Abstract
Penicillins represent most commonly prescribed and salient part of antibacterial armamentarium. Due to lack of proper guidance and regulations, especially in developing countries they are indiscriminately applied in agricultural sectors (livestock farming, aquaculture and plants). Major concerns related to remaining residues of penicillins in food or environment are allergy, development of antibacterial resistance, environmental pollution, economical losses to food industries, consumers and food safety. Researchers of different disciplines are developing reliable detection methods for penicillins in various samples. Among them biosensors are attracting considerable attention primarily for their instant detection, convenience in application, on-site monitoring and portability. Nowadays, they are becoming more sophisticated with the help of modern approaches such as nanotechnology. This review article summarizes the research literature on advancements and recent trends in the field of biosensors for penicillins quantification till date. Different domains of biosensors (electrochemical, optical, mass-sensitive and thermal) are discussed for penicillins along with their applicability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Penicillins and its related concerns
Even after 90 years of its discovery, the penicillin group of antibiotics is still the most frequently used antibacterials in the world due to their well tolerance and efficiency against bacterial infections in both humans and animals. The penicillin molecule discovered by Fleming contains a beta (β)-lactam ring carrying a long side-chain fused to a thiazolidine ring carrying a carboxyl and two methyl groups. The various penicillins differ only in the nature of the R group in the formula (Robinson 1947; Smith et al. 1948). The main target of penicillin is the transpeptidase enzyme of bacteria which is responsible for synthesis of cell wall. Penicillin inactivate transpeptidase enzyme by acylating the transpeptidase’s serine side-chain oxygen (Mucsi et al. 2013). Penicillin are distributed among four generations on the basis of their antibacterial spectrum—natural or first-generation penicillin, antistaphylococcal or second-generation penicillin, extended-spectrum or third-generation penicillin and β-lactamase inhibitor or fourth-generation penicillin (Wright 1999). Figure 1 consists of examples of different generations of penicillins.
Penicillins are applicable in various agricultural sectors (livestock farming, plants and crops, and aquaculture) for the treatment. Benzylpenicillin (Penicillin G), phenoxymethylpenicillin (Penicillin V), cloxacillin, nafcillin, dicloxacillin, oxacillin, ampicillin and amoxicillin are some of the approved drugs for veterinary use. In developing countries, due to their low cost and easy availability they are also used for infection prophylaxis and as feed supplements for improving production efficiency and to enhance the development process in food-producing animals. Figure 2 mentions various problems that are associated with the penicillin residues. Due to these indiscriminate applications, the impact of penicillins is now fading because development of multi drug resistance (MDR) bacteria with strong never-before-seen mutation leaves us vulnerable to many common infections (Manyi-Loh et al. 2018). United Nations considers the emergence of MDR bacteria a worldwide threat. Recent studies on antimicrobial resistance clearly indicate that the lower income countries such as China and India are the major hotspots of resistance to antimicrobial agents used both in humans and food animals due to lack of implementation of strict regulations, policies and standard for antibiotic administration in medical and agricultural sectors (Ayukekbong et al. 2017). Another major concern correlated with the appearance of penicillin residues in foodstuffs (e.g., meat, eggs, pork, fish and milk) are allergic reactions in hypersensitive individuals even at smaller dose (as low as 1 ppb), although the penicillin has low toxicity level. However, Immunoglobulin E (IgE)-mediated hypersensitivity reactions i.e. true allergy, found rarely among reported adverse drug reactions. An estimated frequency is 1–5 per 10,000 cases (Bhattacharya. 2010). For the regulation of penicillins; the European Union (EU) has defined safe maximum residue limits (MRLs) regarding different penicillins in foodstuffs. MRLs for benzylpenicillin, phenoxymethylpenicillin, cloxacillin, nafcillin, dicloxacillin, oxacillin, ampicillin and amoxicillin regulated by EU ranges from 4 to 30 µg/kg (Díaz-Bao et al. 2018).
Apart from damaging consumer’s health the antibiotic residues in food products are causing losses to food processing industries because it is known that existence of penicillin residues in raw milk inhibit the starter cultures and delay lactic acid production which is essential in cultured dairy products (Wilkowske and Krienke 1951). Therefore, the detection and quantification of penicillin is crucial in different fields such as for quality control in medicines, pharmaceutical production, food industries, environmental monitoring, clinical laboratories and fermentation bioprocesses control in biotechnology.
Conventional analytical methods
To date, numerous analytical strategies have been developed for identification and evaluation of penicillin antibiotics such as capillary electrophoresis (CE), chromatographic methods including liquid chromatography (LC), high performance liquid chromatography (HPLC), and spectrometric methods such as mass spectrometry (MS), and coupled analytical methods including high performance liquid chromatography combined with tandem mass spectrometry (HPLC-MS2), electrospray ionization combined with tandem mass spectrometry (ESI-MS2), liquid chromatography with multiple mass spectrometry (LC–MSn) and pressurized liquid extraction and high pressure liquid chromatography with ultraviolet detection (PLE–HPLC–UV). Although these methods are quite sensitive and specific but there are some limitations such as longer and complicated samples pretreatment and operating process, require expensive equipment, trained technicians and thus mostly used in centralized laboratories. Except these conventional methods, various rapid test kits are also commercially available based on microbial growth inhibition (Delvotest SP-NT, Black Reduction Test (BRT) MRL test and Eclipse Farm), lateral flow-based assays (Beta Star, Charm II and Unisensor) and immunoassays (Fluorescent immunoassay (FIA), Enzyme linked immunosorbent assays (ELISA)). These test kits can be applied easily. However, lack of adequate sensitivity, semi-quantitative results and long incubation period are some of the drawbacks (Babington et al. 2012; Kantiani et al. 2009). Therefore, the development of methods or devices that are convenient for instant detection, selective, sensitive and capable of on-site monitoring of antibiotic residues in different samples is very challenging and highly in demand.
Biosensors are capable in accomplishing these requirements due to their specificity, sensitivity, affordability, and rapid screening. They are widely applicable in different domains of research and are gradually becoming more sophisticated with the help of nanotechnology. Biosensors designed with nanomaterials e.g., metal nanoparticles, graphene, carbon nanotubes, nano-dendrimers, nanorods, nanodiamonds, nanocluster, magnetic beads and nanocomposite exhibit lower detection limit, reagent less, single-step evaluation, and, in few examples, shows label-free detection and require very low amount of sample with greater sensitivity (Majdinasab et al. 2019). In this review we summarize the research literature on biosensors that were developed based on different methods and modalities for the analysis of a most important class of antibiotics, penicillin in different samples to date. Figure 3 mentions different modalities used in the development of penicillin biosensors.
Biosensors
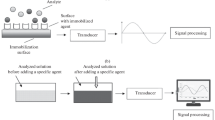
In general, the term biosensor refers to an analytical device, which combines a sensitive biological element with a detector element known as transducer for the detection of particular analyte in any kind of sample. A biosensor is usually classified according to type of bioreceptors utilized or by the kind of transducer employed. Antibodies (monoclonal, polyclonal and recombinant), enzymes, proteins, nucleic acids (DNA or RNA), microbial cells and biomimetics (aptamers and molecular imprinted polymers; MIPs) are the examples of bioreceptors that are employed in penicillin biosensors. Another key part of a biosensor i.e., transducer transforms the physico-chemical change associated with the biorecognition event into measurable electrical signals. The transducers can be electrochemical, optical, gravimetric (mass-sensitive) and thermometric (calorimetric). These four major classes are further divided into various subclasses (Gaudin 2017).
Among various bioreceptors, anti-penicillins antibodies (monoclonal/polyclonal), enzymes β-lactamases (also known as penicillinase) and aptamers are the most common bioreceptors that are found in penicillin biosensors with different transducers.
Electrochemical biosensors
Currently, electrochemical biosensors are dominating in the biosensors field due to their simplicity, easy miniaturization, robustness and low detection limits. They are generally based on the enzymatic reaction with the analyte and generation of an electrical signal in the form of electrons or ions i.e. proportional to the concentration of analyte. Figure 4 illustrates the mechanism of an electrochemical penicillin biosensor. Different electrochemical approaches are used in penicillin detection such as potentiometric (measuring potential/pH variation), amperometric (measuring current) or impedimetric sensors (measuring impedance which is the combination of resistance and reactance) (Grieshaber et al. 2008).
Potentiometric
In potentiometric biosensors, a bioreceptor (commonly an enzyme) is combined with a transducer that senses the variations in ions concentration, the recorded analytical signal being logarithmically correlated with the analyte concentration. A considerable amount of research literature of penicillin potentiometric biosensors is reported using biosensing scheme based on bio-catalysis. Many potentiometric sensors such as glass pH electrode (most common), gas electrode (CO2 electrode), ion selective electrode (ISE), ion selective field effect transistors (ISFETs) and enzyme linked field effect transistors (EnFETs) are employed for penicillin detection. The ion-selective gates of EnFETs are covered by enzyme membranes and the biosensor responds to the electrical potential change via the current output (Caras and Janata 1980; Brand et al. 1989). They have attracted huge attention due to their capability for miniaturization and integration in portable devices. Few examples of reported potentiometric biosensors using pH electrode with different immobilized bioreceptors such as whole microbial cells (e.g. recombinant E. coli producing penicillinase enzyme) (Galindo et al.1990; Kiran and Kale 2002) and enzyme penicillinase for penicillin detection in different matrices such as food and fermentation broth (Koncki et al. 1996; Li et al. 1995; Eppelsheim et al. 1995; Leszczyńska et al. 1998; Park et al. 2004). The use of whole microbial cells as bioreceptor makes the immobilization procedure easier and also reduces the production cost of biosensor. However, microbial biosensors are less specific than enzymatic biosensors. Limit of detection (LOD) of these biosensors in fermentation broth lies in the range of 0.001–1 mM range (greater than the MRLs). Poghossian et al. (2001a, b, c) developed several biosensors by combination of enzymes and silicon-based field effect devices (FED) for detection of penicillins because silicon-based sensors exhibit the advantages in terms of miniaturization and integration of sensors and signal processor devices into a single chip. EnFET, capacitive electrolyte-insulator-semiconductor (EIS) and light addressable potentiometric sensor (LAPS) are examples of microelectronic potentiometric biosensors. All these three types of sensors have relatively common structure and common detection principle (semiconductor field effect). Nevertheless, the sensor preparation and the sensor configuration are specific for each kind. The EnFETs are more sensitive and suitable for miniaturization as compared to capacitive EIS and LAPS, but their long-term stability is lower because they require corrosion resistant passivation. In contrast, capacitive EIS and LAPS are easier and cheaper in production than EnFETs due to their simple layout and no need of corrosion resistant encapsulation material. However, the larger sensitive area in case of capacitive EIS and integration of light source on sensor chip in case of LAPS makes them unsuitable for miniaturization. The properties of these penicillin biosensors mainly depend on the method of immobilization, enzyme membrane thickness, pH and capacity of buffer solution and pH sensitive material. With respect to transducer material, nanomaterials such as carbon nanotubes (CNTs) have been widely applied as a building layer of multilayer films in biosensors due to their high surface area and unique electrical properties combined with polyelectrolytes and highly branched dendritic macromolecules such as polyamidoamine (PAMAM). One simple and versatile technique known as layer by layer (LbL) technique is used to assemble the dispersed CNTs (Siqueira et al. 2009a, b, 2010). The incorporation of PAMAM/CNT LbL film improve the sensitivity, response time and stability. A different technique for immobilization of penicillinase enzyme known as nano-spotting is employed for spatially resolved and patterned deposition of small volume of enzyme on EIS sensor (Beging et al. 2015).
An EnFET was developed with nanocrystalline-diamond (NCD) films for the first time in the detection of penicillin G by directly immobilizing penicillinase enzyme onto the O-terminated NCD surface. Diamond is an outstanding material as electrochemical electrode. Electrochemical reactions generally take place at the surface of diamond. So, there is no need for large volumes of diamond bulk, because NCD inherits most of the superior characteristics of diamond bulk and provides them at the nanoscale. This penicillin biosensor exhibits wider linear concentration range from 0.005 mM to 2.5 mM and LOD of 5 µM (Abouzar et al. 2008). Furthermore, a penicillin biosensor was reported for detection in fermentation broth with charge transfer technique (CTTPS). It consists of charge accumulation technique for penicilloic acid and H+ ion perception system. With the suggested CTTPS, it is feasible to increase the sensing signals in the absence of external amplifier by utilizing the charge accumulation cycles. Under optimum conditions, the suggested biosensor surpassed the capabilities of the generally utilized ISFET penicillin sensor and exhibited eight times greater sensitivity with LOD of 0.1 mM (Lee et al. 2009). A label free capacitive immunosensor is proposed for determination of human serum albumin (HSA), Penicillin G and microcystin-LR. Monoclonal antibodies are used for penicillin detection. The electrodes are modified with silver nanoparticles for improving performance by increasing the loading concentration of antibodies. The detection limit for penicillin G was lowered from 10 to 0.7 fM (Dawan et al. 2011). Another potentiometric biosensor is developed with penicillinase enzyme immobilized via an ANB-NOS (N-5-azido-2-nitrobenzoyloxysuccinimide) cross-linker on zinc oxide (ZnO) nanorods (single-crystal) which are grown over gold (Au) coated glass (Ibupoto et al. 2011). They reported wider linear concentration range i.e., 100 μM to 100 mM. This sensor is useful in the fields of medicine and fermentation. A new concept for EnFET is reported for penicillin G based on tobacco mosaic virus (TMV) nanotubes (penicillinase nanocarrier) modified gate surface for enhancing the performance of biosensor. The biosensor exhibited linear range from 0.1 to 10 mM (Jablonski et al. 2017). With the help of TMV nanotubes high number of enzymes is immobilized on surface without substantial loss of their activity. Although the potentiometric biosensors are simple, cost effective, easily automated, can be miniaturized and does not require labeling still there are some limitation such as they only detect free ions, lack specificity, drifting of signal, slower and less sensitive. A list showing various other potentiometric biosensors for penicillins detection has been illustrated in Table 1.
Amperometric
Amperometric biosensors measures the change in current during a fixed time period at a particular potential. The concentration of the target analyte is proportional to the change in current. Amperometric biosensors for penicillins detection, depending upon pH change is less reported. Other techniques such as voltammetry and chronoamperometry also belong to class of amperometric detection. In voltammetry, information about a target analyte is acquired by shifting a potential and then evaluating the subsequent current. The target analyte can be easily identified with high sensitivity and selectivity by its voltammetric peak potential. The primary kind of voltammetric methods includes cyclic voltammetry (CV), differential pulse voltammetry (DPV) and square wave voltammetry (SWV) (Lan et al. 2017). All these methods are widely utilized for electrochemical detection of antibiotics. Cyclic voltammetry is most common among them and it is helpful in getting data about the redox potential and electrochemical response pace of analyte arrangements (Grieshaber et al. 2008). In chronoamperometry the information about target analyte is acquired by applying a square-wave potential to the working electrode and then measuring alternating current as a component of time. In chronoamperometry there is no need of labeling the reactants. Some of the reported penicillin biosensors based on amperometric, voltammetric and chronoamperometric detection are briefly discussed. Stred’anský et al. (2000) initiated the amperometric detection of penicillin and reported a biosensor based on penicillinase enzyme and hematein (a pH sensitive redox probe) with 4 µM LOD and 0.004–2 mM linear range.
An enzyme and nanomaterial based amperometric biosensor is reported for penicillin detection in buffer solution and milk with co-immobilization of multi-walled carbon nanotubes (MWCNTs) as an enhancer of electron transfer, penicillinase and hematein on glassy carbon electrode using LbL assembly technique. The LOD of 50 nM was reported (Chen et al. 2010). An immobilized Class B penicillinase enzyme on a Au electrode by means of a cysteine SAM (Self Assembled Monolayer) is used for chronoamperometric detection of penicillin G. The obtained LOD was 4.5 nM (Gonçalves et al. 2014).
Apart from enzymatic biosensor based on amperometric detection, immunosensor with amperometric detection was also constructed to detect penicillin G in milk by immobilizing horseradish-peroxidase (HRP)-labeled penicillin polyclonal antibody (PePAb) and new methylene blue (NMB) on GCE. The results are obtained by using cyclic voltammetry and impedance spectroscopy. This biosensor has a linear concentration range of 5.20–41.6 nM with the LOD 1.82 nM (Wu et al. 2014a, b). Recently, a simple and rapid sensor based on voltammetry (SWV) detection is developed by using 8,9-dihydroxy-7-methyl 12H-benzothiazolo[2,3-b]quinazolin-12-one (DMBQ) and ZnO/CNTs nanocomposite modified carbon paste electrode (CPE) for amoxicillin and glutathione. The linear ranges of glutathione and amoxicillin were 0.002–720 µM and 1.0–950 μM with LOD as 0.0008 µM and 0.5 μM. The developed sensor provides satisfactory results when applied to real samples such as blood and urine (Karimi-Maleh et al. 2014). Aptamers, innovative bio (mimetic) materials and alternatives to antibodies are gaining lot of attention from past few years. They are short peptides or oligonucleotides which are chosen using method called Systematic Evolution of Ligand by EXponential enrichment (SELEX) and are identified with greater specificity by the analyte for which they are chosen. Selection of aptamers and antibodies against small target analytes are difficult. Limited aptamers are available against penicillin (Bottari et al. 2018). An electrochemical sensor based on aptamers (biomimetics) and DPV were developed for ampicillin detection by using nicking endonuclease (Nt.AlwI) and DNA polymerase (phi29) assisted target induced quadratic recycling amplification for the first time to increase the sensitivity of aptasensor and reported the LOD as low as 1.09 pM (Wang et al. 2015). The sensor had LOD below the MRL of ampicillin. However, due to inherent complexity of this strategy it is difficult for the commercial application.
A different sequence of aptamer AMP18 is utilized (initially used in 2012 by Song et al.) for the ampicillin aptasensor, based on “signal-on” strategy. The signaling of aptasensor is based on conformational changes in methylene blue (MB) modified thiolated aptamer which is immobilized on gold electrodes due to the binding of target. The change in conformation of aptamer is responsible for decrease in distance between electrode surface and redox probe. Hence, increases the electron transfer which is recorded using alternate current voltammetry (ACV) and SWV. The authors demonstrated that the sensor had good specificity and regenerability. However, this sensor does not fulfill the necessary characteristic regarding LOD (1 μM) and linear range (5–5 × 103 μM) for ampicillin evaluation in food samples (Yu et al. 2018). In a subsequent paper (Yu and Lai 2018) by same authors the initially proposed aptasensor was improved by including a displacement probe (DP-12base S7 Probe) which increases the signal gain induced by target upon binding with aptamer. Hence, the linear dynamic range (0.2–15 × 103 μM) and the lower LOD (30 nM) of the aptasensor are now beneficial for ampicillin evaluation in real samples (milk, urine, saliva and water). However, the aptasensor is not regenerable anymore due to this sacrificial displacement probe.
A multiplexed aptamer-based probe labeled electrochemical sensor using DSBTR (double stirring bar-assisted signal amplified strategy) were successfully developed for simultaneous detection of ampicillin and kanamycin in milk and fishes. The DSBTR can enhance the signal and selectivity by avoiding the background signal and matrix interference. Hence, detection limits for antibiotic residues reached as low as 15fM for ampicillin and 18fM for kanamycin (Shen et al. 2019). Oxacillin was detected by Feier and coresearchers using a DPV technique. The researchers utilized boron-doped diamond electrode (BDD) and 0.2 mol/L acetate buffer (pH 4.5) as electrolyte. The linear concentration range was reported from 5.0 × 10−5 to 1.0 × 10−3 mol/L. The detection limit of 1.1 × 10−5 mol/L was determined. The proposed electrochemical method was successfully applied in the detection of oxacillin in pharmaceutical, environmental and biomedical samples with satisfactory recovery (93–102%) (Feier et al. 2017).
Various other penicillin biosensors based on amperometric, voltammetric and chronoamperometric detection has been listed in Table 2. Amperometric biosensors are more fast, sensitive and suitable for large scale production as compared to potentiometric biosensors. But the major disadvantage is they require labeling of the analyte to enhance the electrochemical reaction.
Impedimetric or conductometric
Electrochemical impedance spectroscopy (EIS), most well-known and sensitive technique utilized in impedimetric biosensors to analyze the interfacial properties associated with biomolecular recognition events. It allows direct and label free detection. A sinusoidal voltage signal of small amplitude is applied and the resulting current (I) is measured. The impedance (Z) is the voltage–current ratio at a particular frequency. Most of the studies in the field of impedimetric biosensors are focused on immunosensors and aptamer-based sensors (Bahadir and Sezgintürk 2016). Conductometric biosensors are related to impedimetric biosensors and are considered as members of this category and they are mainly associated with the enzymes. With the help of conductometric biosensor, the changes in conductivity of a solution is measured which arises due to enzymatic reaction between two electrodes. Some of the example s of biosensor based on these techniques are discussed. A penicillin conductometric biosensor is reported with penicillinase and polypyrrole membrane coated microarray electrode. The enzymatic reaction catalyzed by penicillinase cause the acidification of polypyrrole membrane due to which there is enhancement in the electrical conductivity of polypyrrole (Nishizawa et al. 1992).
An impedimetric immunosensor based on direct determination of benzylpenicillin is reported. The anti-benzylpenicillin monoclonal antibodies are immobilized on working electrode of Au modified by SAM of thioctic acid. For the monitoring of immunoreaction, the optimum frequency was 160 Hz. At this frequency the change in impedance was detected. Under optimum conditions, a wider linear range from 0.1 pM to 10 nM and a very low LOD of 3 fM in milk was obtained. The working system can be reused up to 45 times which further decreases the cost of analysis (Thavarungkul et al. 2007). Another impedimetric sensor based on immobilization of anti-penicillin G antibodies from mouse with gold nanoparticles (AuNPs) modified bilayers lipids membrane was developed (Li et al. 2015). With the help of AuNPs the sensitivity is enhanced and LOD of 2.7 × 10−4 pg/mL is achieved. The hindrance effect of streptomycin and ampicillin is tested and good recovery is obtained in milk sample. The biosensor is also compared with HPLC, and it is concluded that this biosensor is saves more time and convenient to use due to simple preparation of milk sample.
A signaling-probe displacement electrochemical aptasensor of second generation (SD-EAS II) is reported for the detection of lipophilic targets, sulfadimethoxine (SDM) and ampicillin (AMP). For the sensitive and selective detection of ampicillin, the anti-ampicillin DNA aptamer was employed to design the signaling and capture probe. After the immobilization of probe on gold electrode. The electrode was immersed in solution passivation agent i.e., O-(2-mercaptoethyl)-O′-methyl-hexa (ethylene glycol) (2-EG6-OMe) SAM. In the presence of AMP, the current signal was decreased due to the displacement of signaling probe and its release from the surface of electrode. The LOD (0.2 μM) and the linear range (100 pM–1 mM) are certainly great and also the hindrance effect of other antibacterials is minimum. The sensor is practically applied for detection of lipophilic antibiotics in spiked-in lake water (Yang et al. 2017). Another impedimetric aptasensor is designed for the detection of ampicillin with the combination of EIS and interdigitated electrodes (PEDOT:TsO). The geometry of the electrodes and the microchannels is optimized to evaluate the performance of the biosensor. Additionally, the aptamer was directly immobilized on the electrodes due to the presence of an appended poly (T)–poly(C) sequence. The reported linear range and LOD was 100 pM–10 nM and 100 pM (Rosati et al. 2014). Several other impedimetric biosensors for penicillin detection have been illustrated in Table 3. Impedimetric biosensors are relatively fast, cost effective, label free but they have poor reproducibility.
Optical biosensors
Another popular kind of biosensors that are frequently applied from the last two decades for the detection of penicillin antibiotics are optical biosensor. They work on the principle of detecting changes in properties of light or photons. Optical biosensors are fast, highly sensitive, specific and allow real time monitoring. The LODs obtained from optical biosensors are much lower than MRLs. Various signal transducing mechanisms are employed in optical biosensor due to which they are further sub-classified into vast number of groups such as fluorescent, colorimetric, surface plasmon resonance (SPR), localized SPR, surface enhanced Raman scattering (SERS), ellipsometric and reflectometric interference spectroscopy (RIfS). Among these various optical biosensors, SPR based biosensor are most commonly employed and they allow direct detection of the changes occurring in the refractive index of light at the surface of the sensor i.e. proportional to the concentration of analyte. The first commercial SPR-based biosensor instrument was launched by Biacore (Damborsky et al. 2016). SPR sensors are also able to register changes in mass associated with the refractive index changes at the surface. These biosensors are less expensive, free of labels, smaller in dimensions and operate in real time (Song et al. 2008).
Two related SPR based biosensor are developed for the detection of penicillins in milk by immobilizing the DD-carboxypeptidase producing R39 bacteria. The biosensors are based on catalytic activity of carboxypeptidase which convert 3-peptide (substrate) into 2-peptide (product) and this reaction is inhibited in the presence of beta-lactams. The concentration of penicillin G was measured by using antibodies against the 2-peptide product or against the remaining 3-peptide substrate. The results agreed well with HPLC. The LOD of both biosensors was 1.2 µg/kg and 1.5 µg/kg (Gustavsson et al. 2004).
A simple and inexpensive biosensor known as holographic biosensor is reported using pH-sensitive holographic transducers to monitor the pH change which is related to enzymatic reaction catalyzed by penicillinase enzyme for the detection of penicillin concentration in fermentation broths. The color produced in holographic biosensors is affected by shrinkage and swelling behavior of these biosensors. The concentration of penicillin is proportional to the color or diffraction wavelength (Marshall et al. 2004). The response time is similar to electrochemical and optical biosensors and it exhibited a linear range of 1–25 mM.
Recently, first ampicillin aptasensor using AuNPs based combination of two methods (fluorescence and colorimetry) is developed for its detection with the help AMP4, AMP17 and AMP18. These aptamers are selected by SELEX and are also used by other researchers. The modified aptamer act as dual probe for simultaneous observation of changes in color and fluorescence differences. The lower LODs were a 2 ng/mL (fluorescence) and a 10 ng/mL (colorimetry) in the milk and distilled water (Song et al. 2012). Another sensitive fluorescent biosensor for ampicillin detection was reported using thiol modified aptamers, composite of magnetic beads (MB) coated with AuNPs and an endonuclease (Nb.BbvCl) (Luo et al. 2017). The aptasensor exhibited more sensitivity with the LOD of 70 ng/L in comparison to first aptasensor suggested by Song et al. (2012).
Aptamers against penicillin G were also reported recently by using rGO-SELEX (reduced Graphene Oxide-SELEX). This method utilizes π–π stacking interaction of rGO and DNA to select aptamers with high binding affinity and it is immobilization free. This aptasensor had a LOD of 9.2 nM. Selectivity tests indicated that the aptamer against penicillin G can also bind with less affinity to amoxicillin and ampicillin (Lee et al. 2017).
MIPs (biomimetic materials) are employed as pre-concentration or extraction steps in other analytical applications. An optical biosensor is reported for label-free detection of benzylpenicillin in fermentation broths based on nanoparticles of benzylpenicillin imprinted polymer (MIP) forming sensitive layer and non-imprinted particles (NIPs) as reference. Both MIPs and NIPs are modified with azide and then immobilized on glass transducer (alkyne modified). This sensitive layer together with RIfS instrumental setup served as quick, easy and direct optical sensing method for in-line monitoring of Penicillin G concentration. The linear range was 0.0015–0.0195 mol/L. RIfS depends on measurement of white light interference at thin layers. It is highly flexible and less expensive than the SPR (Weber et al. 2018).
In milk, the presence of Penicillin G detected with dual optrode biosensor i.e. glucose optrode and oxygen optrode as a reference. In this biosensor, concentration of glucose in milk acts as an indicator for penicillin presence. The penicillin G inhibits the activity of β-galactosidase resulting in decrease of glucose concentration as compared to high quality milk (Kagan et al. 2016). Various other optical biosensors for the penicillins quantification has been described in Table 4.
Mass-sensitive (gravimetric) biosensors
Detection of penicillins using mass-sensitive biosensors is scarcely reported. Mass-sensitive or gravimetric biosensors evaluate the concentration of the target analyte by measuring the changes in property that is related to the mass bound on its sensitive surface due to biorecognition events. Gravimetric biosensors allow label free detection. Gravimetric biosensors mainly use piezoelectric crystals as transducers. The piezoelectric crystals such as quartz lack center of symmetry and produce voltage (an electrical signal) when they are mechanically stressed. Methods of mechanical transduction mainly depend on production and detection of mechanical or acoustic waves. Therefore, piezoelectric crystals-based sensors are known as acoustic wave sensors. The acoustic wave sensors are distinguished into two main categories on the basis of direction of propagation of acoustic wave namely surface acoustic waves (SAW) sensor and bulk acoustic wave (BAW) sensor or quartz crystal microbalances (QCM) (Fogel et al. 2016). SAW sensors detect the variations in surface wave velocity occurring due to the biorecognition events (causing mass adsorption and variation in viscoelasticity) taking place at biosensor surface. These devices are suitable for large scale production as they have cheap and disposable sensor components. Quartz Crystal Microbalance is a stable oscillator that can detect the changes in mass in nanogram magnitude, highly precise and most popular mass-sensitive biosensor. The QCM has been commonly used due to its low cost (particularly as compared to SPR) and relatively simple instrumentation. When a ferromagnetic material is mechanically stressed, they experience the changes in magnetic flux which is known as magnetoelastic effect. The biosensors based on magnetoelastic effect are known as magnetoelastic biosensors. They are generally composed of amorphous ferromagnetic ribbon like thick film strips and have attracted a considerable attraction as being wireless and passive (Ren et al. 2019).
Some examples of reported mass-sensitive biosensors for penicillins detection are discussed. A wireless magnetoelastic biosensor for penicillin detection in milk is reported with wider linear range of 1.9 mM–5.0 mM and LOD of 1.3 mM under optimum conditions. The sensor was fabricated with a sensing film of penicillinase and BSA over the magnetoelastic ribbon with a pH-sensitive polymer layer. Due to hydrolysis of penicillin G by enzyme penicillinase, the pH of solution decreases. As a result, polymer shrinks which increases the resonance frequency of magnetoelastic biosensor (Gao et al. 2009).
A piezoelectric immunosensor is developed for the detection of penicillin G and ampicillin in real samples (milk and meat). The piezoelectric biosensor monitors the change in resonance frequency with and without adding the target analyte. Both polyclonal and monoclonal antibodies are tested using polypyrrole modified electrode immobilized with antibiotic–protein conjugate. The biosensor exhibited good recovery (Karaseva and Ermolaeva 2014). Same researchers developed piezoelectric biosensor for penicillin G and ampicillin using nanoparticles of MIPs for direct detection. The study showed that MIPs are selective enough but cross reactivity is high with close structurally related antibiotics. The biosensor showed narrow linear range 102–103 ng/mL with LOD of 40 ng/mL (penicillin G) and 90 ng/mL (ampicillin) (Karaseva et al. 2016).
For the first time the direct detection of penicillin G in milk using SAW biosensor was reported based on binding inhibition assay. The basic principle of binding inhibition assay is based on the determination of the target-specific antibodies instead of target molecule itself. The biosensor is coated with the target molecule providing the required epitopes for the target-specific antibody. Then antibodies are allowed to bind with the analyte and the residual antibodies having one or two free sites for binding are determined by the biosensor. Higher concentration of analyte produces low sensor signal and vice-versa. This biosensor demonstrated the LOD of 2.2 ng/mL in milk and 2 ng/mL in buffer solution (Gruhl and Länge 2013).
Thermal (calorimetric) biosensors
Thermal or calorimetric biosensors evaluate the concentration of the target analyte by measuring the changes in energy (generation or absorption) related to biochemical reaction (e.g. enzymatic reaction). The energy which is released or absorbed scales proportionally to the number of molecules generated in the biochemical reaction. Thermistors and thermocouples (thermopiles) are the commonly employed transducers in thermal biosensors (Xie and Danielsson 2008). Microcalorimetry is used to measure the change in temperature. The most commonly applied biological elements in thermal biosensors are enzymes. Enzyme based thermal biosensors can be fabricated for the simultaneous multiplex detection. Indeed, a column may be fractioned into various regions; each region is immobilized with a specific enzyme for the identification of a specific analyte. Thermal biosensors are applied for the monitoring of penicillin V production with the help of β-lactamase by fermentation (Rank et al. 1992). A split-flow modified thermal biosensor is applied with the more specific enzyme penicillin amidase, especially in fermentation broths to monitor penicillin V production by fermentation. The sensitivity is; however, lower although sufficient for fermentation process monitoring (Rank et al. 1993).
An integrated silicon thermopile is reported as biosensor for the monitoring of multiple analytes such as glucose, urea and penicillin. Penicillinase is utilized for penicillin detection. The sensor was operated together with flow injection analysis (FIA). Detection limits between 1 and 2 mM were reported (Bataillard et al. 1993).
Other biosensors
A study is reported based on real-time detection of penicillin V (phenoxymethylpenicillin) in the extracellular fluid (ECF) by applying a microneedle biosensor to the forearm. Microneedles are minimally invasive transdermal medical devices that are utilized for various applications, including drug delivery, fluid sampling, micro-dialysis, and electrochemical sensing. Microneedle biosensor-based quantification of ECF penicillin has shown comparable results to the standard methods employed (micro-dialysis and serum concentration measurement in blood samples) (Rawson et al. 2019).
Hybrid methods such as photoelectrochemistry are also applied for better understanding and evaluation of biochemical processes. Photoelectrochemistry is an innovative method which has gained considerable attention in detection of significant compounds. In photoelectrochemical (PEC) biosensor the photoactive material is used as sensing species and electric signal are generated by conversion of photo irradiation. The PEC biosensor is a combination of both traditional electrochemical and optical biosensors. Recently, perovskite-type BiFeO3 has gained considerable attention owing to its non-toxicity and good chemical stability as comparison to UV light. An effective sensing method is developed for detection of ampicillin by using perovskite-type BiFeO3 nanoparticles (Ge et al. 2019).
Another sensor based on photoelectrochemical sensing strategy using zinc-phthalocyanine (ZnPc) photo-sensitizers as enzyme mimics for electrochemical sensing of amoxicillin. The perfluorinated ZnPc photo-sensitizer are reactive but chemically resistant produces singlet oxygen from air when illuminated under visible light and analytes gets oxidized into products which are easily detectable by electrochemistry. The sensor requires small sample volumes (in mL) and exhibited low LOD of 20 nM with enhanced selectivity as compared to enzyme-based sensors (Trashin et al. 2017).
Conclusion and future perspectives
Food safety is the most important for human’s health, economy and tourism and to ensure its safety, monitoring of food is essential. But, for monitoring of food, limited number of tools are commercially available due to which the need of developing faster, sensitive and precise analytical tools continue to become more evident. Researchers have developed various tools based on different methods and modalities. Among these, biosensors show impressive capabilities for the detection of antibiotics residues. Various potential prototypes have been developed for the detection of penicillins based different modalities such as optical, electrochemical, mass-sensitive and thermometric that are sensitive and precise. However, till now, most of them are applied in the controlled lab environment and have not yet reached the customer. The main obstacle to the realistic implementation of biosensors is their application in complex and real samples. In most of the cases, their sensitivity and specificity are influenced by real sample conditions, such as ionic strength, interfering components (e.g., lipids, proteins etc.), viscosity and pH. Moreover, nonspecific interactions of bioreceptors with components of sample can lead to false positive results. The design and application of receptors that specifically bind to the target antibiotic is crucial to develop biosensors with high sensitivity and specificity. As discussed in this review, enzymes, antibodies and aptamers are the most commonly used bioreceptors in the design of various types of penicillins biosensor. Each of these receptors has its own pros and cons. Pure enzymes are expensive and less stable in extreme conditions. Antibodies are difficult to produce against small molecules such as antibiotics. However, few anti-penicillin antibodies are commercially available. Aptamers are more stable in adverse environmental conditions as compared to enzymes and antibodies, can be easily modified, shows higher affinity and have low production cost. There is growing interest in developing synthetic bioreceptors, such as MIPs, which are expected to offer strong recognition but so far lack the specificity offered by their natural counterparts. To overcome the lack of specificity, a combination of various bioreceptors is recommended for the development of a biosensor with higher selectivity. Simultaneous application of multiple bioreceptors increases the sensitivity, selectivity and precision of the bioassay.
Biosensors based on optical detection have better prospects for customization due to the availability of many fluorescent molecules. However, they also suffer from some drawbacks, such as the sample pre-treatment needed to remove the background noise from the intrinsic fluorescence properties many molecules have. The prime aim of biosensing device is to allow layperson to utilize it in a portable manner with ease for raw environmental or real samples without any preparation, so florescence-based biosensors are often not ideal in this aspect. Electrochemical biosensors have the highest capabilities for portability and miniaturization, but their development alone can be money and time consuming. Many electrochemical prototypes are developed that are portable and accurate, but these techniques still have a long way to go before they are applied in the field. Nanomaterials in biosensors are also facing many technical obstacles, such as biocompatibility and suitable connection between nanomaterials and bioreceptors, the effect of non-uniform nanomaterials on the performance of biosensors, and cross-reactions within antibiotics which should be taken in consideration. Furthermore, research efforts should focus on improving the bioreceptors to increase their tolerance toward environmental conditions. Overall, each method has the capacity for transforming into an effective analytical tool but need further testing and optimization before they attain their full potential.
References
Abouzar MH, Poghossian A, Razavi A et al (2008) Penicillin detection with nanocrystalline-diamond field-effect sensor. Phys Status Solidi A 205(9):2141–2145. https://doi.org/10.1002/pssa.200879713
Abouzar MH, Poghossian A, Siqueira JR et al (2010) Capacitive electrolyte-insulator-semiconductor structures functionalised with a polyelectrolyte/enzyme multilayer: new strategy for enhanced field-effect biosensing. Phys Status Solidi A 207(4):884–890. https://doi.org/10.1002/pssa.200983317
Ayukekbong JA, Ntemgwa M, Atabe AN (2017) The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 6(1):47. https://doi.org/10.1186/s13756-017-0208-x
Babington R, Matas S, Marco MP et al (2012) Current bioanalytical methods for detection of penicillins. Anal Bioanal Chem 403(6):1549–1566. https://doi.org/10.1007/s00216-012-5960-4
Bahadır EB, Sezgintürk MK (2016) A review on impedimetric biosensors. Artif Cells Nanomed Biotechnol 44(1):248–262. https://doi.org/10.3109/21691401.2014.942456
Bataillard P, Steffgen E, Haemmerli S et al (1993) An integrated silicon thermopile as biosensor for the thermal monitoring of glucose, urea and penicillin. Biosens Bioelectron 8(2):89–98. https://doi.org/10.1016/0956-5663(93)80057-V
Beging S, Leinhos M, Jablonski M et al (2015) Studying the spatially resolved immobilization of enzymes on a capacitive field-effect structure by means of nano-spotting. Phys Status Solidi A 212(6):1353–1358. https://doi.org/10.1002/pssa.201431891
Bhattacharya S (2010) The facts about penicillin allergy: a review. J Adv PharmTechnol Res 1:11–17
Bottari F, Blust R, De Wael K (2018) Bio(inspired) strategies for the electro-sensing of β-lactam antibiotics. Curr Opin Electrochem 10:136–142. https://doi.org/10.1016/j.coelec.2018.05.015
Brand U, Scheper T, Schügerl K (1989) Penicillin G sensor based on penicillin amidase coupled to a field effect transistor. Anal Chim Acta 226(1):87–97. https://doi.org/10.1016/S0003-2670(00)80906-X
Cacciatore G, Petz M, Rachid S et al (2004) Development of an optical biosensor assay for detection of β-lactam antibiotics in milk using the penicillin-binding protein 2x*. Anal Chim Acta 520(1–2):105–115. https://doi.org/10.1016/j.aca.2004.06.060
Caras S, Janata J (1980) Field effect transistor sensitive to penicillin. Anal Chem 52(12):1935–1937. https://doi.org/10.1021/ac50062a035
Chan PH, Liu HB, Chen YW et al (2014) Rational design of a novel fluorescent biosensor for β-Lactam antibiotics from a class A β-Lactamase. J Am Chem Soc 126(13):4074–4075. https://doi.org/10.1021/ja038409m
Chao HP, Lee WC (2000) A bioelectrode for penicillin detection based on gluten-membrane-entrapped microbial cells. Biotechnol Appl Biochem 32(1):9. https://doi.org/10.1042/BA20000003
Chen B, Ma M, Su X (2010) An amperometric penicillin biosensor with enhanced sensitivity based on co-immobilization of carbon nanotubes, hematein, and β-lactamase on glassy carbon electrode. Anal Chim Acta 674(1):89–95. https://doi.org/10.1016/j.aca.2010.06.014
Damborsky P, Svitel J, Katrlik J (2016) Optical biosensors. Essays Biochem 60(1):91–100. https://doi.org/10.1042/EBC20150010
Daprà J, Lauridsen LH, Nielsen AT et al (2013) Comparative study on aptamers as recognition elements for antibiotics in a label-free all-polymer biosensor. Biosens Bioelectron 43:315–320. https://doi.org/10.1016/j.bios.2012.12.058
Dawan S, Kanatharana P, Wongkittisuksa B et al (2011) Label-free capacitive immunosensors for ultra-trace detection based on the increase of immobilized antibodies on silver nanoparticles. Anal Chim Acta 699(2):232–241. https://doi.org/10.1016/j.aca.2011.05.038
Díaz-Bao M, Barreiro R, Miranda JM et al (2018) Fast HPLC-MS/MS method for determining penicillin antibiotics in infant formulas using molecularly imprinted solid-phase extraction. J Anal Methods Chem. https://doi.org/10.1155/2015/959675
Eppelsheim C, Aubeck R, Hampp N (1995) Comparison of potentiometric enzyme sensors for urea and penicillin-G: differential thick-film sensors versus classical electrodes. J Membr Sci 100(2):131–137. https://doi.org/10.1016/0376-7388(94)00256-X
Feier B, Ionel I, Cristea C et al (2017) Electrochemical behavior of several penicillins at high potential. New J Chem 41(21):12947–12955. https://doi.org/10.1039/C7NJ01729D
Ferrini AM, Mannoni V, Carpico G et al (2008) Detection and identification of β-Lactam residues in milk using a hybrid biosensor. J Agric Food Chem 56(3):784–788. https://doi.org/10.1021/jf071479i
Fogel R, Limson J, Seshia AA (2016) Acoustic biosensors. Essays Biochem 60(1):101–110. https://doi.org/10.1042/EBC20150011
Galindo E, Bautista D, García J et al (1990) Microbial sensor for penicillins using a recombinant strain of Escherichia coli. Enzyme Microb Technol 12(9):642–646. https://doi.org/10.1016/0141-0229(90)90001-7
Gamella M, Campuzano S, Conzuelo F et al (2013) An amperometric affinity penicillin-binding protein magnetosensor for the detection of β-lactam antibiotics in milk. Analyst. https://doi.org/10.1039/C3AN36727D
Gao X, Zhen R, Zhang Y et al (2009) Detecting penicillin in milk with a wireless magnetoelastic biosensor. Sens Lett 7(1):6–10. https://doi.org/10.1166/sl.2009.1002
Gaudin V (2017) Advances in biosensor development for the screening of antibiotic residues in food products of animal origin—a comprehensive review. Biosens Bioelectron 90:363–377. https://doi.org/10.1016/j.bios.2016.12.005
Ge L, Xu Y, Ding L et al (2019) Perovskite-type BiFeO3/ultrathin graphite-like carbon nitride nanosheets pn heterojunction: boosted visible-light-driven photoelectrochemical activity for fabricating ampicillin aptasensor. Biosens Bioelectron 124:33–39. https://doi.org/10.1016/j.bios.2018.09.093
Gonçalves LM, Callera WFA, Sotomayor MDPT et al (2014) Penicillinase-based amperometric biosensor for penicillin G. Electrochem Commun 38:131–133. https://doi.org/10.1016/j.elecom.2013.11.022
Grieshaber D, MacKenzie R, Vörös J et al (2008) Electrochemical biosensors—sensor principles and architectures. Sensors 8(3):1400–1458. https://doi.org/10.3390/s80314000
Gruhl FJ, Länge K (2013) Surface acoustic wave (SAW) biosensor for rapid and label-free detection of penicillin G in milk. Food Anal Methods 7(2):430–437. https://doi.org/10.1007/s12161-013-9642-4
Gustavsson E, Degelaen J, Bjurling P et al (2004) Determination of β-Lactams in milk using a surface plasmon resonance-based biosensor. J Agric Food Chem 52(10):2791–2796. https://doi.org/10.1021/jf0344284
Ibupoto ZH, Ali SMU, Khun K et al (2011) ZnO nanorods based enzymatic biosensor for selective determination of penicillin. Biosensors 1(4):153–163. https://doi.org/10.3390/bios1040153
Ismail F, Adeloju SB (2010) Galvanostatic entrapment of penicillinase into polytyramine films and its utilization for the potentiometric determination of penicillin. Sensors 10(4):2851–2868. https://doi.org/10.3390/s100402851
Ismail F, Adeloju SB (2014) The use of poly (vinyl alcohol) to cross-link penicillinase for the fabrication of a penicillin potentiometric biosensor. Electroanalysis 26(12):2701–2709. https://doi.org/10.1002/elan.201400437
Ismail F, Adeloju SB (2015) Comparison of single layer and bilayer biosensors based on crosslinking of penicillinase for potentiometric detection of penicillin in milk and antibiotics. Electroanalysis 27(6):1523–1531. https://doi.org/10.1002/elan.201500037
Ismail F, Adeloju SB, Moline AN (2014) Fabrication of a single layer and bilayer potentiometric biosensors for penicillin by galvanostatic entrapment of penicillinase into polypyrrole films. Electroanalysis 26(12):2607–2618. https://doi.org/10.1002/elan.201400452
Jablonski M, Koch C, Bronder TS et al (2017) Field-effect biosensors modified with tobacco mosaic virus nanotubes as enzyme nanocarrier. Proceedings 1(4):505. https://doi.org/10.3390/proceedings1040505
Jablonski M, Poghossian A, Koch C et al (2018) Field-effect biosensor using virus particles as scaffolds for enzyme immobilization. Biosens Bioelectron 110:168–174. https://doi.org/10.1016/j.bios.2018.03.036
Kagan M, Printsmann G, Kivirand K et al (2016) Determination of penicillins in milk by a dual-optrode biosensor. Anal Lett 50(5):819–828. https://doi.org/10.1080/00032719.2016.1202957
Kantiani L, Farré M, Barceló D et al (2009) Analytical methodologies for the detection of β-lactam antibiotics in milk and feed samples. Trends Anal Chem 28(6):729–744. https://doi.org/10.1016/j.trac.2009.04.005
Karaseva NA, Ermolaeva TN (2014) Piezoelectric immunosensors for the detection of individual antibiotics and the total content of penicillin antibiotics in foodstuffs. Talanta 120:312–317. https://doi.org/10.1016/j.talanta.2013.12.018
Karaseva NA, Ermolaeva T, Mizaikoff B (2016) Piezoelectric sensors using molecularly imprinted nanospheres for the detection of antibiotics. Sens Actuators B 225:199–208. https://doi.org/10.1016/j.snb.2015.11.045
Karimi-Maleh H, Tahernejad-Javazmi F, Gupta VK et al (2014) A novel biosensor for liquid phase determination of glutathione and amoxicillin in biological and pharmaceutical samples using a ZnO/CNTs nanocomposite/catechol derivative modified electrode. J Mol Liq 196:258–263. https://doi.org/10.1016/j.molliq.2014.03.049
Kiran BR, Kale KU (2002) Transformed E. coli JM109 as a biosensor for penicillin. Indian J Pharm Sci 64(3):205–208
Koch C, Poghossian A, Schöning MJ et al (2018) Penicillin detection by tobacco mosaic virus-assisted colorimetric biosensors. Nanotheranostics 2(2):184–196
Koncki R, Leszczyńska E, Cybulska A et al (1996) Penicillin enzyme biosensors based on pH membrane electrodes. Anal Chim Acta 321(1):27–34. https://doi.org/10.1016/0003-2670(95)00547-1
Lan L, Yao Y, Ping J et al (2017) Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens Bioelectron 91:504–514. https://doi.org/10.1016/j.bios.2017.01.007
Lee SR, Rahman MM, Sawada K et al (2009) Fabrication of a highly sensitive penicillin sensor based on charge transfer techniques. Biosens Bioelectron 24(7):1877–1882. https://doi.org/10.1016/j.bios.2008.09.008
Lee AY, Ha NR, Jung IP et al (2017) Development of a ssDNA aptamer for detection of residual benzylpenicillin. Anal Biochem 531:1–7. https://doi.org/10.1016/j.ab.2017.05.013
Leszczyńska E, Głąb S, Sokół A et al (1998) Potentiometric biosensor for control of biotechnological production of penicillin G. Anal Chim Acta 368(3):205–210. https://doi.org/10.1016/S0003-2670(98)00206-2
Li QS, Zhang SL, Yu JT (1995) Amperometric penicillin biosensor and its application in flow injection analysis system for determination of penicillin in broth. Microchem J 52(2):166–173. https://doi.org/10.1006/mchj.1995.1081
Li JL, Pan DD, Zhu HJ et al (2013) Determination of penicillin in milk by the nanocomposite immunosensor (MWCNTs-CS). Mod Food Sci Technol 9:47
Li H, Xu B, Wang D et al (2015) Immunosensor for trace penicillin G detection in milk based on supported bilayer lipid membrane modified with gold nanoparticles. J Biotechnol 203:97–103. https://doi.org/10.1016/j.jbiotec.2015.03.013
Li Z, Liu C, Sarpong V et al (2019) Multisegment nanowire/nanoparticle hybrid arrays as electrochemical biosensors for simultaneous detection of antibiotics. Biosens Bioelectron 126:632–639. https://doi.org/10.1016/j.bios.2018.10.025
Luo Z, Wang Y, Lu X et al (2017) Fluorescent aptasensor for antibiotic detection using magnetic bead composites coated with gold nanoparticles and a nicking enzyme. Anal Chim Acta 984:177–184. https://doi.org/10.1016/j.aca.2017.06.037
Majdinasab M, Mitsubayashi K, Marty JL (2019) Optical and electrochemical sensors and biosensors for the detection of q uinolones. Trends Biotechnol 37(8):898–915. https://doi.org/10.1016/j.tibtech.2019.01.004
Manyi-Loh C, Mamphweli S, Meyer E et al (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23(4):795. https://doi.org/10.3390/molecules23040795
Marshall AJ, Young DS, Blyth J et al (2004) Metabolite-sensitive holographic biosensors. Anal Chem 76(5):1518–1523. https://doi.org/10.1021/ac030357w
Merola G, Martini E, Tomassetti M et al (2014) New immunosensor for β-lactam antibiotics determination in river waste waters. Sens Actuators B 199:301–313. https://doi.org/10.1016/j.snb.2014.03.083
Merola G, Martini E, Tomassetti M et al (2015) Simple and suitable immunosensor for β-lactam antibiotics analysis in real matrixes: milk, serum, urine. J Pharm Biomed Anal 106:186–196. https://doi.org/10.1016/j.jpba.2014.08.005
Mohammad-Razdari A, Ghasemi-Varnamkhasti M, Izadi Z et al (2019) An impedimetric aptasensor for ultrasensitive detection of penicillin G based on the use of reduced graphene oxide and gold nanoparticles. Microchim Acta 186(6):372. https://doi.org/10.1007/s00604-019-3510-x
Mucsi Z, Chass GA, Ábrányi-Balogh P et al (2013) Penicillin's catalytic mechanism revealed by inelastic neutrons and quantum chemical theory. Phys Chem Chem Phys 15(47):20447–20455. https://doi.org/10.1039/C3CP50868D
Nishizawa M, Matsue T, Uchida I (1992) Penicillin sensor based on a microarray electrode coated with pH-responsive polypyrrole. Anal Chem 64(21):2642–2644. https://doi.org/10.1021/ac00045a030
Paniel N, Istamboulié G, Triki A et al (2017) Selection of DNA aptamers against penicillin G using capture-SELEX for the development of an impedimetric sensor. Talanta 162:232–240. https://doi.org/10.1016/j.talanta.2016.09.058
Park IS, Kim DK, Kim N (2004) Characterization and food application of a potentiometric biosensor measuring β-lactam antibiotics. J Microbiol Biotechnol 14:698–706
Poghossian A, Thust M, Schroth A et al (2001a) Penicillin detection by means of silicon-based field-effect structures. Sens Mater 13:207–223
Poghossian A, Schöning MJ, Schroth P et al (2001b) An ISFET-based penicillin sensor with high sensitivity, low detection limit and long lifetime. Sens Actuators B 76(1–3):519–526. https://doi.org/10.1016/S0925-4005(01)00609-8
Poghossian A, Yoshinobu T, Simonis A et al (2001c) Penicillin detection by means of field-effect based sensors: ENFET, capacitive EIS sensor or LAPS? Sens Actuators B 78:237–242. https://doi.org/10.1016/S0925-4005(01)00819-X
Prado TMD, Foguel MV, Gonçalves LM et al (2015) β-Lactamase-based biosensor for the electrochemical determination of benzylpenicillin in milk. Sens Actuators B 210:254–258. https://doi.org/10.1016/j.snb.2014.12.108
Rahman MM, Asiri AM (2015) Development of penicillin G biosensor based on penicillinase enzymes immobilized onto bio-chips. Biomed Microdevices 17(1):9. https://doi.org/10.1007/s10544-014-9910-0
Rank M, Danielsson B, Gram J et al (1992) Implementation of a thermal biosensor in a process environment: on-line monitoring of penicillin V in production-scale fermentations. Biosens Bioelectron 7(9):631–635. https://doi.org/10.1016/0956-5663(92)85020-B
Rank M, Gram J, Danielsson B (1993) Industrial on-line monitoring of penicillin V, glucose and ethanol using a split-flow modified thermal biosensor. Anal Chim Acta 281(3):521–526. https://doi.org/10.1016/0003-2670(93)85010-H
Rawson TM, Gowers SA, Freeman DM et al (2019) Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit Health 1(7):e335–e343. https://doi.org/10.1016/S2589-7500(19)30131-1
Ren L, Yu K, Tan Y (2019) Applications and advances of magnetoelastic sensors in biomedical engineering: a review. Materials 12(7):1135. https://doi.org/10.3390/ma12071135
Robinson FA (1947) Chemistry of penicillin. Analyst 72(856):274–276. https://doi.org/10.1039/AN9477200274
Rosati G, Daprà J, Cherré S et al (2014) Performance improvement by layout designs of conductive polymer microelectrode based impedimetric biosensors. Electroanalysis 26(6):1400–1408. https://doi.org/10.1002/elan.201400062
Rosati G, Ravarotto M, Scaramuzza M et al (2018) Silver nanoparticles inkjet-printed flexible biosensor for rapid label-free antibiotic detection in milk. Sens Actuators B 280:280–289. https://doi.org/10.1016/j.snb.2018.09.084
Scholl FA, Morais PV, Gabriel RC et al (2017) Carbon nanotubes arranged as smart interfaces in lipid langmuir–blodgett films enhancing the enzymatic properties of penicillinase for biosensing applications. ACS Appl Mater Interfaces 9(36):31054–31066. https://doi.org/10.1021/acsami.7b08095
Shen Z, Cao Y, Hong F et al (2019) Multiplexed electrochemical aptasensor for antibiotics detection using metallic-encoded apoferritin probes and double stirring bars-assisted target recycling for signal amplification. Talanta 197:491–499. https://doi.org/10.1016/j.talanta.2018.12.018
Siqueira JR, Abouzar MH, Poghossian A et al (2009a) Penicillin biosensor based on a capacitive field-effect structure functionalized with a dendrimer/carbon nanotube multilayer. Biosens Bioelectron 25(2):497–501. https://doi.org/10.1016/j.bios.2009.07.007
Siqueira JR, Werner CF, Bäcker M et al (2009b) Layer-by-layer assembly of carbon nanotubes incorporated in light-addressable potentiometric sensors. J Phys Chem C 113(33):14765–14770. https://doi.org/10.1021/jp904777t
Siqueira JR, Bäcker M, Poghossian A et al (2010) Associating biosensing properties with the morphological structure of multilayers containing carbon nanotubes on field-effect devices. Phys Status Solidi A 207(4):781–786. https://doi.org/10.1002/pssa.200983301
Smith EL, Boon WR, Patterson SJ, Emery WB, Twigg GH (1948) Methods of penicillin assay: their purpose, scope and validity. Analyst 73(865):197–216
Song S, Wang L, Li J et al (2008) Aptamer-based biosensors. Trends Anal Chem 27(2):108–117. https://doi.org/10.1016/j.trac.2007.12.004
Song KM, Jeong E, Jeon W et al (2012) Aptasensor for ampicillin using gold nanoparticle based dual fluorescence–colorimetric methods. Anal Bioanal Chem 402(6):2153–2161. https://doi.org/10.1007/s00216-011-5662-3
Song E, Yu M, Wang Y et al (2015) Multi-color quantum dot-based fluorescence immunoassay array for simultaneous visual detection of multiple antibiotic residues in milk. Biosens Bioelectron 72:320–325. https://doi.org/10.1016/j.bios.2015.05.018
Stred’anský M, Pizzariello A, Stred’anská S et al (2000) Amperometric pH-sensing biosensors for urea, penicillin, and oxalacetate. Anal Chim Acta 415(1–2):151–157. https://doi.org/10.1016/S0003-2670(00)00869-2
Taghdisi SM, Danesh NM, Nameghi MA et al (2019) An electrochemical sensing platform based on ladder-shaped DNA structure and label-free aptamer for ultrasensitive detection of ampicillin. Biosens Bioelectron 15(133):230–235. https://doi.org/10.1016/j.bios.2019.111674
Thavarungkul P, Dawan S, Kanatharana P et al (2007) Detecting penicillin G in milk with impedimetric label-free immunosensor. Biosens Bioelectron 23(5):688–694. https://doi.org/10.1016/j.bios.2007.08.003
Tomassetti M, Conta G, Campanella L et al (2016) A flow SPR immunosensor based on a sandwich direct method. Biosensors 6(2):22. https://doi.org/10.3390/bios6020022
Tomassetti M, Merola G, Martini E et al (2017) Comparison between a direct-flow SPR immunosensor for ampicillin and a competitive conventional amperometric device: analytical features and possible applications to real samples. Sensors 17(4):819. https://doi.org/10.3390/s17040819
Trashin S, Rahemi V, Ramji K et al (2017) Singlet oxygen-based electrosensing by molecular photosensitizers. Nat Commun 8:16108. https://doi.org/10.1038/ncomms16108
Wang H, Wang Y, Liu S et al (2015) Target–aptamer binding triggered quadratic recycling amplification for highly specific and ultrasensitive detection of antibiotics at the attomole level. Chem Commun 522:8377–8380. https://doi.org/10.1039/C5CC01473E
Wang X, Dong S, Gai P et al (2016) Highly sensitive homogeneous electrochemical aptasensor for antibiotic residues detection based on dual recycling amplification strategy. Biosens Bioelectron 82:49–54. https://doi.org/10.1016/j.bios.2016.03.055
Wang J, Ma K, Yin H et al (2018) Aptamer based voltammetric determination of ampicillin using a single-stranded DNA binding protein and DNA functionalized gold nanoparticles. Microchim Acta 185(1):68. https://doi.org/10.1007/s00604-017-2566-8
Wang T, Yin H, Zhang Y et al (2019) Electrochemical aptasensor for ampicillin detection based on the protective effect of aptamer-antibiotic conjugate towards DpnII and Exo III digestion. Talanta 197:42–48. https://doi.org/10.1016/j.talanta.2019.01.010
Weber P, Riegger BR, Niedergall K et al (2018) Nano-MIP based sensor for penicillin G: Sensitive layer and analytical validation. Sens Actuators B 267:26–33. https://doi.org/10.1016/j.snb.2018.03.142
Wilkowske HH, Krienke WA (1951) Influence of penicillin on the lactic acid production of certain lactobacilli. J Dairy Sci 34(10):1030–1033. https://doi.org/10.3168/jds.S0022-0302(51)91819-X
Wright AJ (1999) The Penicillins. Mayo Clin Proc 74(3):290–307. https://doi.org/10.4065/74.3.290
Wu H, Fan S, Zhang W et al (2014a) Amperometric immunosensor based on covalent immobilization of new methylene blue and penicillin polyclonal antibody for determination of penicillin G in milk. Anal Methods 6(2):497–502. https://doi.org/10.1039/C3AY41624K
Wu Y, Tang L, Huang L et al (2014b) A low detection limit penicillin biosensor based on single graphene nanosheets preadsorbed with hematein/ionic liquids/penicillinase. Mater Sci Eng C 39:92–99. https://doi.org/10.1016/j.msec.2014.02.012
Xie B, Danielsson B (2008) Thermal biosensor and microbiosensor techniques. In: Lowe C, et al. (eds) Handbook of biosensors and biochips. Wiley, New York
Yang Z, Ding X, Guo Q et al (2017) Second generation of signaling-probe displacement electrochemical aptasensor for detection of picomolar ampicillin and sulfadimethoxine. Sens Actuators B 253:1129–1136. https://doi.org/10.1016/j.snb.2017.07.119
Yola ML, Eren T, Atar N (2014) Molecular imprinted nanosensor based on surface plasmon resonance: Application to the sensitive determination of amoxicillin. Sens Actuators B 195:28–35. https://doi.org/10.1016/j.snb.2014.01.011
Yu ZG, Lai RY (2018) A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of ampicillin in complex samples. Talanta 176:619–624. https://doi.org/10.1016/j.talanta.2017.08.057
Yu ZG, Sutlief AL, Lai RY (2018) Towards the development of a sensitive and selective electrochemical aptamer-based ampicillin sensor. Sens Actuators B 258:722–729. https://doi.org/10.1016/j.snb.2017.11.193
Zhang ZF, Xu KX, Liu J et al (2008) Detection of ampicillin residues in milk by surface plasmon resonance-based optical biosensor assay method. Food Sci 6:6
Zhao J, Guo W, Pei M et al (2016) GR–Fe3O4NPs and PEDOT–AuNPs composite based electrochemical aptasensor for the sensitive detection of penicillin. Anal Methods 8(22):4391–4397. https://doi.org/10.1039/C6AY00555A
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kharewal, T., Verma, N., Gahlaut, A. et al. Biosensors for penicillin quantification: a comprehensive review. Biotechnol Lett 42, 1829–1846 (2020). https://doi.org/10.1007/s10529-020-02970-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02970-6