentration) is measured at a fixed potential, or the potential can be measured at zero current (this gives a logarithmic response). Setford et al. [42] described an assay whereby screen-printed devices, incorporating working electrode immobilised β-lactam-specific receptor binding protein, were used to measure penicillin G levels in milk. Quantification was achieved by use of an ELISA-based affinity-assay format coupled to amperometric determination of bound enzyme label activity. The receptor binding protein was specific for the major β-lactam antibiotics.
Conclusions
Let us review the information we have gathered. To critically assess these detection methods we must determine what qualities we are seeking in a rapid detection method. Evidently, that they should be performed rapidly is important. In this regard, lateral flow assays are a good choice. They are performed within minutes and are user-friendly, because interpretation of the results is easy. Therefore, these tests are suitable for use on site. Nevertheless, for meat products, previous preparation steps are required, reducing the practicality of such a method. They are only semi-quantitative and not selective, meaning they do not differentiate between different β-lactams.
Microbial inhibition tests are less expensive but require the initial cost of buying an incubator. However, they can be easily performed by farmers on site. Results are seen within a few hours. These tests would be a good choice on dairy farms. For meat products, the agar plate assays could be performed in large slaughterhouses, with the capacity to perform many tests at once, but have little value for the individual farmer. Sample preparation is simple—rapid liquidisation of the meat is all that is required. Microbial inhibition tests are the only rapid tests recognized by the European Union as screening methods for penicillins [6]. The main problem associated with these tests is that they sometimes give false positives, because they are, of course, not selective among antibiotics.
Immunoassays are good screening methods for detection of analytes because they can be highly specific. For penicillins, sufficient specificity has not yet been achieved. Researchers have not been able to design immunogens capable of producing antibodies sufficiently selective against the β-lactams. These tests can be developed and performed rapidly once this has been overcome.
Biosensors are an interesting new means of detection. By applying both biological and physicochemical elements analytes can be detected and quantified. This quantification is important, because it enables the user to eliminate false positives and negatives. Biosensors based on receptor-ligand detection would be a better choice, because antibody–antigen assays for penicillins are difficult to design. The best option would be a biosensor that takes advantage of the fact that penicillin binds to penicillin-binding proteins (PBP). Biosensors are very expensive to design. The need for a biosensor for detection of penicillins could only be justified by a large factory (e.g. in the production of yoghurts or cheese or mass production of meat products), and even then it would be a very expensive choice. A few biosensors are commercially available e.g. for detection of glucose by diabetes sufferers, and most papers on biosensors deal with those. For the moment it would seem that the biosensors are not feasible for detection of antibiotics. Their greatest advantage is that the results can be quantified.
To conclude, all of the aforementioned tests have their own uses, depending on the type of detection required. Further development within these fields is of utmost importance. Although each has limitations researchers continue to look for ways of overcoming them and we are looking toward better screening of the penicillins in food products.
References
Foley EG, Lee SW, Epstein GA (1946) The effect of penicillin on staphylococci and streptococci commonly associated with bovine mastitis. Milk Food Technol 8:5
Mitchell JM, Griffiths MW, McEven SA, McNab WB, Yee J (1998) Antimicrobial drug residues in milk and meat: causes, concerns, prevalence, regulations, tests, and test performance. Food Production 61:15
Yamaki M, Berruga MI, Althaus RL, Molina MP, Molina A (2004) Occurrence of antibiotic residues in milk from Manchega Ewe Dairy Farms. J Dairy Sci 87(10):3132–3137
Thavarungkul P, Dawan S, Kanatharana P, Asawatreratanakul P (2007) Detecting penicillin G in milk with impedimetric label-free immunosensor. Biosens Bioelectron 23(5):688–694
Wilkowske HH, Krienke WA (1951) Influence of penicillin on the lactic acid production of certain lactobacilli. J Dairy Sci 34(10):1030–1033
Agency EM (2008) Committee for veterinary medicinal products penicillins summary report. European Medicines Agency, London
Water Framework Directive 2000/60/EC (2000) Off J Eur Communities
Richardson SD (2003) Water analysis: emerging contaminants and current issues. Anal Chem 75(12):2831–2857. doi:10.1021/ac0301301
Hirsch R, Ternes T, Haberer K, Kratz K-L (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225(1–2):109–118
Campagnolo ER, Johnson KR, Karpati A, Rubin CS, Kolpin DW, Meyer MT, Esteban JE, Currier RW, Smith K, Thu KM, McGeehin M (2002) Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci Total Environ 299(1–3):89–95
Mojica ERE, Aga DS (2011) Antibiotics Pollution in Soil and Water: Potential Ecological and Human Health Issues. In: Jerome ON (ed) Encyclopedia of Environmental Health. Elsevier, Burlington, pp 97–110
Thong BYH, Tan T-C (2011) Epidemiology and risk factors for drug allergy. Br J Clin Pharmacol 71(5):684–700
Mediavilla A, Garcia-Lobo JM (2001) Antibioticos [Beta]-lactamicos. In: Farmacologia Humana. MASSON, S.A., pp 1085–1106
Elander RP (2003) Industrial production of [beta]-lactam antibiotics. Appl Microbiol Biotechnol 61:8
Council Regulation 2377/90/EC laying down a community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin (1990) Off J Eur Communities
Council Directive 96/23/EC on measures to monitor certain substances and residues thereof in live animals and animal products and repealing Directives 85/358/EEC and 86/469/EEC and Decisions 89/187/EEC and 91/664/EEC (1996) Off J Eur Communities
Commission Decision 97/747/EC fixing the levels and frequencies of sampling provided for by Council Directive 96/23/EC for the monitoring of certain substances and residues thereof in certain animal products (1997) Off J Eur Communities
Commission Decision 2002/657/EC implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (2002) Off J Eur Communities
Roca M, Villegas L, Kortabitarte ML, Althaus RL, Molina MP (2011) Effect of heat treatments on stability of β-lactams in milk. J Dairy Sci 94(3):1155–1164
Kantiani L, Farré M, Barceló D (2009) Analytical methodologies for the detection of β-lactam antibiotics in milk and feed samples. Trends Anal Chem 28(6):729–744
Bailon-Perez ML, Garcia-Campana AM, Cruces-Blanco C, Iruela MD (2008) Trace determination of P-lactam antibiotics in environmental aqueous samples using off-line and on-line preconcentration in capillary electrophoresis. J Chromatogr A 1185(2):273–280
Santos SM, Henriques M, Duarte AC, Esteves VI (2007) Development and application of a capillary electrophoresis based method for the simultaneous screening of six antibiotics in spiked milk samples. Talanta 71(2):731–737
Juan-Garcia A, Font G, Pico Y (2007) Simultaneous determination of different classes of antibiotics in fish and livestock by CE–MS. Electrophoresis 28(22):4180–4191
Urraca JL, Moreno-Bondi MC, Orellana G, Sellergren B, Hall AJ (2007) Molecularly imprinted polymers as antibody mimics in automated on-line fluorescent competitive assays. Anal Chem 79(13):4915–4923
Wan FW, Yu JH, Dai P, Ge SG (2010) Molecular imprinting-chemiluminescent sensor for the determination of amoxicillin. Anal Lett 43(6):1033–1045
Zhang XP, Chen LG, Xu Y, Wang H, Zeng QL, Zhao Q, Ren NQ, Ding L (2010) Determination of beta-lactam antibiotics in milk based on magnetic molecularly imprinted polymer extraction coupled with liquid chromatography–tandem mass spectrometry. J Chromatogr B 878(32):3421–3426
Diserens JM, Beck Henzelin A, Le Breton MH, Savoy Perroud MC (2010) Current situation and compilation of commercially available screening methods for the detection of inhibitors/antibiotic residues in milk. Bulletin of the International Dairy Federation, vol 442. Int Dairy Fed
Messer JW, Leslie JE, Houghtby GA, Peeler JT, Barnett JE (1982) Bacillus stearothermophilus disc assay for detection of inhibitors in milk: collaborative study. J Assoc Off Anal Chem 65:1208–1214, Copyright (C) 2011 U.S. National Library of Medicine
Lamar J, Petz M (2007) Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal Chim Acta 586(1–2):296–303
Broughton A, Strong JE (1976) Radioimmunoassay of antibiotics and chemotherapeutic agents. Clin Chem 22(6):726–732
Benito-Pena E, Moreno-Bondi MC, Orellana G, Maquieira A, van Amerongen A (2005) Development of a novel and automated fluorescent immunoassay for the analysis of β-lactam antibiotics. J Agric Food Chem 53(17):6635–6642
Cliquet P, Cox E, Van Dorpe C, Schacht E, Goddeeris BM (2001) Generation of class-selective monoclonal antibodies against the penicillin group. J Agric Food Chem 49(7):3349–3355
Samsonova Z, Shchelokova O, Ivanova N, Rubtsova M, Egorov A (2005) Enzyme-linked immunosorbent assay of ampicillin in milk. Appl Biochem Microbiol 41(6):589–595
Zhang Y, Jiang Y, Wang S (2010) Development of an enzyme-linked immunosorbent assay to detect benzylpenicilloic acid, a degradation product of penicillin G in adulterated milk. J Agric Food Chem 58(14):8171–8175
Kress C, Schneider E, Usleber E (2011) Determination of penicillin and benzylpenicilloic acid in goat milk by enzyme immunoassays. Small Ruminant Res 96(2–3):160–164
Grubelnik A, Padeste C, Tiefenauer L (2001) Highly sensitive enzyme immunoassays for the detection of beta-lactam antibiotics. Food Agric Immunol 13(3):161–169
Strasser A, Usleber E, Schneider E, Dietrich R, Burk C, Martlbauer E (2003) Improved enzyme immunoassay for group-specific determination of penicillins in milk. Food Agric Immunol 15(2):135–143
Fitzgerald SP, O’Loan N, McConnell RI, Benchikh EO, Kane NE (2007) Stable competitive enzyme-linked immunosorbent assay kit for rapid measurement of 11 active beta-lactams in milk, tissue, urine, and serum. J AOAC Int 90(1):334–342
Duan H, Liu ZF, Liu SP, Yi A (2008) Resonance Rayleigh scattering, second-order scattering and frequency doubling scattering methods for the indirect determination of penicillin antibiotics based on the formation of Fe(3)Fe(CN)(6) (2) nanoparticles. Talanta 75(5):1253–1259
Khalilzadeh MA, Gholami F, Karimi-Maleh H (2009) Electrocatalytic determination of ampicillin using carbon-paste electrode modified with ferrocendicarboxylic acid. Anal Lett 42(3):584–599
Hu YF, Li JX, Zhang ZH, Zhang HB, Luo LJ, Yao SZ (2011) Imprinted sol–gel electrochemical sensor for the determination of benzylpenicillin based on Fe(3)O(4)@SiO(2)/multi-walled carbon nanotubes-chitosans nanocomposite film modified carbon electrode. Anal Chim Acta 698(1–2):61–68
Setford SJ, Van Es RM, Blankwater YJ, Kröger S (1999) Receptor binding protein amperometric affinity sensor for rapid β-lactam quantification in milk. Anal Chim Acta 398(1):13–22
Gustavsson E, Bjurling P, Sternesjö Å (2002) Biosensor analysis of penicillin G in milk based on the inhibition of carboxypeptidase activity. Anal Chim Acta 468(1):153–159
Cliquet P, Goddeeris BM, Bonroy K, Cox E (2005) Penicillin-specific antibodies: monoclonals versus polyclonals in ELISA and in an optical biosensor. Food Agric Immunol 16(2):101–115
Gaudin V, Fontaine J, Maris P (2001) Screening of penicillin residues in milk by a surface plasmon resonance-based biosensor assay: comparison of chemical and enzymatic sample pre-treatment. Anal Chim Acta 436(2):191–198
Adrian J, Pasche S, Voirin G, Pinacho DG, Font H, Sánchez-Baeza F, Marco M-P, Diserens J-M, Granier B (2009) Wavelength-interrogated optical biosensor for multi-analyte screening of sulfonamide, fluoroquinolone, β-lactam and tetracycline antibiotics in milk. Trends Anal Chem 28(6):769–777
Jiang Z, Li Y, Liang A, Qin A (2008) A sensitive and selective immuno-nanogold resonance-scattering spectral method for the determination of trace penicillin G. Luminescence 23(3):157–162
Odonnance du DFI du 26 juin 1995 sur les substances étrangères et les composants dans les denrées alimentaires, 817.021.23 (1995) Confederation Suisse
Food and Drugs. Chapter 1-Food and drug administration. Subchapter E – Animal drugs, feeds and related products part 556 (2011) US Food Drug Adm
Veterinary Drugs Directorate. Administrative Maximum Residue Limits (AMRLs) and Maximum Residue Limits (MRLs) set by Canada (2011) Health Canada
The Japanese positive list system for agricultural chemical residues in foods, Maximum Residue Limits (MRLs) of agricultural chemicals in foods (2006) Jpn Food Chem Res Found
Regulations Governing the Maximum Limits for Veterinary Medicine and Stock Remedy Residues that may be present in foodstuffs (2006) South African Government. Department of Health
New Zealand (Maximum Residue Limits of Agricultural Compounds) Food Standards 2005 (No. 2) (2011) New Zealand Food Safety Authority
Veterinary Drug Residue Limits in Foods. Taiwan Department of Health
Sierra D, Contreras A, Sánchez A, Luengo C, Corrales JC, Morales CT, de la Fe C, Guirao I, Gonzalo C (2009) Short communication: detection limits of non-β-lactam antibiotics in goat’s milk by microbiological residues screening tests. Journal of Dairy Science 92(9):4200–4206
Andrew SM, Frobish A, Paape MJ, Maturin LJ (1997) Evaluation of selected antibiotic residue screening tests for milk from individual cows and examination of factors that affect the probability of false-positive outcomes. J Dairy Sci 80(11):3050–3057
Carlsson, Björck L, Johnsson G (1992) The use of different microbial assays in combination with the Charm II test in the detection of antibiotic residues in herd milk. Int Dairy J 2(2):109–119
Stead SL, Ashwin H, Richmond SF, Sharman M, Langeveld PC, Barendse JP, Stark J, Keely BJ (2008) Evaluation and validation according to international standards of the Delvotest® SP-NT screening assay for antimicrobial drugs in milk. Int Dairy J 18(1):3–11
Okerman L, van Hoof J, Debeuckelaere W (1998) Evaluation of the European four-plate test as a tool for screening antibiotic residues in meat samples from retail outlets. J AOAC Int 81(1):51–56
Currie D, Lynas L, Kennedy DG, McCaughey WJ (1998) Evaluation of a modified EC Four Plate Method to detect antimicrobial drugs. Food Addit Contam 15(6):651–660
USDA (1979) A self instructional guide: performing the SWAB test (On Premises) for antibiotic residues. United States Department of Agriculture, Washington, DC
Koenen-Dierick K, De Beer JO (1998) Optimization of an antibiotic residue screening test, based on inhibition of Bacillus subtilis BGA, with experimental design. Food Addit Contam 15(5):528–534
Nouws JFM, Broex NJG, Den Hartog JMP, Driessens F (1988) The New Dutch Kidney Test. Arch Lebensmittelhyg 39:133–156
USDA (1994) Fast Antimicrobial Screen Test for detection of antibiotic and sulfonamide residues in livestock kidney tissue. A self-instructional guide. United States Department of Agriculture, Washington, DC
Abouzied M, Sarzynski M, Walsh A, Wood H, Mozola M (2009) Validation study of a receptor-based lateral flow assay for detection of beta-lactam antibiotics in milk. J AOAC Int 92(3):959–974
Žvirdauskienė R, Šalomskienė J (2007) An evaluation of different microbial and rapid tests for determining inhibitors in milk. Food Control 18(5):541–547
Bacigalupo MA, Meroni G, Secundo F, Lelli R (2008) Time-resolved fluoroimmunoassay for quantitative determination of ampicillin in cow milk samples with different fat contents. Talanta 77(1):126–130
Cliquet P, Goddeeris BM, Okerman L, Cox E (2007) Production of penicillin-specific polyclonal antibodies for a group-specific screening ELISA. Food Agric Immunol 18(3–4):237–252
Dietrich R, Usleber E, Martlbauer E (1998) The potential of monoclonal antibodies against ampicillin for the preparation of a multi-immunoaffinity chromatography for penicillins[dagger]. Analyst 123(12)
Kloth K, Rye-Johnsen M, Didier A, Dietrich R, Martlbauer E, Niessner R, Seidel M (2009) A regenerable immunochip for the rapid determination of 13 different antibiotics in raw milk. Analyst 134(7):1433–1439
Gustavsson E, Bjurling P, Degelaen J, Sternesjö A (2002) Analysis of beta-lactam antibiotics using a microbial receptor protein-based biosensor assay. Food Agric Immunol 14(2):121–131
Sternesjö A, Gustavsson E Biosensor analysis of beta-lactams in milk using the carboxypeptidase activity of a bacterial penicillin binding protein. J AOAC Int 89(3):832–837
Cacciatore G, Petz M, Rachid S, Hakenbeck R, Bergwerff AA (2004) Development of an optical biosensor assay for detection of β-lactam antibiotics in milk using the penicillin-binding protein 2x*. Anal Chim Acta 520(1–2):105–115
Knecht BG, Strasser A, Dietrich R, Märtlbauer E, Niessner R, Weller MG (2004) Automated microarray system for the simultaneous detection of antibiotics in milk. Anal Chem 76(3):646–654
Dawan S, Kanatharana P, Wongkittisuksa B, Limbut W, Numnuam A, Limsakul C, Thavarungkul P (2011) Label-free capacitive immunosensors for ultra-trace detection based on the increase of immobilized antibodies on silver nanoparticles. Anal Chim Acta 699(2):232–241
Poghossian A, Schöning MJ, Schroth P, Simonis A, Lüth H (2001) An ISFET-based penicillin sensor with high sensitivity, low detection limit and long lifetime. Sens Actuators B 76(1–3):519–526
Poghossian A, Yoshinobu T, Simonis A, Ecken H, Lüth H, Schöning MJ (2001) Penicillin detection by means of field–effect based sensors: EnFET, capacitive EIS sensor or LAPS? Sens Actuators B 78(1–3):237–242
Stred’anský M, Pizzariello A, Stred’anská S, Miertuš S (2000) Amperometric pH-sensing biosensors for urea, penicillin, and oxalacetate. Anal Chim Acta 415(1–2):151–157
Ferrini AM, Mannoni V, Carpico G, Pellegrini GE (2008) Detection and identification of beta-lactam residues in milk using a hybrid biosensor. J Agric Food Chem 56(3):784–788
Acknowledgments
This work was supported by the Ministry of Science and Innovation (SAF2008-03082). The AMR group is a consolidated research group (Grup de Recerca) of the Generalitat de Catalunya and has support from the Departament d’Universitats, Recerca i Societat de la Informació la Generalitat de Catalunya (expedient 2009 SGR 1343). CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008-2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions and financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babington, R., Matas, S., Marco, MP. et al. Current bioanalytical methods for detection of penicillins. Anal Bioanal Chem 403, 1549–1566 (2012). https://doi.org/10.1007/s00216-012-5960-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-5960-4