Abstract
A new vapor generation system for mercury (Hg) species based on the irradiation of mercaptoethanol (ME) with UV was developed to provide an effective sample introduction unit for atomic fluorescence spectrometry (AFS). Preliminary investigations of the mechanism of this novel vapor generation system were based on GC–MS and FT–IR studies. Under optimum conditions, the limits of determination for inorganic divalence mercury and methyl mercury were 60 and 50 pg mL−1, respectively. Certified reference materials (BCR 463 tuna fish and BCR 580 estuarine sediment) were used to validate this new method, and the results agreed well with certified values. This new system provides an attractive alternative method of chemical vapor generation (CVG) of mercury species compared to other developed CVG systems (for example, the traditional KBH4/NaOH–acid system). To our knowledge, this is the first systematic report on UV/ME-based Hg species vapor generation and the determination of total and methyl Hg in environmental and biological samples using UV/ME–AFS.

A new vapor generation system for mercury species using mercaptoethanol under UV irradiation was developed as an effective sample introduction unit for atomic fluorescence spectrometry
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical vapor generation (CVG), including hydride generation, alkylation, halide generation, metal carbonyl and elemental vapor generation, is frequently employed as a method of introducing the sample analyzed in atomic fluorescence spectrometry [1–4]. CVG has several advantages over the conventional pneumatic nebulization system used for sample introduction, such as higher transport efficiency of target elemental species to the detector and efficient on-line matrix separation. Such merits are important not only for enhancing detection power and for eliminating possible physicochemical and spectral interferences from the matrix of a sample, but also for meeting the requirements of the interface linking hyphenated techniques used for elemental speciation, because only trace amounts of the target elemental species are usually present in the sample. Besides the most frequently used KBH4/NaOH–Acid system, some new advances in CVG have recently been reported in terms of understanding the mechanism involved, developing new methodologies, and designing new vapor generation systems, including UV alkylation in the presence of low-molecular-weight organic compounds as well as photocatalytical reduction using the electrons in the conduction band of a nano TiO2 semiconductor [5–11]. These approaches open up new routes to elemental determination and speciation analysis [12].
Mercury (Hg)—a well known and dangerous element—is a typical example of an element that is amenable to CVG, and Hg determination and speciation analyses have been widely performed and extensively reviewed [13–18]. In this study, we developed a new on-line vapor generation system for Hg species using the UV irradiation of mercaptoethanol (ME), used as a reductive radical precursor. The vapor generation mechanism associated with this approach was investigated based on the photochemistry of ME and evidence from capillary gas chromatography coupled with electron impact ionization mass spectrometry (GC–MS) and FT–IR studies. It was used as a sample introduction unit for atomic fluorescence spectrometry (AFS) and applied to total Hg and methyl Hg determinations in biological and environmental samples. To our knowledge, this is the first report of the systematic use of UV/ME–AFS for Hg species analysis.
Experimental
Instrumentation
A nondispersive atomic fluorescence spectrometer (ND-AFS 610A) (Beijing BRAIC Analytical Instrument Corporation, People’s Republic of China) equipped with a high-performance Hg hollow cathode lamp (253.7 nm, Beijing Institute of Vacuum Electronics Research, People’s Republic of China) and a sunlight-blinded photomultiplier tube (PMT) (Hamamatsu, Hamamatsu City, Japan) was used for Hg determination; signal acquisition and processing were carried out by HWH software Version 1.0 [19]. A LEAD-1 peristaltic pump (Baoding Longer Precision Pump Co., Ltd, People’s Republic of China) was used to introduce reagents during the experiments, and a 40-W low-pressure Hg lamp (Xinyuan Appliance Lighting Co., Ltd., People’s Republic of China) surrounded by a PTFE tube (1.5 m in length × 0.8 mm i.d. × 1.6 mm o.d., Cole-Parmer, Vernon Hills, IL, USA) was employed as a light source. These were used as a sample introduction unit for AFS on-line photolysis and to generate the vapor of the Hg species. Each part of the hyphenated system was connected with PTFE tubes (0.8 mm i.d. and 1.6 mm o.d.), as shown schematically in Fig. 1, and the optimum operating conditions are listed in Table 1. A Shimadzu (Kyoto, Japan) QP 2010 GC-MS and a Nicolet (Thermo Fisher Scientific Inc., Waltham, MA, USA) Avatar 360 FT-IR spectrometer were also used when studying the UV/ME Hg species vapor generation mechanism.
Chemical reagents
All chemicals used were of at least analytical-reagent grade, and ultrapure water (UPW) with a resistivity of 18 MΩ (Millipore, Bedford, MA, USA) was used throughout in this study. Methyl mercury chloride (methyl Hg) and mercury nitrate [Hg(NO3)2] were obtained, respectively, from Dr. Ehrenstorfer, Germany and Shanghai Chemicals, China. Methyl Hg and Hg(NO3)2 stock solutions were prepared using methanol (MeOH) (HPLC grade, Tedia, Fairfield, OH, USA) and UPW, respectively, and stored at 4 °C in the dark before use. Working solutions were prepared by further stepwise dilution with MeOH and UPW, respectively. Mercaptoethanol (ME) was purchased from Sigma (St. Louis, MO, USA). The Standard Reference materials BCR 463 (tuna fish) and BCR 580 (estuarine sediment) were obtained from the Community Bureau of Reference (BCR, Brussels, Belgium), and were subsequently used for method validation.
Sample pretreatment
A microwave-assisted digestion method was used for total Hg determination. Appropriate amounts of samples dried at room temperature (0.1–0.2 g) were weighed and put into PTFE bombs, to each of which 5 mL of ultrapure HNO3 was added. The samples were subjected to a cold predigestion step overnight in order to reduce any organic materials in the samples. The sealed bombs were then digested for 5 min (for biological samples) or 10 min (for the sediments) using a MK-III optical fiber pressure-controlled microwave decomposition system (Shinco, Shanghai, China) with a full power of 1200 W, placed inside a fume cupboard. After cooling the sealed bombs, the resulting transparent solutions were diluted with an appropriate amount of UPW to 10 mL and filtered through a 0.45-μm filter. An alkaline extraction procedure was applied to selectively extract the methyl Hg [20–22]. Briefly, 0.1 g of a biological sample or 1 g of a sediment sample and 25% (m/v) KOH (in methanol) were added to each 50-mL centrifuge tube. This mixture was sonicated and shaken mechanically and then CH2Cl2 and concentrated HCl were added to the tube in sequence. Shaking extracted the methyl Hg into the CH2Cl2 phase, while the inorganic Hg remained in the water phase. After centrifuging at 3500 rpm for about 10 min, the CH2Cl2 phase was transferred into a 7-mL centrifuge tube, and 1 mL of a 10 mM sodium thiosulfate solution was added to strip the methyl Hg along with vigorous shaking for 45 min. After the water phase had been filtered through a 0.45-μm membrane, it was injected directly into the (UV/ME)–AFS system for determination.
Results and discussion
Possible mechanism of the generation of Hg species vapor by UV irradiation of ME
When the mixtures of Hg(NO3)2 and/or methyl Hg with ME were irradiated with UV light, a signal from Hg could be detected by AFS after sweeping with argon from a liquid–gas separator. Cowan and Drisko [23] noted that the electron of the ketyl radical generated by the UV light might undergo intermolecular migration to the sulfur atom of the mercaptan in the solution, and a hydrogen could be transferred from the mercaptan to the ketyl radical. ME (1) contains both hydroxyl and mercapto in its molecule and, under UV irradiation, the ME radical (HO\({\mathop {\text{C}}\limits^ \cdot }\)HCH2SH) (2) is generated. Then the electron on the carbon atom may transfer to the sulfur atom through intramolecular migration to form the HOCH2CH2 \({\mathop {\text{S}}\limits^ \cdot }\) radical, which is then further oxidized by the Hg2+ in the reaction medium to form the HOOCCH2 \({\mathop {\text{S}}\limits^ \cdot }\) radical (3), while Hg2+ is reduced to form Hg0, as detected by AFS. The resulting radicals (3) combine to form HOCH2CH2S-SCH2COOH (4) and a cyclic disulfide ester (5) by intramolecular esterification, as shown in Scheme 1. This mechanism was verified by analyzing the solution obtained after the photochemical reaction using GC–MS, as shown in Fig. 2, which shows that signals from HOCH2CH2S–SCH2COOH (m/z 167) and the cyclic disulfide ester (m/z 149) are present in the mass spectrum. Further evidence to support the proposed mechanism is provided by the pH of the Hg2+ and ME mixture, which decreases from 5.3 to 4.5 after reduction under UV irradiation, as well as the presence of typical absorption peaks of ν C = O 1709 cm−1, ν C–O 1048 cm−1 and ν OH 3334 cm−1 as well as δ OH 1292 cm−1 in the FT-IR spectrum of the solution.
Mass spectrum of the solution analyzed using GC–MS. Column: DB-XLB 15 m × 0.25 mm × 0.25 μm; injection temperature, 280 °C; oven temperature program beginning at 120 °C and holding for 2 min, then rising to 200 °C at 20 °C min−1; carrier gas (He) flow rate: 1.2 mL min−1; temperature of interface between GC and MS: 280 °C; electron impact (EI): 70 eV; ion source temperature: 200 °C; detector voltage: 1.2 kV. The solution (3 mL) was prepared by mixing 0.1% ME in methanol and 10 ng Hg2+ irradiated with UV light for 30 s
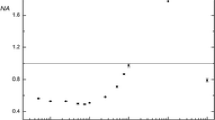
Moreover, comparison of the use of CH3COOH [9], CH3CH2OH [24], HOCH2CH2OH, cysteine and thiourea [25] with ME for Hg vapor generation indicated that the ME is the most effective for this purpose (Fig. 3). A possible reason for this is that only ME contains both hydroxyl and mercapto in its molecule.
Effectiveness of some radical precursors at generating the cold vapors of mercury species: 1% thiourea, 0.1% cysteine, 0.1% C2H5OH, 0.1% CH3COOH, 0.1% ME and 0.1% CH2OH–CH2OH. The experimental conditions used are indicated in Table 1
Performance of the UV/ME–AFS system
Both the ME concentration and the flow rate employed strongly influence the efficiency of Hg species vapor generation. These parameters were optimized using AFS in flow injection mode, and the results indicated that 0.1% ME at a flow rate of 2.0 mL min−1 was optimum for Hg species vapor generation, as shown in Figs. 4 and 5. Although the chemical bond between the carbon atom and Hg in methyl Hg can be effectively broken under UV irradiation [24, 26, 27], it should be noted that the AFS signal intensity of methyl Hg is much higher than that of Hg2+. Obviously, this phenomenon needs further investigation. Under optimum operating conditions, the detection limits (3σ) for Hg2+ and methyl Hg are 60 and 50 pg mL−1, respectively. The signals for both Hg2+ (R 2 = 0.9954) and methyl Hg (0.9992) were linear in the range 1.0–100 ng mL−1, and the RSDs (n = 9; the concentrations of both Hg2+ and methyl Hg were 100 ng mL−1) were less than 3.2%. A comparison of the UV/ME–AFS system with the conventional (KBH4/NaOH–HCl)–AFS system indicated that UV/ME offered an attractive alternative for the generation of Hg species vapor (Fig. 6). On the other hand, a comparison of the detection limits obtained using our UV/ME–AFS system with previously published limits obtained using CV–AAS [28, 29] also indicated that the DL of the UV/ME–AFS system is lower than that of the CV–AAS system. Moreover, because ME is usually used as a component in the mobile phase during the HPLC separation of Hg species [30, 31], UV/ME is more convenient since no additional reagent is needed when it is used as an interface between HPLC and an element-specific detector for Hg speciation analysis.
Effect of ME concentration on the vapor generation of Hg species. Experimental conditions are indicated in Table 1. Uncertainties, shown by the error bars, are expressed as the standard deviation when n = 9
Effect of ME flow rate on the vapor generation of Hg species. The other experimental conditions were the same as in Fig. 4. Uncertainties, shown by the error bars, are expressed as the standard deviation when n = 9
Comparison of the KBH4/NaOH–HCl–AFS system and the UV/ME–AFS system. Experimental conditions: KBH4/NaOH, 1.5% containing 0.5% NaOH; HCl, 2%; the flow rate of both KBH4 /NaOH and HCl was 2.0 mL min−1. The other experimental conditions are indicated in Table 1
Method validation and sample analysis
Two Certified Reference Materials—CRM 463 (tuna fish) and CRM 580 (estuarine sediment)—were used for method validation. The total Hg and methyl Hg concentrations determined by (UV/ME)–AFS agreed well with the certified values (as shown in Table 2), and the recoveries of methyl Hg ranged from 92.7 to 96.7%. This method was applied also to a shellfish (Ostrea) and a lake sediment, which were collected, respectively, off the coast of and in Xiamen Island in September 2006. Both methyl Hg and inorganic Hg were found in the Ostrea and lake sediment samples (Table 2), indicating Hg pollution, probably from local thermoelectric power plants.
Conclusions
A new system based on the UV irradiation of mercaptoethanol was developed in order to generate Hg species vapor. Compared with the conventional KBH4/NaOH–HCl system, UV/ME offers an attractive alternative for the generation of Hg species vapor, and it can be used as an effective sample introduction unit when determining Hg species by atomic spectrometry. Furthermore, UV/ME can be expected to be utilized in the near future, after appropriate modification, as an on-line interface between HPLC and various element-specific detectors used for Hg speciation analysis.
References
Dedina J, Tsalev DL (1995) Hydride generation atomic absorption spectrometry. Wiley, New York
Sturgeon RE, Mester Z (2002) Appl Spectrosc 56:202A–212A
Pohl P (2004) Trends Anal Chem 23:87–101
Pohl P (2004) Trends Anal Chem 23:21–27
D’Ullivo A, Onor M, Pitzalis E (2004) Anal Chem 76:6342–6352
Denkhaus E, Golloch A, Guo X, Huang BL (2001) J Anal Atom Spectrom 16:870–878
Chatterjee A, Shibata Y, Yoneda M, Banerjee R, Uchida M, Kon H, Morita M (2001) Anal Chem 73:3181–3186
Villano M, Padro A, Rubio R, Rauret G (1998) J Chromatogr A 819:211–220
Guo XM, Sturgeon RE, Mester Z, Gardner GJ (2004) Anal Chem 76:2401–2405
Wang QQ, Liang J, Qiu J, Huang BL (2004) J Anal Atom Spectrom 19:715–716
Liang J, Wang QQ, Huang BL (2005) Anal Bioanal Chem 381:366–372
Sturgeon RE, Guo XM, Mester Z (2005) Anal Bioanal Chem 382:881–883
Wilken RD (1992) Fresenius J Anal Chem 342:795–801
Quevauviller P, Draback I, Muntau H, Griepink B (1993) Appl Organom Chem 7:413–420
Puk R, Weber JH (1994) Appl Organom Chem 8:293–302
Lobinski R (1997) Appl Spectrosc 51:260A–278A
Sanchez Uria JE, Sanz-Medel A (1998) Talanta 47:509–524
Leermakers M, Baeyens W, Quevauviller P, Horvat M (2005) Trends Anal Chem 24:383–393
Hong YC, Wang QQ, Yan H, Liang J, Guo XM, Huang BL (2003) Spectrosc Spect Anal 23:354–357
Ramalhosa E, Rio Segade S, Pereira E, Vale C, Duarte A (2001) Anal Chim Acta 448:135–143
Liang LN, Jiang GB, Liu JF, Hu JT (2003) Anal Chim Acta 477:131–137
Ortiz AIC, Albarran YM, Rica CC (2002) J Anal Atom Spectrom 17:1595–1601
Cowan DO, Drisko RL (eds) (1976) Elements of organic photochemistry. Plenum, New York
Zheng CB, Li Y, He YH, Ma Q, Hou XD (2005) J Anal Atom Spectrom 20:746–750
Qiu JH, Wang QQ, Ma YN, Yang LM, Huang BL (2006) Spectrochim Acta B 61:803–809
Falter R, Scholer HF (1996) Fresenius J Anal Chem 354:492–493
Krishna MVB, Ranjit M, Karunasagar D, Arunachalam J (2005) Talanta 67:70–80
Rio-Segade S, Bendicho C (1999) Talanta 48:477–484
Torres DP, Vieira MA, Ribeiro AS, Curtius AJ (2005) J Anal Atom Spectrom 20:289–294
Hintelmann H, Hempel H, Wilken RD (1995) Environ Sci Technol 29:1845–1850
Bloxham MJ, Gachanja A, Hill SJ, Worsfold PJ (1996) J Anal Atom Spectrom 11:145–148
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (No. 20535020, 21075019) and the National Basic Research Program (No. 2003CD415001). The loans of a BRAIC 610A atomic fluorescence spectrometer and Shimadzu QP2010 GC–MS are very much appreciated. We thank Miss YL Zhao for her assistance in GC-MS experiment. Professor John Hodgkiss is thanked for his assistance with the English in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, Y., Qiu, J., Yang, L. et al. A new vapor generation system for mercury species based on the UV irradiation of mercaptoethanol used in the determination of total and methyl mercury in environmental and biological samples by atomic fluorescence spectrometry. Anal Bioanal Chem 388, 831–836 (2007). https://doi.org/10.1007/s00216-007-1122-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-007-1122-5