Abstract
Rationale
Strategies are needed to decrease the abuse liability of mu opioid receptor (MOR) agonists. One strategy under consideration is to combine MOR agonists with kappa opioid receptor (KOR) agonists.
Objectives
The effects of KOR agonists (U50488, nalfurafine) on fentanyl-vs.-food choice were compared under conditions where the KOR agonists were added to the intravenously self-administered fentanyl (contingent delivery) or administered as subcutaneous pretreatments (non-contingent delivery) in male and female rats.
Methods
Rats were trained to respond under a concurrent schedule of fentanyl (0, 0.32–10 μg/kg/infusion) and food reinforcement. In experiment 1, U50488 and nalfurafine were co-administered with fentanyl as fixed-proportion mixtures (contingent administration). In experiment 2, U50488 (1–10 mg/kg) and nalfurafine (3.2–32 μg/kg) were administered as acute pretreatments (non-contingent administration). The selective KOR antagonist, nor-BNI (32 mg/kg), was administered prior to contingent and non-contingent KOR-agonist treatment in experiment 3.
Results
Both U50488 and nalfurafine decreased fentanyl choice when administered contingently, demonstrating that KOR agonists punish opioid choice. However, evidence for punishment corresponded with an elimination of operant responding in the majority of rats. Non-contingent U50488 and nalfurafine administration only decreased the number of choices made during the behavioral session without altering fentanyl choice. Contingent and non-contingent KOR-agonist effects on fentanyl choice were both attenuated by nor-BNI.
Conclusions
These results illustrate that the effects of KOR agonists on fentanyl reinforcement are dependent upon the contingencies under which they are administered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2018, the number of mu opioid receptor (MOR) agonist-related overdose deaths decreased in the USA for the first time in decades, attributable primarily to a decline in fatalities involving prescription opioids over the prior year (Wilson et al. 2020). This welcome reduction in MOR agonist-related overdoses was preceded by a 19% reduction in rates of opioid prescriptions in the USA from 2006 to 2017 (CDC, Prevention USCfDCa 2019), suggesting that decreasing the supply of prescription opioids was an effective approach for reducing the frequency of overdose. However, concerns have been raised that this reduction in prescription opioid availability has led to the inadequate treatment of pain (Pergolizzi Jr et al. 2019). One strategy for addressing the interdependent clinical issues of opioid abuse and pain management includes decreasing the abuse liability of prescription MOR agonists (CDER 2015), with the goal of minimizing the likelihood of misuse while maintaining appropriate access to opioid analgesics to those in pain.
Previous work suggests that kappa opioid receptor (KOR) agonists may decrease the abuse liability of drugs of abuse, including MOR agonists. Stimulation of KOR promotes inhibitory signal transduction through Gi/o processes, locally decreasing neuronal excitability and neurotransmitter release (Di Chiara and Imperato 1988; Walker et al. 1987). KORs are highly enriched throughout mesocorticolimbic structures (ventral tegmental area, nucleus accumbens, prefrontal cortex), and KOR agonists inhibit dopamine accumulation within this pathway (Devine et al. 1993; Donzanti et al. 1992; Spanagel et al. 1990). Administration of KOR agonists can also diminish the ability of drugs of abuse to promote mesocorticolimbic dopamine accumulation (Margolis et al. 2003; Thompson et al. 2000), illustrating an opposing interaction between KOR and dopaminergic neurotransmitter systems (Escobar et al. 2020; Tejeda and Bonci 2019).
An implication of this KOR/dopamine interaction is that KOR agonists would be expected to counteract the dopaminergic reinforcing effects of drugs of abuse. Consistent with this hypothesis, KOR agonists decrease rates of cocaine and MOR agonist self-administration under a variety of circumstances (Bowen et al. 2003; Cosgrove and Carroll 2002; Glick et al. 1995; Glick et al. 1998; Mello and Negus 1998; Negus et al. 1997; Negus et al. 2008; Schenk et al. 1999; Schenk et al. 2001; Townsend et al. 2017; Zamarripa et al. 2020) but see (Kuzmin et al. 1997). However, one interpretive complication of this literature is that reinforcement was determined using rate-based schedules of reinforcement. This is an important consideration when evaluating the effects of KOR-agonist effects, because previous works have shown KOR agonists to similarly decrease rates of drug- and food-maintained responding under rate-based schedules of reinforcement (Cosgrove and Carroll 2002; Mello and Negus 1998; Negus et al. 1997; Negus et al. 2008). Thus, the aforementioned studies do not exclude the possibility that KOR agonists decrease rates of drug self-administration through non-selective effects on operant responding.
One method to minimize the influence of non-selective effects on operant responding includes the use of concurrent “choice” schedules of reinforcement. The primary dependent measure under concurrent schedules is behavioral allocation, which has been shown to be relatively insensitive to alterations in response rate (Banks and Negus 2012). Three studies have utilized choice procedures to evaluate KOR-agonist effects on drug reinforcement. In the earliest study, acute pretreatment with the KOR agonist enadoline failed to attenuate cocaine-vs.-money choice in humans (Walsh et al. 2001a). A later study using rhesus monkeys as subjects found continuous 3-day treatment with the KOR agonist U50488 to increase choice of cocaine over a food alternative (Negus 2004). These findings do not support the hypothesis that KOR agonists selectively decrease the reinforcing effects of drugs of abuse, with the latter results suggesting that KOR-agonist maintenance may actually enhance the reinforcing effects of cocaine. However, a more recent study suggests that the experimental conditions under which a KOR agonist is administered are an important determinant of its effects on drug reinforcement. Using a drug-vs.-drug choice procedure in rhesus monkeys, the KOR-agonist salvinorin A decreased drug + salvinorin A choice, irrespective of whether the reinforcer was remifentanil or cocaine (Freeman et al. 2014). This finding suggests that contingent KOR-agonist administration (i.e., operant responding results in KOR agonist delivery) punishes drug self-administration, as the introduction of the KOR agonist as a consequent stimulus decreased the probability that drug self-administration would occur, consistent with the definition of punishment (Azrin 1966).
A translational implication of the aforementioned results is that a KOR agonist would only be expected to selectively decrease the reinforcing effects of a MOR agonist if it were administered contingently, which could be accomplished by combining a KOR agonist and a MOR agonist into a single medication. However, previous reports of poor tolerability of KOR agonists introduce a potential obstacle to the clinical utility of this approach. Namely, dysphoric, psychotomimetic, and sedative effects have been reported following acute KOR-agonist administration in humans (Kumor et al. 1986; Pfeiffer et al. 1986; Walsh et al. 2001b). These unpleasant KOR-agonist effects could contribute to medication non-compliance, resulting in inadequate pain management.
In recent years, a new class of G protein biased KOR agonists have emerged, and evidence suggests that these compounds may produce fewer adverse effects than traditional KOR agonists (Mores et al. 2019). Relative to traditional KOR agonists that activate intracellular G protein and beta-arrestin pathways with similar potency (e.g., U50488), G protein biased KOR agonists exhibit greater potency to activate the G protein pathway. In light of previous work that supports a role of beta-arrestin activation in the aversive effects of KOR agonists (Bruchas and Chavkin 2010; Bruchas et al. 2007), G protein biased KOR agonists may produce fewer untoward effects and have a greater likelihood of tolerability. One of these drugs is nalfurafine, which functions as a G protein biased agonist at both human and rat KOR (Kaski et al. 2019; Liu et al. 2019; Schattauer et al. 2017). Nalfurafine is the only selective KOR agonist approved for clinical usage, and it has been available for the treatment of uremic pruritus in Japan since 2009. No clinical reports of psychiatric side effects have been reported (Kozono et al. 2018), providing evidence that nalfurafine is well tolerated. Recent preclinical studies have reported that contingently administered nalfurafine can punish oxycodone self-administration in rats and rhesus monkeys when the two drugs are self-administered as a mixture (Townsend et al. 2017; Zamarripa et al. 2020). While encouraging, these studies used rate-based schedules to evaluate oxycodone reinforcement. Therefore, it remains unclear whether nalfurafine decreased rates of MOR agonist self-administration through a non-selective effect other than punishment.
The current study compared the effects of contingent and non-contingent KOR agonist administration on fentanyl-vs.-food choice in male and female rats. We hypothesized that contingent KOR-agonist administration would punish opioid choice, and non-contingent KOR-agonist pretreatment would fail to selectively affect opioid self-administration. The current study also sought to evaluate whether a G protein biased KOR agonist (nalfurafine) produced similar effects on fentanyl-vs.-food choice relative to an unbiased compound (U50488). Pretreatment with nor-BNI (KOR antagonist) evaluated whether contingent and non-contingent effects of U50488 and nalfurafine were each KOR-mediated.
Methods
Subjects
Twelve Sprague-Dawley rats (6 male, 6 female) were acquired at 10 weeks of age (Envigo Laboratories, Frederick, MD, USA) and surgically implanted with vascular access ports (Instech, Plymouth Meeting, PA) with custom-made jugular catheters as described previously (Huskinson et al. 2017). Rats were administered subcutaneous (s.c.) ketoprofen (5 mg/kg) immediately following surgery and again 24 h after surgery. Rats recovered for at least 5 days after surgery before behavioral testing. Although all rats completed the nalfurafine portion of experiment 1, only ten rats completed the U50488 portion of experiment 1, and only eight of the eleven rats completed experiments 2 and 3 (3 male, 5 female), see Supplementary Table 1 for further detail. Notably, the 6th male rat was catheterized but did not recover from surgery. Rats were singly housed in a temperature and humidity-controlled vivarium, maintained on a 12-h light/dark cycle (lights off at 6:00 PM). Water and food (Teklad Rat Diet, Envigo) were provided ad libitum in the home cage. Behavioral testing was conducted 5 days per week from approximately 2:00 PM–4:00 PM. Catheter patency was verified at the conclusion of each experiment by instantaneous muscle tone loss following intravenous (IV) methohexital (0.5 mg) administration. Animal maintenance and research were conducted in accordance with the 2011 guidelines for the care and use of laboratory animals and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Apparatus and catheter maintenance
Eleven modular operant chambers located in sound-attenuating cubicles (Med Associates, St. Albans, VT, USA) were equipped with two retractable levers, a set of three LED lights (red, yellow, green) mounted above each lever, and a retractable “dipper” cup (0.1 ml) located between the levers for presenting diluted Ensure® (18% v/v vanilla flavor Ensure® in tap water; Abbott Laboratories, Chicago, IL, USA). Intravenous drug solutions were delivered by activation of a syringe pump (PHM-100, Med Associates; 0.177 ml/s from 10-ml syringe) located inside the sound-attenuating cubicle as described previously (Townsend et al. 2017). After each behavioral session, catheters were flushed with gentamicin (0.4 mg), followed by 0.1 ml of heparinized saline (10 U/ml).
Drugs
Fentanyl HCl and nalfurafine HCl were provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD) and dissolved in sterile saline. (±) U50488 HCl was purchased commercially (Tocris, Pittsburgh, PA) and dissolved in sterile saline. nor-BNI di-HCl (synthesized and generously provided by K Cheng and K Rice, National Institutes of Health, Bethesda, MD) was dissolved in 1% lactic acid: sterile water at a concentration of 22 mg/ml. Methohexital sodium was purchased from the Virginia Commonwealth University pharmacy (Richmond, VA) and dissolved in sterile water (16 mg/ml). All solutions were passed through a 0.22-μm sterile filter (Millex GV, Millipore Sigma, Burlington, MA) before administration. All drug doses were expressed as the salt forms listed above and delivered based on weights collected at least weekly.
Procedure
Self-administration training
Rats were trained to respond in a fentanyl-vs.-food choice procedure as described previously (Townsend et al. 2019a; Townsend et al. 2019b). Briefly, rats were first trained to respond on the right lever for IV fentanyl (3.2 μg/kg/infusion) under a fixed-ratio 1, 20s timeout (FR1, TO20) schedule of reinforcement, signaled by the illumination of a green stimulus light. After rats earned at least ten fentanyl infusions under the FR1, TO20 schedule, the response requirement for fentanyl infusions was increased to FR5. This FR5, TO20 schedule, was in place for at least five sessions and until the number of earned infusions was stable, defined as the number of earned fentanyl injections differing by less than 20% of the running mean for three consecutive sessions with no trends. Next, rats were trained to respond on the left lever for a 5-s presentation of 18% Ensure® under a FR1, TO20 schedule of reinforcement, signaled by the illumination of a red stimulus light. After rats earned at least fifty food presentations under the FR1, TO20 schedule, the response requirement for food presentations was increased to FR5. The FR5, TO20 schedule, of food reinforcement was in place until three consecutive days with at least 50 earned reinforcers and with no upward- or downward-facing trends. Unit doses of fentanyl and the dilution of Ensure® (18%) were informed by our previous studies (Townsend et al. 2019a; Townsend et al. 2019b).
Once rats were trained to respond for fentanyl and 18% Ensure® in isolation, both reinforcers were made available under a concurrent FR5, TO20: FR5, TO20 schedule of reinforcement. Here, the behavioral session consisted of five 20-min response components each preceded by a “non-contingent component.” Each non-contingent component started with an infusion of the unit fentanyl dose available during the upcoming response component followed by a 2-min time out. Next, a 5-s presentation of liquid food was programmed followed by a 2-min time out. Following this second time out, the response component began. During each response component, both levers were extended, a red stimulus light above the left lever was illuminated to signal liquid food availability, and a green stimulus light above the right lever was illuminated to signal IV fentanyl availability. Response requirement (FR5) completion on the left lever resulted in a 5-s presentation of liquid food whereas response requirement (FR5) completion on the right lever resulted in the delivery of the IV fentanyl dose available for that component. Responding on one lever reset the ratio requirement for the other lever. The Ensure® concentration was held constant throughout the session. A different fentanyl dose was available during each of the five successive response components (0 (no injection), 0.32, 1.0, 3.2, and 10 μg/kg/inf during components 1–5, respectively). Fentanyl dose was varied by changing the infusion duration (e.g., 315 g rat; 0, 0.5, 1.56, 5, and 15.6 s during components 1–5, respectively) and the green light above the fentanyl-lever flashed on and off in 3-s cycles (i.e., longer flashes corresponded with larger fentanyl doses). The resulting injection volumes for a 315-g rat were as follows: 0, 0.09, 0.28, 0.89, and 2.76 ml/injection during components 1–5, respectively.
During each response component, rats could complete up to 10 total ratio requirements between the food- and fentanyl-associated levers. Each ratio requirement completion initiated a 20-s time out, the retraction of both levers, and extinction of the red and green stimulus lights. If all 10 ratio requirements were completed before 20 min had elapsed, then both levers retracted, and stimulus lights were extinguished for the remainder of that component. Choice was considered stable when the smallest fentanyl unit dose that maintained at least 80% of completed ratio requirements on the fentanyl-associated lever (typically 3.2 or 10 μg/kg/injection) was within a 0.5 log unit for three consecutive days with no trends (i.e., stability criteria). During the training phase, stability criteria were not assessed until rats responded in at least 5 choice sessions. A maximum number of choice sessions in a given condition was capped at 10. However, stability criteria were met in all rats for each condition in less than 10 sessions in the current report.
Experiment 1: effects of contingent U50488 and nalfurafine administration on fentanyl vs. food choice
Once the fentanyl-vs.-food-training criteria were met, the punishing effects of contingently administered U50488 and nalfurafine were evaluated. Each fentanyl/KOR agonist mixture was tested for at least 5 sessions and until stability criteria were met. Fentanyl:nalfurafine mixtures were tested in an irregular dosing order (fentanyl:nalfurafine, 1:0.1, 1:0.32, 1:1). Dosing orders are depicted for individual rats in Supplementary Table 1. After completion of the fentanyl:nalfurafine mixtures, fentanyl:U50488 mixtures were similarly tested in an irregular dosing order (fentanyl:U50488, 1:10, 1:32, 1:100). Fentanyl-vs.-food choice was assessed for at least three sessions and until aforementioned stability criteria were met. The stable fentanyl-vs.-food choice data preceding each of the fentanyl:nalfurafine or fentanyl:U50488 mixtures were averaged and depicted as either “fentanyl:nalfurafine: 1:0” or “fentanyl:U50488: 1:0” (i.e., fentanyl alone), respectively.
Experiment 2: effects of non-contingent U50488 and nalfurafine administration on fentanyl vs. food choice
The effects of non-contingent KOR agonist administration on fentanyl-vs.-food choice were also evaluated. Rats received a s.c. injection of KOR agonist or vehicle (saline) 10 min prior to every other choice session, with a fentanyl-vs.-food choice session in the absence of KOR-agonist pretreatment separating each test condition. Nalfurafine (vehicle (saline), 3.2, 10, and 32 μg/kg) and U504488 (vehicle (saline), 1, 3.2, 10 mg/kg) pretreatments were tested in an irregular dosing order (see Supplementary Table 1). All nalfurafine conditions were tested before those of U50488. The 10-min pretreatment time was based on previous U50488 and nalfurafine intracranial self-stimulation studies (Faunce and Banks 2020; Lazenka et al. 2018).
Experiment 3: effects of nor-BNI on U50488 and nalfurafine administration on fentanyl vs. food choice
The effects of nor-BNI (selective KOR antagonist) treatment on both contingent and non-contingent U50488 and nalfurafine administration were determined as a final experiment. A single intraperitoneal injection of nor-BNI (32 mg/kg, i.p.) occurred 24 h prior to behavioral studies, accounting for the slow onset and long duration of action of the compound (Endoh et al. 1992). nor-BNI effects were evaluated across 5 daily testing sessions, wherein (1) KOR-agonist vehicle (saline, s.c.), (2) 1:100 (fentanyl:U50488, i.v.), (3) 1:1 (fentanyl:nalfurafine, i.v.), (4) 10 mg/kg U50488 (s.c.), and (5) 32 μg/kg nalfurafine (s.c.) were tested for a single session in an irregular dosing order (see Supplemental Materials). Pretreatment times for non-contingent KOR-agonist injections were 10 min.
Data analysis
The two primary dependent measures were (1) percent drug choice, defined as (number of ratio requirements, or “choices,” completed on the fentanyl-associated lever/total number of choices completed on both levers) × 100, and (2) number of choices per component. For experiment 1, data from the last three stable testing sessions were averaged for analysis. These stable data were also compared to those observed on the first day of the condition. For experiments 2 and 3, analysis was performed from the single test session of each condition. Results were plotted as a function of unit fentanyl dose and analyzed using a two-way ANOVA or a mixed-model analysis in instances of missing data (noted in figures) (GraphPad Prism 8, La Jolla, CA). Baseline choice functions were re-determined for each experiment and KOR agonist. These five baseline functions were compared within using a mixed-model analysis. For each experiment, data were separated by sex and analyzed using a distinct two-way ANOVA or mixed-model analysis for each experimental condition. For these sex comparisons, the p values of related tests were corrected for type I error using the Benjamini and Hochberg procedure (Keselman et al. 2002) within each experiment. In the presence of a significant main effect or interaction, a Dunnett post hoc test was conducted as appropriate. Statistical significance was set a priori at the 95% confidence level (p < 0.05).
Results
Baseline fentanyl vs. food choice
Under baseline conditions, liquid food was almost exclusively chosen when no fentanyl or the smallest unit dose of fentanyl (0.32 μg/kg/infusion) was available (dashed lines; Figs. 1a, 1b, 2a, 2b, 3a). As the fentanyl dose increased, behavior was reallocated to the fentanyl lever and the largest fentanyl dose (10 μg/kg/infusion) maintained near exclusive fentanyl choice. Additionally, choices per component decreased as a function of increasing fentanyl doses (dashed lines; Figs. 1c, 1d, 2c, 2d, 3b). Baseline choice behavior remained stable across experiments 1, 2, and 3 (percent fentanyl choice, fentanyl dose F1.2, 10.9 = 103.5, p < 0.0001; experiment F1.6, 14.4 = 1, p = 0.36; interaction F3.4, 23.1 = 1.2, p = 0.32; choices per component, fentanyl dose F2.7, 24.3 = 230.4, p < 0.0001; experiment F2.5, 22.5 = 1.2, p = 0.31; interaction F3.3, 23.3 = 0.75, p = 0.54). No effect of sex was detected on either dependent measure under baseline or vehicle-treatment conditions. Data are plotted separately for each sex in Supplementary Figures 1–5.
Effectiveness of KOR agonists to punish choice of fentanyl over food in male and female rats. Abscissa: IV fentanyl unit dose in μg/kg. Top row ordinates: percentage of completed ratio requirements on the fentanyl-associated lever. Bottom row ordinates: number of choices completed per component. Left panels: effects of contingently administered U50488 on percent fentanyl choice (a) and the number of choices completed per component (c) (n = 6 female, 4 male). Right panels: effects of contingently administered nalfurafine on percent fentanyl choice (b) and the number of choices completed per component (d) (n = 6 female, 5 male). Points represent mean ± SEM, numbers in parenthesis denote the number of rats that completed at least one ratio requirement at a given data point in instances wherein a subset of rats did not respond, and filled symbols denote significant difference relative to baseline. * denotes a significant fentanyl unit dose × fentanyl:nalfurafine mixture proportion interaction. Significance defined as p < 0.05
Effectiveness of non-contingent administration of KOR agonists on fentanyl-vs.-food choice in male and female rats. Abscissa: IV fentanyl unit dose in μg/kg. Top row ordinates: percentage of completed ratio requirements on the fentanyl-associated lever. Bottom row ordinates: number of choices completed per component. Left panels: effects of non-contingent U50488 pretreatment on percent fentanyl choice (a) and the number of choices completed per component (c) (n = 5 female, 3 male). Right panels: effects of non-contingent nalfurafine pretreatment on percent fentanyl choice (b) and the number of choices completed per component (d) (n = 5 female, 3 male). Points represent mean ± SEM, numbers in parenthesis denote the number of rats that completed at least one ratio requirement at a given data point in instances wherein a subset of rats did not respond, and filled symbols denote significant difference relative to baseline. Of note, none of the rats responded during the 0 μg/kg/injection component following injection of 10 mg/kg U50488. Significance defined as p < 0.05
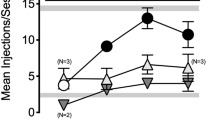
Effectiveness of nor-BNI to block the effects of contingent and non-contingent KOR-agonist administration on fentanyl-vs.-food choice in male and female rats (n = 5 female, 3 male). Abscissa: IV fentanyl unit dose in μg/kg. Top row ordinate (a): percentage of completed ratio requirements on the fentanyl-associated lever. Bottom row ordinate (b): number of choices completed per component. Points represent mean ± SEM, numbers in parenthesis denote the number of rats that completed at least one ratio requirement at a given data point in instances wherein a subset of rats did not respond (i.e., 1:100 fentanyl:U50488), and filled symbols denote significant difference relative to baseline. Significance defined as p < 0.05
Experiment 1: effects of contingent U50488 and nalfurafine administration on fentanyl vs. food choice
The unbiased KOR agonist, U50488, functioned as a punisher of fentanyl choice (Fig. 1a; fentanyl dose F2, 17.9 = 0.5, p < 0.0001; fentanyl:U50488 mixture proportion F2.3, 20.7 = 0.76, p = 0.003; interaction F5, 40 = 0.41, p = 0.0007). Although the majority of rats did not respond when the largest unit dose of fentanyl was combined with U50488 or nalfurafine (4/10 and 2/11, respectively), choice of the largest unit dose of the 1:100 (fentanyl:U50488) proportion was significantly decreased. In addition, a “unit fentanyl dose and fentanyl/nalfurafine mixture” interaction was detected (Fig. 1b; fentanyl dose F1.7, 17.2 = 0.43, p < 0.0001; fentanyl:nalfurafine mixture proportion F1.8, 18 = 2.5, p = 0.12; interaction F2.4, 20 = 4.8, p = 0.016). However, post hoc analysis did not detect significant changes in fentanyl choice relative to fentanyl alone. The average number of choices completed per component was not significantly affected by contingent KOR agonist administration (Fig. 1c and d, respectively). Neither percent fentanyl choice nor the number of choices completed per component were significantly different between the first day of the condition and the stable average. When the data were separated by sex, the number of choices completed per component was lower in male rats relative to female rats when the 1:100 (fentanyl:U50488) proportion was available (adjusted p = 0.04). No other effect of sex was detected on either dependent measure following contingent administration of U50488 or nalfurafine. Data are plotted separately for each sex in Supplementary Figures 1 and 2.
Experiment 2: effects of non-contingent U50488 and nalfurafine administration on fentanyl vs. food choice
Pretreatment with U50488 or nalfurafine did not significantly alter behavioral allocation between fentanyl and food (U50488, Fig. 2a; nalfurafine, Fig. 2b). However, both KOR agonists decreased the number of choices completed per component (U50488, Fig. 2c; fentanyl dose, F2.8, 19.6 = 39.9, p < 0.0001; fentanyl:U50488 mixture proportion F1.8, 12.6 = 31, p < 0.0001; interaction F2.9, 20.4 = 6.6, p = 0.003; nalfurafine, Fig. 2d; fentanyl dose F1.7, 11.6 = 41.9, p < 0.0001; fentanyl:nalfurafine mixture proportion F2.2, 15.2 = 10.9, p = 0.001; interaction F3.8, 26.5 = 4, p = 0.013). Percent fentanyl choice was greater in male rats relative to female rats following pretreatment with 3.2 μg/kg nalfurafine (adjusted p = 0.03). No other effect of sex was detected on either dependent measure following non-contingent U50488 or nalfurafine administration. Data are plotted separately for each sex in Supplementary Figures 3 and 4.
Experiment 3: effects of nor-BNI on U50488 and nalfurafine administration on fentanyl vs. food choice
Administration of the KOR antagonist nor-BNI blocked the effects of contingent and non-contingent KOR agonist administration on fentanyl choice and the number of choices completed per component (Fig. 3). An effect of sex was detected for fentanyl choice of the 1:1 (fentanyl:nalfurafine) mixture following nor-BNI treatment (adjusted p = 0.011), with increased fentanyl choice in male rats. No other effect of sex was detected on either dependent measure in experiment 3. Data are plotted separately for each sex in Supplementary Figure 5.
Discussion
The current study demonstrated that KOR agonists punished fentanyl self-administration under a choice procedure when administered contingently as a fixed-proportion of fentanyl/KOR agonist. In addition, KOR agonists did not significantly attenuate fentanyl self-administration when administered as non-contingent pretreatments up to KOR agonist doses that decreased rates of operant responding. This demonstration of KOR-mediated punishment under an opioid-vs.-food choice procedure is in agreement with previous results of salvinorin A punishing cocaine and remifentanil choice (Freeman et al. 2014), and extends the generality of this finding to include the KOR agonists U50488 and nalfurafine. When considered alongside previous reports of nalfurafine’s tolerability in clinical populations (Kozono et al. 2018), nalfurafine-mediated punishment of fentanyl self-administration may be encouraging, as nalfurafine is clinically available and could be repurposed to decrease the abuse liability of opioid analgesics. However, only two of eleven rats responded in the final component when the 1:1 (fentanyl:nalfurafine) mixture was available, suggesting that KOR-induced punishment may correspond with undesirable effects such as motoric impairment or sedation.
The putative G protein biased KOR agonist nalfurafine and the unbiased KOR agonist U50488 similarly punished fentanyl self-administration. Although weaker evidence of punishment was observed with nalfurafine based on statistical analysis, the overall findings were consistent with a recent report of contingently administered nalfurafine and salvinorin A decreasing rates of oxycodone self-administration under a progressive-ratio schedule of reinforcement in rhesus monkeys (Zamarripa et al. 2020). Considered alongside reports that nalfurafine and other G protein biased KOR agonists attenuate the conditioned rewarding effects of MOR agonists (Kaski et al. 2019; Tsuji et al. 2001), these results suggest that the abuse-limiting effects of KOR agonists may be G protein mediated. Furthermore, in light of evidence that the aversive effects of KOR agonists are beta-arrestin-mediated (Bruchas and Chavkin 2010; Bruchas et al. 2007), these results also suggest that aversion may not be a necessary component of KOR-mediated punishment. However, given that nalfurafine is only moderately G protein biased, particularly at rat KOR (Schattauer et al. 2017), higher doses of nalfurafine may have recruited beta-arrestin-mediated processes. Therefore, the evaluation of additional KOR agonists with greater intracellular bias for the G protein pathway (Mores et al. 2019) as well as beta-arrestin biased KOR agonists (Crowley et al. 2020) could clarify the role of G protein and beta-arrestin signaling in KOR-mediated punishment.
Acute, non-contingent administration of subcutaneous U50488 or nalfurafine failed to selectively decrease fentanyl choice. These findings suggest that KOR agonists would not be effective as standalone treatments for substance use disorders (Banks 2020) and are in agreement with a report of acute enadoline treatment failing to selectively affect cocaine-vs.-money choice in human volunteers (Walsh et al. 2001a). Given that the number of fentanyl and food choices decreased following U50488 and nalfurafine pretreatment (Fig. 2c and d), these data suggest that these KOR agonist treatments would have produced orderly, dose-dependent decreases in fentanyl self-administration if fentanyl reinforcement was assessed in isolation. However, by including an alternative reinforcer (food), these data provide evidence that acute KOR-agonist pretreatment produces non-selective decreases in operant responding, which would be hypothesized not to be a favorable attribute of a substance use disorder treatment. Indeed, previous works have highlighted the importance of evaluating behavioral selectivity of treatment effects of candidate substance use disorder treatments (Czoty et al. 2016; Haney and Spealman 2008; Mello and Negus 1996), arguing that such measures increase the potential for preclinical-to-clinical translation.
The current report found the punishing effectiveness of U50488 and nalfurafine to be most evident when the largest dose of fentanyl (10 μg/kg/infusion) was available. These results are in contrast to previous works investigating the effectiveness of other punishing stimuli to attenuate cocaine reinforcement (Johanson 1975; Johanson 1977; Negus 2005). Here, the punishing effectiveness of electric shock and histamine decreased as the dose of cocaine increased. Differences in the delivery of the punishing stimuli may account for these differences. In the previous studies, the punishing effectiveness of a fixed intensity of electric shock (Johanson 1975; Johanson 1977) or a fixed dose of histamine (Negus 2005) was evaluated across a range of cocaine doses. In the current study, KOR agonists were administered as a fixed mixture proportion with fentanyl, wherein the doses of each drug were increased in tandem. An implication of the current results is that formulating an opioid analgesic with a pharmacological punisher may limit the reinforcing dose range of the opioid analgesic, as consumption of higher doses of the opioid analgesic would be met with higher doses of the pharmacological punisher. However, the proportion of opioid analgesic to punisher would need to be carefully considered, with the amount of punisher being sufficient to discourage overconsumption while being low enough such that appropriate clinical usage is unpunished, else risking medication non-compliance.
Although evidence for punishment was detected with both KOR agonists, it also corresponded with an elimination of operant behavior in most rats. Specifically, only four of eleven rats responded in the final component when the 1:100 (fentanyl:U50488) mixture was available and only two of eleven rats responded in the final component when the 1:1 (fentanyl:nalfurafine) mixture was available. These results differ from those of a previous study that evaluated the effects of naltrexone and a fentanyl-targeted vaccine, which used the same fentanyl-vs.-food choice procedure as the current report. Here, naltrexone- and vaccine-induced decreases in percent fentanyl choice were accompanied by significant increases in the total number of choices completed (Townsend et al. 2019a). The lack of consistent behavioral reallocation away from fentanyl and towards food presentations following contingent KOR-agonist delivery may be the consequence of undesirable KOR-mediated effects such as sedation. For example, a recent study found that combining nalfurafine or U50488 with oxycodone enhanced the sedation-like effects of oxycodone in rhesus monkeys (Huskinson et al. 2020). However, foundational work evaluating the effectiveness of electric shock to punish food-maintained responding noted that rates of responding similarly decrease (Azrin 1959; Azrin 1966; Bergman and Johanson 1981; Grove and Schuster 1974). Given that electric shock does not produce sedative effects, these findings suggest rates of behavior decrease following delivery of punishing stimuli and that the decreases in the number of choices completed in the current study may not necessarily reflect sedation. Future studies could increase the session duration or use a discrete trial choice procedure to limit potential carryover effects from the previous self-administered drug or drug mixture. In addition, preclinical “food-vs.-food+drug” choice procedures could evaluate whether mixtures of MOR and KOR agonists (1) lack reinforcing effects or (2) serve as punishing stimuli themselves (Minervini et al. 2019). Ultimately, human laboratory studies are needed to evaluate the relationship between the reinforcing, punishing, and subjective effects of opioid analgesic/KOR agonist mixtures.
No sex differences in the punishing effectiveness of U50488 or nalfurafine were detected. However, the 1:100 (fentanyl:U5088) mixture was more effective to decrease the number of choices completed in male rats (Supplementary Figure 1), with only one male rat responding when the highest unit dose was available. In addition, no male rats responded when the highest unit dose of the 1:1 (fentanyl:nalfurafine) mixture was available. These data are consistent with previous reports of increased sensitivity to the locomotor-suppressant effects of U50488 in male rats (Craft and Bernal 2001; Kavaliers and Innes 1987). Following non-contingent administration of the smallest tested dose of nalfurafine (3.2 μg/kg), male rats were found to choose more fentanyl than female subjects. However, these effects do not appear to be dose-dependent, as sex differences in fentanyl choice were not detected at larger nalfurafine doses or any tested dose of U50488 (Supplementary Figure 4). Male rats also exhibited increased choice of the 1:1 (fentanyl:nalfurafine) mixture following treatment with nor-BNI (Supplementary Figure 5). The effects of KOR agonist treatment were quantitatively similar between sexes otherwise. Nevertheless, sex differences in KOR agonist effects were detected despite the relatively low sample size per sex. In light of previous reports of increased sensitivity to the punishing effects of electric shock in female rodents (Kutlu et al. 2020; Orsini et al. 2016), these results provide rationale for the further evaluation of the interaction between sex as a biological variable and punishment.
At least two variables besides the contingency underlying KOR-agonist administration were present in experiments 1 and 2. First, the timing of KOR agonist administration differed. In experiment 1, U50488 and nalfurafine were added to the self-administered fentanyl solution, such that a mixture of KOR agonist and fentanyl was only delivered following responding on the fentanyl-associated lever in the response component or during the preceding non-contingent component. In contrast, U50488 and nalfurafine were only administered as 10-min pretreatments to the self-administration session in experiment 2. The differences in the timing of KOR agonist administration could be overcome by adding a yoked group of rats to experiment 1. Here, each time a rat self-administered a fentanyl:KOR agonist mixture, a yoked rat would receive a non-contingent KOR-agonist injection during its fentanyl-vs.-food choice session. This experimental design would leave only the contingency of KOR agonist administration as the between-group variable. However, a strength of the design of experiment 2 (i.e., non-contingent pretreatment) is that it ensures that the KOR agonist was present during each choice between fentanyl and food. In a previous study, acute pretreatment with the KOR agonist enadoline failed to attenuate cocaine-vs.-money choice in humans (Walsh et al. 2001a). These findings are in agreement with those of experiment 2 and suggest that KOR agonist stimulation does not affect the decision to choose fentanyl or cocaine over a non-drug alternative. A second variable that distinguished experiments 1 and 2 besides contingency was the route of KOR-agonist delivery. U50488 and nalfurafine were administered intravenously in experiment 1 and subcutaneously in experiment 2. The decision to deliver KOR agonists subcutaneously in experiment 2 was informed by previous data to suggest a relatively short duration of action of U50488. Specifically, previous antinociceptive studies with U50488 have reported the duration of action to be between 2 and 2.5 h following intraperitoneal administration (Bhargava et al. 1994; Gullapalli and Ramarao 2002; Russell et al. 2014). The sustained significant decrease in the number of choices completed following the highest tested doses of U50488 (Fig. 2c) and nalfurafine (Fig. 2d) provides evidence that the effects of these drugs were present throughout the choice session. However, it is unclear whether intravenous administration would have been sufficient to produce sustained effects throughout the choice session. Finally, the results of experiment 3 illustrate that the effects of both subcutaneous and intravenous U50488 and nalfurafine were similarly antagonized by nor-BNI, providing evidence that the differing effects observed between experiments 1 and 2 were KOR-mediated, irrespective of route of administration.
The current results illustrate that the effects of a drug on behavior can be more complex than its interactions with receptors. Experimenter-administered delivery of KOR agonists to the subject was found to produce non-specific decreases in self-administration of both fentanyl and Ensure®. However, if delivery of the KOR agonist was the consequence of fentanyl self-administration by the subject, the relative reinforcing effects of fentanyl were decreased. A potential clinical implication of this finding is that formulating a KOR agonist with an opioid analgesic (MOR agonist) may decrease the likelihood of misuse and overconsumption. Furthermore, the results of experiment 2 do not support the use KOR agonists as standalone substance use disorder medications. An additional and intriguing question for future research is whether the contingency-dependent effects of KOR agonists can be dissociated from a neurochemical, circuit, or other quantitative neurobiological measure. Recent efforts have addressed similar questions related to the punishing effects of electric shock (Jacobs and Moghaddam 2020; Kutlu et al. 2020; Verharen et al. 2020). Expanding this area of research to include pharmacological punishers such as KOR agonists could elucidate both similarities and differences between KOR- and shock-mediated punishment, which may aid in the identification of substrates that mediate environmental and contingency-dependent determinants of decision-making.
References
Azrin NH (1959) Punishment and recovery during fixed-ratio performance. J Exp Anal Behav 2:301–305. https://doi.org/10.1901/jeab.1959.2-301
Azrin NHH (1966) Punishment. In: Honig WK (ed) Operant behavior: areas of research and application. Appleton-Century-Crofts, New York
Banks ML (2020) The rise and fall of kappa-opioid receptors in drug abuse research. Handb Exp Pharmacol 258:147–165. https://doi.org/10.1007/164_2019_268
Banks ML, Negus SS (2012) Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci 2012:281768. https://doi.org/10.1155/2012/281768
Bergman J, Johanson CE (1981) The effects of electric shock on responding maintained by cocaine in rhesus monkeys. Pharmacol Biochem Behav 14:423–426. https://doi.org/10.1016/0091-3057(81)90413-5
Bhargava HN, Matwyshyn GA, Reddy PL, Veeranna (1994) Brain and spinal cord kappa opiate receptors and pharmacological responses to U-50,488H in rats of differing ages. Pharmacol Biochem Behav 48:87–91. https://doi.org/10.1016/0091-3057(94)90502-9
Bowen CA, Negus SS, Zong R, Neumeyer JL, Bidlack JM, Mello NK (2003) Effects of mixed-action kappa/mu opioids on cocaine self-administration and cocaine discrimination by rhesus monkeys. Neuropsychopharmacology 28:1125–1139. https://doi.org/10.1038/sj.npp.1300105
Bruchas MR, Chavkin C (2010) Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology 210:137–147. https://doi.org/10.1007/s00213-010-1806-y
Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C (2007) Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci 27:11614–11623. https://doi.org/10.1523/JNEUROSCI.3769-07.2007
CDC, Prevention USCfDCa (2019) Trends in annual opioid prescribing rates by overall and high-dosage prescriptions vol https://www.cdc.gov/drugoverdose/data/prescribing/prescribing-practices.html
CDER (2015) Abuse-deterrent opioids —evaluation and labeling vol https://www.fda.gov/regulatory-information/search-fda-guidance-documents/abuse-deterrent-opioids-evaluation-and-labeling. U.S. Department of Health and Human Services
Cosgrove KP, Carroll ME (2002) Effects of bremazocine on self-administration of smoked cocaine base and orally delivered ethanol, phencyclidine, saccharin, and food in rhesus monkeys: a behavioral economic analysis. J Pharmacol Exp Ther 301:993–1002. https://doi.org/10.1124/jpet.301.3.993
Craft RM, Bernal SA (2001) Sex differences in opioid antinociception: kappa and ‘mixed action’ agonists. Drug Alcohol Depend 63:215–228. https://doi.org/10.1016/s0376-8716(00)00209-x
Crowley RS, Riley AP, Alder AF, Anderson RJ III, Luo D, Kaska S, Maynez P, Kivell BM, Prisinzano TE (2020) Synthetic studies of neoclerodane diterpenes from Salvia divinorum: design, synthesis, and evaluation of analogues with improved potency and G-protein activation bias at the mu opioid receptor. ACS Chem Neurosci 11:1781–1790. https://doi.org/10.1021/acschemneuro.0c00191
Czoty PW, Stoops WW, Rush CR (2016) Evaluation of the “pipeline” for development of medications for cocaine use disorder: a review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev 68:533–562. https://doi.org/10.1124/pr.115.011668
Devine DP, Leone P, Pocock D, Wise RA (1993) Differential involvement of ventral tegmental mu, delta and kappa opioid receptors in modulation of basal mesolimbic dopamine release: in vivo microdialysis studies. J Pharmacol Exp Ther 266:1236–1246
Di Chiara G, Imperato A (1988) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080
Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF (1992) Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol 78:193–210
Endoh T, Matsuura H, Tanaka C, Nagase H (1992) Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther 316:30–42
Escobar ADP, Casanova JP, Andres ME, Fuentealba JA (2020) Crosstalk between kappa opioid and dopamine systems in compulsive behaviors. Front Pharmacol 11:57. https://doi.org/10.3389/fphar.2020.00057
Faunce KE, Banks ML (2020) Effects of repeated kappa-opioid receptor agonist U-50488 treatment and subsequent termination on intracranial self-stimulation in male and female rats. Exp Clin Psychopharmacol 28:44–54. https://doi.org/10.1037/pha0000287
Freeman KB, Naylor JE, Prisinzano TE, Woolverton WL (2014) Assessment of the kappa opioid agonist, salvinorin A, as a punisher of drug self-administration in monkeys. Psychopharmacology 231:2751–2758. https://doi.org/10.1007/s00213-014-3436-2
Glick SD, Maisonneuve IM, Raucci J, Archer S (1995) Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res 681:147–152. https://doi.org/10.1016/0006-8993(95)00306-b
Glick SD, Visker KE, Maisonneuve IM (1998) Effects of cyclazocine on cocaine self-administration in rats. Eur J Pharmacol 357:9–14. https://doi.org/10.1016/s0014-2999(98)00548-2
Grove RN, Schuster CR (1974) Suppression of cocaine self-administration by extinction and punishment. Pharmacol Biochem Behav 2:199–208. https://doi.org/10.1016/0091-3057(74)90053-7
Gullapalli S, Ramarao P (2002) Role of L-type Ca(2+) channels in pertussis toxin induced antagonism of U50,488H analgesia and hypothermia. Brain Res 946:191–197. https://doi.org/10.1016/s0006-8993(02)02880-9
Haney M, Spealman R (2008) Controversies in translational research: drug self-administration. Psychopharmacology 199:403–419. https://doi.org/10.1007/s00213-008-1079-x
Huskinson SL, Naylor JE, Townsend EA, Rowlett JK, Blough BE, Freeman KB (2017) Self-administration and behavioral economics of second-generation synthetic cathinones in male rats. Psychopharmacology 234:589–598. https://doi.org/10.1007/s00213-016-4492-6
Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, Freeman KB (2020) Quantification of observable behaviors induced by typical and atypical kappa-opioid receptor agonists in male rhesus monkeys. Psychopharmacology. https://doi.org/10.1007/s00213-020-05519-7
Jacobs DS, Moghaddam B (2020) Prefrontal cortex representation of learning of punishment probability during reward-motivated actions. J Neurosci. https://doi.org/10.1523/JNEUROSCI.0310-20.2020
Johanson CE (1975) Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev 27:343–355
Johanson CE (1977) The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology 53:277–282. https://doi.org/10.1007/BF00492364
Kaski SW et al (2019) Preclinical testing of nalfurafine as an opioid-sparing adjuvant that potentiates analgesia by the mu opioid receptor-targeting agonist morphine. J Pharmacol Exp Ther 371:487–499. https://doi.org/10.1124/jpet.118.255661
Kavaliers M, Innes DG (1987) Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacol Biochem Behav 27:477–482. https://doi.org/10.1016/0091-3057(87)90351-0
Keselman HJ, Cribbie R, Holland B (2002) Controlling the rate of type I error over a large set of statistical tests. Br J Math Stat Psychol 55:27–39. https://doi.org/10.1348/000711002159680
Kozono H, Yoshitani H, Nakano R (2018) Post-marketing surveillance study of the safety and efficacy of nalfurafine hydrochloride (Remitch((R)) capsules 2.5 mug) in 3,762 hemodialysis patients with intractable pruritus. Int J Nephrol Renov Dis 11:9–24. https://doi.org/10.2147/IJNRD.S145720
Kumor KM, Haertzen CA, Johnson RE, Kocher T, Jasinski D (1986) Human psychopharmacology of ketocyclazocine as compared with cyclazocine, morphine and placebo. J Pharmacol Exp Ther 238:960–968
Kutlu MG et al (2020) A novel multidimensional reinforcement task in mice elucidates sex-specific behavioral strategies. Neuropsychopharmacology. https://doi.org/10.1038/s41386-020-0692-1
Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, Van Ree JM (1997) Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur J Pharmacol 321:265–271. https://doi.org/10.1016/s0014-2999(96)00961-2
Lazenka ML, Moerke MJ, Townsend EA, Freeman KB, Carroll FI, Negus SS (2018) Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats. Psychopharmacology 235:203–213. https://doi.org/10.1007/s00213-017-4758-7
Liu JJ et al (2019) Phosphoproteomic approach for agonist-specific signaling in mouse brains: mTOR pathway is involved in kappa opioid aversion. Neuropsychopharmacology 44:939–949. https://doi.org/10.1038/s41386-018-0155-0
Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23:9981–9986
Mello NK, Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14:375–424. https://doi.org/10.1016/0893-133X(95)00274-H
Mello NK, Negus SS (1998) Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther 286:812–824
Minervini V, Osteicoechea DC, Casalez A, France CP (2019) Punishment and reinforcement by opioid receptor agonists in a choice procedure in rats. Behav Pharmacol 30:335–342. https://doi.org/10.1097/FBP.0000000000000436
Mores KL, Cummins BR, Cassell RJ, van Rijn RM (2019) A review of the therapeutic potential of recently developed G protein-biased kappa agonists. Front Pharmacol 10:407. https://doi.org/10.3389/fphar.2019.00407
Negus SS (2004) Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology 176:204–213. https://doi.org/10.1007/s00213-004-1878-7
Negus SS (2005) Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology 181:244–252. https://doi.org/10.1007/s00213-005-2266-7
Negus SS, Mello NK, Portoghese PS, Lin CE (1997) Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282:44–55
Negus SS, Schrode K, Stevenson GW (2008) Micro/kappa opioid interactions in rhesus monkeys: implications for analgesia and abuse liability. Exp Clin Psychopharmacol 16:386–399. https://doi.org/10.1037/a0013088
Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B (2016) Sex differences in a rat model of risky decision making. Behav Neurosci 130:50–61. https://doi.org/10.1037/bne0000111
Pergolizzi JV Jr, Rosenblatt M, LeQuang JA (2019) Three years down the road: the aftermath of the CDC guideline for prescribing opioids for chronic pain. Adv Ther 36:1235–1240. https://doi.org/10.1007/s12325-019-00954-1
Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233:774–776. https://doi.org/10.1126/science.3016896
Russell SE, Rachlin AB, Smith KL, Muschamp J, Berry L, Zhao Z, Chartoff EH (2014) Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry 76:213–222. https://doi.org/10.1016/j.biopsych.2013.07.042
Schattauer SS, Kuhar JR, Song A, Chavkin C (2017) Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal 32:59–65. https://doi.org/10.1016/j.cellsig.2017.01.016
Schenk S, Partridge B, Shippenberg TS (1999) U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology 144:339–346. https://doi.org/10.1007/s002130051016
Schenk S, Partridge B, Shippenberg TS (2001) Effects of the kappa-opioid receptor agonist, U69593, on the development of sensitization and on the maintenance of cocaine self-administration. Neuropsychopharmacology 24:441–450. https://doi.org/10.1016/S0893-133X(00)00190-1
Spanagel R, Herz A, Shippenberg TS (1990) The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem 55:1734–1740. https://doi.org/10.1111/j.1471-4159.1990.tb04963.x
Tejeda HA, Bonci A (2019) Dynorphin/kappa-opioid receptor control of dopamine dynamics: implications for negative affective states and psychiatric disorders. Brain Res 1713:91–101. https://doi.org/10.1016/j.brainres.2018.09.023
Thompson AC, Zapata A, Justice JB Jr, Vaughan RA, Sharpe LG, Shippenberg TS (2000) Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci 20:9333–9340
Townsend EA et al (2017) Effects of nalfurafine on the reinforcing, thermal antinociceptive, and respiratory-depressant effects of oxycodone: modeling an abuse-deterrent opioid analgesic in rats. Psychopharmacology 234:2597–2605. https://doi.org/10.1007/s00213-017-4652-3
Townsend EA et al (2019a) Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats. Neuropsychopharmacology. https://doi.org/10.1038/s41386-019-0385-9
Townsend EA, Negus SS, Caine SB, Thomsen M, Banks ML (2019b) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology. https://doi.org/10.1038/s41386-019-0356-1
Tsuji M, Takeda H, Matsumiya T, Nagase H, Narita M, Suzuki T (2001) The novel kappa-opioid receptor agonist TRK-820 suppresses the rewarding and locomotor-enhancing effects of morphine in mice. Life Sci 68:1717–1725. https://doi.org/10.1016/s0024-3205(01)00957-2
Verharen JPH, Luijendijk MCM, Vanderschuren L, Adan RAH (2020) Dopaminergic contributions to behavioral control under threat of punishment in rats. Psychopharmacology. https://doi.org/10.1007/s00213-020-05497-w
Walker JM, Thompson LA, Frascella J, Friederich MW (1987) Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol 134:53–59. https://doi.org/10.1016/0014-2999(87)90130-0
Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE (2001a) Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther 299:147–158
Walsh SL, Strain EC, Abreu ME, Bigelow GE (2001b) Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology 157:151–162. https://doi.org/10.1007/s002130100788
Wilson N, Kariisa M, Seth P, Smith H, Davis NL (2020) Drug and opioid-involved overdose deaths - United States, 2017-2018. MMWR Morb Mortal Wkly Rep 69:290–297. https://doi.org/10.15585/mmwr.mm6911a4
Zamarripa CA, Naylor JE, Huskinson SL, Townsend EA, Prisinzano TE, Freeman KB (2020) Kappa opioid agonists reduce oxycodone self-administration in male rhesus monkeys. Psychopharmacology. https://doi.org/10.1007/s00213-020-05473-4
Acknowledgments
The manuscript content is solely the responsibility of the author and does not necessarily reflect the official views of the National Institutes of Health. The author would like to thank Dr. Matthew L. Banks, Dr. Kevin B. Freeman, and Mr. C. Austin Zamarripa for comments on an earlier version of the manuscript.
Funding
This work was supported by (1) a Research Grant awarded by the Virginia Commonwealth University Postdoctoral Association, (2) the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers F32DA047026 and P30DA033934, and (3) the National Institute on Alcohol Abuse and Alcoholism and the National Institute on Drug Abuse Intramural Research Programs.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The manuscript content is solely the responsibility of the author and does not necessarily reflect the official views of the National Institutes of Health.
Conflict of interest
The author declares that he has no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Townsend, E.A. Effects of kappa opioid receptor agonists on fentanyl vs. food choice in male and female rats: contingent vs. non-contingent administration. Psychopharmacology 238, 1017–1028 (2021). https://doi.org/10.1007/s00213-020-05749-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05749-9