Abstract
Rationale
Early life stress is a major risk factor for cocaine addiction; however, the underlying molecular mechanisms remain relatively unexplored. MicroRNA-212 (miR-212) and methyl CpG binding protein 2 (MeCP2) have recently emerged as key regulators of brain-derived neurotrophic factor (BDNF) signaling during the acquisition and maintenance of cocaine-seeking behaviors.
Objectives
We therefore investigated the effect of maternal separation (MS) on cocaine-induced conditioned place preference (CPP) during periadolescence and how this influences miR-212, Mecp2, and Bdnf expressions in the prefrontal cortex.
Methods
Male BALB/c mice subjected to MS (3 h/day) from postnatal day 2 to 15 or normal animal facility rearing (AFR) were tested for CPP at postnatal day 45, or not exposed to experimental manipulations (drug-naïve animals). Cultured primary cortical neurons were used to determine miR-212 expression changes following depolarization by KCL treatment.
Results
MS increased cocaine-induced CPP and decreased Bdnf exon IV expression, which correlated with higher CPP scores in such animals. An experience-dependent decrease in miR-212 expression was observed following CPP test. This effect was mimicked in primary cortical neurons in vitro, under activity-dependent conditions. In contrast, increased Mecp2 expression was found after CPP test, suggesting an opposing relationship between miR-212 and Mecp2 expression following cocaine place preference acquisition. However, these effects were not present in mice exposed to MS.
Conclusions
Together, our results suggest that early life stress can enhance the motivational salience for cocaine-paired cues during periadolescence, and that altered expression of miR-212, Mecp2, and Bdnf in the prefrontal cortex is involved in this process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposure to adverse experiences early in life can dramatically affect brain development and neuroendocrine responses to stress, leading to altered cognition and behavioral phenotypes (Anand and Scalzo 2000; McEwen et al. 2015). Enduring changes in neurogenesis (Boku et al. 2015), synaptogenesis (Andersen and Teicher 2004), and hypothalamo-pituitary-adrenal (HPA) axis functioning (Leussis and Andersen 2008) are some of the pronounced effects induced by chronic early life stress. Recently, the molecular pathways that underlie these experience-dependent changes have been explored, and altered brain-derived neurotrophic factor (BDNF) signaling emerged as one potential candidate for mediating the effects elicited by early life adversity on brain and behavior (Blaze et al. 2015; Xiao et al. 2015). This neurotrophin has a major role in neuronal function and synaptic plasticity, and chronic stress exposure can lead to a persistent decrease in the expression of BDNF, particularly in the hippocampus and the prefrontal cortex (PFC) (Blaze et al. 2015; de Lima et al. 2011).
BDNF plays an important role in neuroplasticity elicited by the effects of drugs of abuse in the brain (Choi et al. 2012; Liu et al. 2006). There is abundant evidence that cocaine increases BDNF protein and Bdnf mRNA levels in reward-related regions including the ventral tegmental area, nucleus accumbens, and the PFC, an effect that regulates cocaine-induced behaviors (Choi et al. 2012; Li and Wolf 2015; Liu et al. 2006). Accordingly, altered cocaine-related phenotypes are a major consequence of early life stress. Repeated maternal separation (MS), as an animal model of chronic early life adversity, can induce higher locomotor response to cocaine (Kikusui et al. 2005), as well as enhanced acquisition of cocaine self-administration (Moffett et al. 2007). However, while the role of BDNF on reward-induced neuroplasticity has been widely explored (Li and Wolf 2015), fewer studies investigated how BDNF could be involved with stress-induced enhancement of cocaine-related phenotypes, particularly following early life stress exposure (Martini and Valverde 2012).
The induction of Bdnf gene expression during acquisition and maintenance of cocaine-seeking behaviors has been recently shown to be dynamically regulated by microRNA-212 (miR-212) and methyl CpG binding protein 2 (MeCP2) (Im et al. 2010). MicroRNAs are small non-coding RNA transcripts that elicit post-transcriptional repression of their target genes and have been shown to be critically involved in neuronal functioning (Bredy et al. 2011). The brain-enriched miR-212 plays an important role in neuronal morphogenesis and synaptogenesis particularly through gene expression regulation of extracellular signal-regulated kinase (ERK), cAMP response element-binding protein (CREB), and neurotrophic factors (Remenyi et al. 2010). Specifically following cocaine administration, higher levels of miR-212 levels in the striatum of rats were observed, whereas overexpression of miR-212 decreased responsiveness to the motivational properties of cocaine (Hollander et al. 2010). Furthermore, miR-212 targets and inhibits MeCP2 signaling during the maintenance of cocaine-related phenotypes (Im et al. 2010). MeCP2 is an epigenetic regulator that binds to methylated cytosines in DNA, including Bdnf promoter regions, thereby regulating Bdnf gene expression (Chahrour et al. 2008; Lonetti et al. 2010). However, despite the role of miR-212 and MeCP2 in Bdnf gene expression, the detailed effects of early life stress in their transcriptional activity remain unknown, particularly in the context of cocaine-associated behaviors.
Therefore, we investigated the effects of MS on cocaine-induced conditioned place preference (CPP) during periadolescence and how this influences miR-212, Mecp2, and Bdnf expressions in the PFC. The CPP is a classical conditioning procedure that provides a measure of the motivational effects of a particular stimulus previously paired with the effects of cocaine (Yap and Miczek 2008). We focused our molecular experiments specifically in the PFC, since cocaine-induced neuroplasticity in the PFC correlates with CPP acquisition scores (Munoz-Cuevas et al. 2013) and exposure to previously drug-paired cues induces hyperactivity of this brain region (Ciccocioppo et al. 2001).
In addition, given that epidemiological studies indicated that the association between childhood maltreatment and drug use manifests at an alarmingly young age (Andersen and Teicher 2009), resulting in 2- to 4-fold increase in the risk of illicit drug use by age 14 (Dube et al. 2003), as well as enhanced drug cue-induced craving symptoms (Elton et al. 2015), we hypothesize that animals exposed to MS would present increased cocaine-induced CPP during periadolescence. Moreover, we propose that alterations in Bdnf gene expression following CPP would be associated with the effects of early life stress on enhanced cocaine place preference, and differential expression of miR-212 and its target gene Mecp2 would be involved in subsequent downstream changes in Bdnf expression.
Material and methods
Animals
The study was performed with male BALB/c mice, since previous evidence indicated that male, but not female, mice are more sensitive to the effects of cocaine after repeated exposure to MS (Kikusui et al. 2005). All animals were housed under a 12 h/12 h light–dark cycle in ventilated cages with temperature maintained at 21 ± 1 °C. Food and water were available ad libitum. The experiments were conducted in accordance with the NIH laboratory animal care guidelines and approved by the Ethical Committee on the Use of Animals of the Pontifical Catholic University of Rio Grande do Sul, Brazil.
Maternal separation
The MS model of early life stress consists of exposing infant animals to daily episodes of maternal care deprivation during the first days of life (Plotsky and Meaney 1993). Thus, pregnant females were visually checked daily for the presence of pups. On the day of birth, the litters were randomly assigned to one of two groups: MS or animal facility rearing (AFR) control animals. The AFR litters were left undisturbed until weaning, except for cage cleaning at postnatal day (PND) 10. The MS litters were subjected to a procedure that was used in previous studies with BALB/C mice (Bhansali et al. 2007; Wang et al. 2011). In this procedure, pups were separated from their dams daily for 180 min (15:00–18:00), from PND 2 to PND 15. To do this, first, the dam was transferred to another cage. Then, the whole litter was transferred to another clean cage with bedding material and placed in another room, to prevent vocal communication between the dam and pups. The temperature of pups’ cage (33 ± 2 °C) was controlled using a digital heating pad placed under the cage to compensate for the dams’ body heat. After the separation period, the pups were returned to their home cage, followed by the dam. All pups were weaned at PND 21 and remained together with their same-sex littermates (two or three animals per cage) under standard housing conditions.
In this study, 14 different mice litters were used, in which 7 were assigned to MS group and 7 were assigned to AFR condition, and each litter contributed with one to three animals for each experimental group (MS-CPP group: MS-litter 1 n = 1, MS-litter 2 n = 1, MS-litter 3 n = 1, MS-litter 4 n = 1, MS-litter 5 n = 3, MS-litter 6 n = 2, MS-litter 7 n = 1; AFR-CPP group: AFR-litter 1 n = 1, AFR-litter 2 n = 3, AFR-litter 3 n = 1, AFR-litter 4 n = 1, AFR-litter 5 n = 1, AFR-litter 6 n = 3, AFR-litter 7 n = 3; MS drug-naïve group: MS-litter 1 n = 2, MS-litter 2 n = 1, MS-litter 3 n = 1, MS-litter 4 n = 1, MS-litter 5 n = 1, MS-litter 6 n = 0, MS-litter 7 n = 1; AFR drug-naïve group: AFR-litter 1 n = 1, AFR-litter 2 n = 1, AFR-litter 3 n = 1, AFR-litter 4 n = 1, AFR-litter 5 n = 1, AFR-litter 6 n = 1, AFR-litter 7 n = 1).
Cocaine-induced CPP
The CPP is a Pavlovian conditioning task, in which drug exposure is repeatedly paired with a neutral environment. Thus, rodents learn to associate a specific spatial context with the effects of cocaine and later animals have an opportunity to choose, in the absence of the drug state, to enter and explore the environment paired with the drug or a non-paired environment (Eisener-Dorman et al. 2011). Cocaine-induced CPP was performed using an unbiased two-chambered acrylic apparatus (30 cm long × 15 cm wide × 15 cm high), similar to that described in Cunningham et al. (2006). The apparatus consisted of two choice compartments (15 × 15 cm) divided by an acrylic barrier (3.2 mm tick). Each compartment had a specific visual cue pattern printed in a white background. One cue consisted of black circles while the other cue consisted of wide black lines arranged in parallel. Visual cue patterns were covered with a smooth plastic and were placed directly under the apparatus, serving as the floor during conditioned place preference sessions. Cue patterns were also printed and taped to the outside of the acrylic walls of each chamber.
The CPP was performed following three sequential phases: habituation (PND 34), conditioning (PND 35 to PND 44), and post-conditioning test (PND 45) (Fig. 1a), which developmentally represents the mice periadolescence (Laviola et al. 1999). For all phases, mice were placed into the chambers for a total of 30 min each day. In the habituation, mice were placed at the center of the apparatus and had free access to both chambers and no injections were administered. The time spent in both compartments was recorded in order to calculate the pre-conditioning value for place preference analysis. In addition, such values were analyzed to establish a potential side preference of each animal prior to conditioning training. Preference bias was defined as more than 70 % of the habituation time spent in only one chamber. No animals exceeded this threshold.
a Schematic procedure of cocaine-induced CPP experiment. b Time spent in cocaine-paired chamber, exploration time in seconds (n = 24). c CPP score comparison between animal facility rearing (AFR) and maternal separation (MS) group (AFR n = 13, MS n = 11). CPP scores were calculated by subtracting the time spent in the cocaine-paired chamber during the post-conditioning test by the percent time spent during the habituation pre-test phase. d CPP score comparison between CPP training order 1 (saline first and cocaine last) and CPP training order 2 (cocaine first and saline last) (n = 12 per condition). e CPP score comparison between cocaine-paired cue 1 (strips) and cue 2 (dots) (n = 12 per condition). f Body weight comparisons between AFR and MS at PND 21 and PND 45 (n = 21 per condition). Between group comparisons using Student’s t tests. Data expressed as mean ± SEM. Single asterisk represents p value below 0.05 and double asterisks represents p value below 0.001
For the conditioning procedure, mice were assigned to receive cocaine in one of the two conditioning chambers and saline in the other. The chamber in which each animal spent less time during the habituation was selected as the cocaine-paired side/cue chamber. Mice were given five pairing sessions of cocaine and five pairing sessions of saline in an alternating manner over 10 subsequent days, with one pairing session per day. Half of animals received cocaine on conditioning days 1, 3, 5, 7, and 9 and saline on days 2, 4, 6, 8, and 10. The other half received cocaine on conditioning days 2, 4, 6, 8, and 10 and saline on days 1, 3, 5, 7, and 9. On the following day, after the last conditioning session, to test for cocaine-induced CPP, mice were allowed free access to the two conditioning chambers with no drug/saline administration. CPP scores were calculated in seconds by subtracting the time spent in the cocaine-paired chamber during the post-conditioning test by the time spent during the pre-test habituation phase, as previously described (Eisener-Dorman et al. 2011).
The CPP apparatus was cleaned using 70 % isopropyl before each behavioral session. To minimize stress reaction due to animal relocation, mice were transported to the test room 30 min before any behavioral experiment. In addition, animals were only handled by two researchers during all procedures.
Drugs
Cocaine hydrochloride was dissolved in a sterile 0.9 % saline solution at a concentration of 20 mg/ml and administered at a dose of 20 mg/kg (i.p.). The 20-mg/kg dose was chosen based on previous studies with BALB/c mice showing that this concentration optimally induces place preference (Eisener-Dorman et al. 2011).
Brain sample preparation
Animals were euthanized by cervical dislocation without anesthesia at the PND 45 following 2 h after the CPP test. We selected this time point since previous evidence indicated enhanced Bdnf gene expression and epigenetic modifications around Bdnf promoter regions in the PFC after 2 h of behavioral training (Bredy et al. 2007). The brains were removed immediately by decapitation and then were directly submerged in RNAlater (Ambion) solution (1 ml). After overnight tissue incubation in RNAlater at 4 °C, PFC was dissected on ice by a coronal cut made at 3 mm anterior to Bregma and included all frontal areas (medial, ventral, and orbital PFC) without the olfactory bulb. Samples were then stored at −80 °C and hemispheres were alternated so that within a given group, half of the samples were from the left hemisphere and half from the right hemisphere. Additionally, in order to only investigate the effects of MS on transcriptional levels without the effects of CPP or drug administration, a group of drug-naïve animals were submitted to MS or AFR rearing conditions, but were not exposed to any experimental manipulations during periadolescence (CPP, drug administration, or handling), and brain tissue was harvested on the same day (PND 45) as for the other animals.
Primary cortical neuron culture
Cortical tissue was isolated from E18 mouse embryos following cervical dislocation of a normally reared pregnant female in a sterile atmosphere. To dissociate the tissue, it was finely chopped followed by digestion in 0.125 % Trypsin (GIBCO 25200) for 12 min. Cells went through the 40-μm cell strainer (BD Falcon 352340) and were plated onto six-well plate coated with poly-l-ornithine (Sigma P2533) and fibronectin (GIBCO 33016-015) at a density of 1 × 106 cells per well. The medium used was neurobasal medium (GIBCO 21103) containing 5 % serum, B27 supplement (GIBCO 17504-044), and 0.5–1 % Pen/Strep (GIBCO 15140). After 2 weeks of growth, primary cortical neurons were treated with KCl (concentration of 20 mM) to induce neural activity and depolarization (Li et al. 2014).
Transcript mRNA and miRNA levels
Total RNA was extracted using QIAzol (Qiagen) according to the reagent manufacturer’s protocol and reconstituted in 25 μl of RNase-free water. The concentration of RNA was measured using Qubit RNA Broad Range Assay in a Qubit Fluorometer 2.0 (Life Technologies), and RNA purity was assessed using the Nanodrop 2000 Spectrophotometer (Thermo Scientific). Two hundred fifty nanograms of RNA from each sample was reverse transcribed using the miScript II RT Kit (Qiagen) and cDNA generated was used for both mRNA and miRNA gene expressions. Specifically, template RNA was combined with 4 μl 5× miScript HiFlex Buffer, 2 μl 10× miScript Nucleics Mix, 2 μl miScript Reverse Transcriptase Mix, and RNase-free water to a final reaction volume of 20 μl. Reactions were incubated for 60 min at 37 °C and for 5 min at 95 °C. The resulting 20 μl of reverse transcription products were diluted with 80 μl of RNase-free water to a total volume of 100 μl, and then samples were stored at −20 °C.
One microliter of cDNA samples was used in each RT-qPCR reaction, performed in a StepOne PCR machine (Applied Biosystems), using the miScript SYBR Green PCR kit (Qiagen). Messenger RNA assays were performed using 7.5 μl of SYBR Green, 1.5 μl of QuantiTect primer (Qiagen), 5 μl of RNase-free water, and 1 μl of cDNA template. MicroRNA assays were performed using 7.5 μl of SYBR Green, 1.5 μl of miScript miRNA primer (Qiagen), 1.5 μl of miRNA-specific universal primer (Qiagen), 3.5 μl of RNase-free water, and 1 μl of cDNA template.
The following Qiagen primers were used: BDNF exon I (n° QT00097118), MeCP2 (n° QT00268555), GAPDH (n° QT01658692), and mature miR-212-3p (n° MS00024570), while the following Invitrogen primers were also used: BDNF exon IV (forward, 5′-GCAGCTGCCTTGATGTTTAC-3′; reverse, 5′-CCGTGGACGTTTACTTCTTTC-3′) (Baker-Andresen et al. 2013), BDNF exon IX (forward, 5′-GCAGCTGGAGTGGATCAGTAA-3′; reverse, 5′-CATTCACGCTCTCCACAGTCCC-3′) (Dong et al. 2015), and U6 (forward, 5′-CTCGCTTCGGCAGCACA-3′; reverse, 5′-AACGCTTCACGAATTTGCGT-3′) (Baroukh et al. 2007). To verify primer specificities, melting curve analyses were performed. Each PCR reaction was run in duplicate for each sample and was repeated one time. Three negative (no template) controls were performed to verify genomic DNA contamination for each PCR plate. The fold change relative expression was calculated using the ∆∆Ct method (Livak and Schmittgen 2001) with the AFR drug-naïve group as a reference for in vivo and primary cortical neurons with no KCl for in vitro analyses. GADPH ct values were used as endogenous control for mRNAs analysis, and U6 snRNA ct values for miR-212 analysis.
Data analyses and statistics
All data were normally distributed, and differences between groups in miRNA and mRNA expression were investigated using two-way analysis of variance (ANOVA) followed by Bonferroni post-tests. Student’s independent t tests, paired sample t tests, one-way ANOVA, and Pearson correlations were used when appropriate. Statistical analyses were conducted using α of 0.05 in the SPSS version 20.0.
Results
MS increased cocaine-induced CPP at the end of periadolescence
We found a significant effect of cocaine treatment regardless of group condition, showing that periadolescent male BALB/c mice exhibited cocaine-induced place preference (20 mg/kg) with an increase in time spent in the drug-paired chamber after conditioning training (Fig. 1b, t (23) = 5.75; p < 0.001). Comparisons between the two postnatal rearing conditions revealed that the MS group presented significant higher CPP scores (time spent in the cocaine-paired chamber during the post-conditioning test, minus time spent during the pre-test habituation phase) compared with the AFR group (Fig. 1c, t (22) = 2.21; p < 0.05). Additionally, to analyze the effects of potential confounds on CPP paradigm, conditioning training sequence bias (cocaine or saline as the first substance administered) (Fig. 1d, t (22) = 0.91; n.s.) and visual cue bias (Fig. 1e, t (22) = 0.67; n.s.) were examined using these conditions as independent variables on CPP scores and no significant difference was found. Regarding body weight, MS produced a significant difference at the weaning day (Fig. 1f, PND 21; t (39) = 3.48; p < 0.01). However, there was no significant body weight difference at the end of periadolescence when CPP test was performed (Fig. 1f, PND 45; t (39) = 1.02; n.s.), suggesting that body weight did not play a role in CPP score differences between rearing conditions.
Effects of MS and cocaine-induced CPP on Bdnf mRNA levels
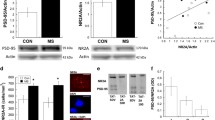
The Bdnf gene contains multiple promoters that are used to generate distinct mRNA transcripts (Choi et al. 2012; Liu et al. 2006). We identified decreased Bdnf exon I mRNA levels in AFR mice following CPP test relative to AFR drug-naïve animals (Fig. 2a, 2-way ANOVA, significant interaction effect F (3,24) = 12.98, p < 0.01; Bonferroni, AFR-CPP < AFR-naïve, p < 0.01). In addition, in drug-naïve conditions, animals exposed to MS had lower Bdnf exon I levels in comparison with AFR animals (Bonferroni, p < 0.01). No significant differences were found between AFR and MS animals following CPP test (Bonferroni, n.s.).
a Two-way ANOVA, significant interaction effect on Bdnf exon 1 mRNA levels, F (3,24) = 12.98, p < 0.01. b Two-way ANOVA on Bdnf exon IV mRNA levels, significant treatment effect F (3,24) = 5.24, p < 0.05; significant group effect F (3,24) = 17.27, p < 0.001. c No significant effects were detected on mRNA levels of Bdnf exon IX. d Pearson’s correlation, R = −0.55, R 2 = 0.30, p < 0.05. AFR-naïve n = 7, MS-naïve n = 7, AFR-CPP n = 7—samples collected from the animals with higher CPP scores, MS-CPP n = 7—samples from the animals with higher CPP scores. Data expressed as mean ± SEM. Single asterisk represents p value below 0.05 and double asterisks represents p value below 0.01 in Bonferroni multiple comparisons
Increased levels of Bdnf exon IV mRNA were observed following CPP test (Fig. 2b, 2-way ANOVA, significant treatment effect F (3,24) = 5.24, p < 0.05), while MS exposure strongly decreased Bdnf exon IV levels in both drug-naïve and CPP trained conditions (significant group effect F (3,24) = 17.27, p < 0.001; Bonferroni AFR-naïve > MS-naïve, p < 0.05; Bonferroni AFR-CPP > MS-CPP, p < 0.01). Multiple comparison analyses revealed higher Bdnf exon IV levels in AFR group only following CPP test (Bonferroni AFR-CPP > AFR-naïve, p < 0.01), but not in maternally separated animals (Bonferroni, n.s.). There were no significant treatments or group effects on Bdnf exon IX mRNA levels (Fig. 2c, F (3,24) = 1.64; n.s.).
Given that both cocaine-induced CPP and MS exposure independently altered Bdnf exon I and IV gene expressions, we performed correlational analysis and found a negative association between Bdnf exon IV mRNA levels and CPP scores (Fig. 2d, Pearson’s correlation, R = −0.55, R 2 = 0.30, p < 0.05), indicating that lower Bdnf exon IV levels in the PFC of animals exposed to MS were correlated with higher cocaine-induced place preference during periadolescence.
Divergent effects of cocaine-induced CPP on miR-212 and Mecp2 transcript levels
To assess the role of neuronal activation on transcriptional levels of miR-212, we first applied an in vitro approach using primary cortical neurons. A time-dependent decrease in miR-212 levels was observed in response to KCl-induced neural activation compared with no KCl treatment (Fig. 3a, one-way ANOVA, F (5,13) = 77.49, p < 0.001) after 30 min (Bonferroni, p < 0.001), 1 h (Bonferroni, p < 0.001), 3 h (Bonferroni, p < 0.001), 5 h (Bonferroni, p < 0.001), and 7 h (Bonferroni, p < 0.001). In vivo, a similar decrease in miR-212 levels was observed following CPP test (Fig. 3b), but such effect was evident in AFR mice only, resulting in significant treatment and interaction effects in a two-way ANOVA (treatment effect F (3,24) = 6.31, p < 0.01; interaction effect F (3,24) = 4.39, p < 0.05; Bonferroni, AFR-CPP < AFR-naïve, p = 0.05). There were no significant differences between AFR and MS groups following CPP test in miR-212 expression, as well as in drug-naïve conditions (Bonferroni, n.s.).
a One-way ANOVA showing reduced miR-212 levels following KCl treatment in primary cortical neurons with different time-points after stimulation (n = 3 per group). b Two-way ANOVA on miR-212 levels showed a treatment effect F (3,24) = 6.31, p < 0.05, and interaction effect F (3,24) = 4.39, p < 0.05. c Two-way ANOVA on Mecp2 mRNA levels showed a significant effect of treatment F (3,24) = 8.68, p < 0.01. AFR-naïve n = 7, MS-naïve n = 7, AFR-CPP n = 7—samples collected from the animals with higher CPP scores, MS-CPP n = 7—samples from the animals with higher CPP scores. Data expressed as mean ± SEM. Single asterisk represents p value below 0.05 and double asterisks represents p value below 0.001 in Bonferroni multiple comparisons
In contrast, Mecp2 mRNA levels were increased following CPP test (Fig. 3c, two-way ANOVA, significant treatment effect F (3,24) = 8.68, p = 0.01; Bonferroni, AFR-CPP > AFR-naïve, p = 0.05), but similarly, such effect was significant in the AFR group only and not in maternally separated animals (Bonferroni, n.s.). No significant differences in Mecp2 levels were found between AFR and MS animals following CPP test and in drug-naïve conditions (Bonferroni, n.s.). Therefore, this suggests that after 2 h of cocaine-induced CPP test, miR-212 levels are decreased while Mecp2 expression is increased in the PFC of normally reared animals, an effect that is blunted in maternally separated mice.
Discussion
Here, we examined the effects of MS on cocaine-induced CPP and how such conditions could affect cortical Bdnf, miR-212, and Mecp2 expression. We found that MS increased CPP during periadolescence and that decreased Bdnf exon IV expression correlated with the higher CPP scores observed in maternally separated animals. Additionally, significant and opposite changes in miR-212 and Mecp2 levels following cocaine-induced CPP were revealed in normally reared animals, while these effects did not occur in mice exposed to MS.
Higher cocaine-induced CPP in mice exposed to MS
Previous studies demonstrated that early life stress can alter cocaine-seeking behaviors. For instance, MS can induce a heightened locomotor response to cocaine regardless of sex (Kikusui et al. 2005), as well as rapid acquisition of cocaine self-administration with lower drug doses (Moffett et al. 2007). The observation that MS increases cocaine-induced CPP during periadolescence, however, is novel and relevant. We report that during a critical developmental period to drug experimentation and abuse, previous exposure to early life stress might enhance the motivational salience evoked by drug-paired environmental stimuli. However, while CPP reflects the rewarding properties of a drug through conditioned association of the incentive properties of cocaine with environmental cues (Schechter and Calcagnetti 1993, 1998), our conclusions are limited to the effects of early life stress in controlled cocaine use and cannot be generalized to compulsive drug taking or drug seeking as observed in self-administration models of drug addiction.
Furthermore, when the effects of early life stress on cocaine-induced CPP were studied with lower drug doses (2 mg/kg), reduced CPP scores following MS were observed (Hays et al. 2012). Furthermore, another study that investigated CPP in adolescent rodents found no differential sensitivity to drug rewarding effects in animals exposed to MS using morphine instead of cocaine (Roma et al. 2007). These findings suggest that MS effects on CPP acquisition could depend on the drug and dose used. This is supported by a recent finding showing that glucocorticoid receptor signaling in dopamine neurons is key for cocaine-induced molecular and behavioral responses, but not for morphine (Barik et al. 2010), and altered glucocorticoid receptor functioning is a well-known consequence elicited by early life stress (Kikusui et al. 2005). Despite the discrepancies, it is evident that there are significant effects of MS on CPP, which appears to be both task and strain specific. Corroborating with this, human research found that cocaine users who were previously exposed to childhood maltreatment demonstrate heightened anticipatory responses to drug cues (Elton et al. 2015) and a more pronounced withdrawal severity during cocaine abstinence (Francke et al. 2013).
Exon-specific effect on Bdnf gene expression associated with MS and cocaine-induced CPP
The observation that MS resulted in lower Bdnf exon I and IV mRNA levels is in agreement with previous studies (Blaze et al. 2015; de Lima et al. 2011; Kundakovic et al. 2013). Evidence suggests a long-lasting epigenetic influence of early life stress, through permanently increased 5-methylcytosine occupancy at multiple Bdnf promoter regions in the PFC, which results in reduced Bdnf gene expression (Roth et al. 2009). Therefore, altered BDNF signaling attributed to early life stress has been associated with learning and memory deficits (de Lima et al. 2011), as well as with increased depression- and anxiety-like behaviors (Kundakovic et al. 2013; Roth et al. 2009).
Regarding cocaine effects, a series of previous studies revealed the key role of BDNF-signaling in the PFC on cocaine-induced neuroadaptations that can suppress cue-induced drug craving and drug-seeking behavior (Berglind et al. 2007; Sun et al. 2015; Whitfield et al. 2011). For instance, elevation of cortical BDNF levels through infusions of exogenous BDNF into the medial PFC reduces acquisition of cocaine-induced CPP, attenuates motivation to cocaine seeking following self-administration training, and diminishes drug relapse (Li and Wolf 2015). These protective effects are mediated by the high-affinity BNDF receptor TrkB and through downstream regulation of CREB, ERK, and glutamatergic and GABAergic signaling (Choi et al. 2012; Lu et al. 2010; Sun et al. 2015; Whitfield et al. 2011).
Furthermore, cocaine selectively induces transcription of Bdnf exon IV in the PFC and such effect is evident from 30 min to 24 h after cocaine injections (Fumagalli et al. 2007; Li and Wolf 2015; Sadri-Vakili et al. 2010). Our data corroborates such previous findings, since relative to drug-naïve animals, we observed increased Bdnf exon IV gene expression in the PFC 2 h after cocaine-induced CPP test. Given the protective role of cortical BDNF signaling and that shRNA-mediated suppression of Bdnf exon IV expression in the PFC results in higher cocaine seeking (Sadri-Vakili et al. 2010), we propose that one mechanism whereby early life stress can enhance associations between cocaine exposure and drug-paired cues may be via interference with cortical Bdnf exon IV expression. Specifically, we observed that reduced Bdnf exon IV levels found in maternally separated animals correlated with higher CPP scores. Such idea is supported by previous findings showing that chronic stress exposure prevents cocaine-induced activation of BDNF signaling in the PFC, which is a potential mechanism for higher vulnerability to cocaine effects following repeated stress exposure (Fumagalli et al. 2009). In this sense, upregulation of BDNF-signaling in the PFC might have potential therapeutic value for the treatment of cocaine addiction (McGinty et al. 2010), particularly when baseline BDNF levels are decreased as a consequence of early life adversity exposure. However, given that the medial PFC and the orbital PFC have differential sensitivities to cocaine and Bdnf expression during reward-related behavior (Crombag et al. 2005; Sun et al. 2012), it is important to consider that our data were generated in whole PFC tissue, which is a limitation of the current study.
Changes in miR-212 and Mecp2 transcription levels following cocaine-induced CPP and MS in the PFC
Recently, a key role of miRNAs in drug-induced remodeling of brain reward systems was revealed, particularly with respect to miR-212 (Bali and Kenny 2013). Here, we observed that cocaine-induced CPP led to decreased miR-212 levels in the PFC of normally reared animals, an effect that was also produced by KCl-induced depolarization in primary cortical neurons. In contrast, increased Mecp2 mRNA levels were observed following CPP, suggesting a plausible relationship between miR-212-mediated silencing of Mecp2 expression following cocaine place preference test, an effect that was similarly observed in the striatum of rats following cocaine self-administration training (Im et al. 2010; Quinn et al. 2015). Since miR-212 binds to Mecp2 mRNA inhibiting protein translation, our data of activity-dependent decrease in miR-212 expression complements previous data showing higher MeCP2 protein levels following neuronal activation (Zhou et al. 2006). Specifically, MeCP2 phosphorylation is triggered by the release of glutamate at excitatory synapses of cultured neurons, suggesting that synaptic activation may regulate MeCP2 function as part of an adaptive response to neuronal stimulation (Chen et al. 2003; Zhou et al. 2006).
However, we found no such effect on miR-212 and Mecp2 transcriptional activities following CPP test in animals exposed to MS. This is interesting because MeCP2 induces Bdnf gene expression while miR-212 would fine-tune drug-induced neuroplasticity by regulating BDNF signaling indirectly through MeCP2 (Wanet et al. 2012). A blunted response of this regulatory mechanism would result in reduced Bdnf expression following cocaine-induced CPP, an effect observed in MS animals specifically on Bdnf exon IV mRNA levels. Indeed, MeCP2 phosphorylation relieves its transcriptional repressor function on Bdnf promoter IV, which provides evidence that MeCP2 selectively regulates Bdnf exon IV isoform gene expression (Rousseaud et al. 2015). Furthermore, reduced brain MeCP2 protein levels in animals exposed to MS were previously associated with higher frequency of methamphetamine self-administration (Lewis et al. 2013), while impaired MeCP2 signaling was shown to induce excitation/inhibition imbalance and impaired synaptic functioning in neurons following stimulation (Calfa et al. 2015). In this sense, the present findings support a role of miR-212 and MeCP2 on the acquisition of cocaine-induced CPP and that MS exposure can alter this regulatory mechanism. Future experiments will shed further light on the effects of early life stress and subsequent cocaine-induced CPP on the functional relationship between miR-212 and MeCP2 on the regulation of Bdnf gene expression.
Conclusion
The acquisition of cocaine-induced CPP is associated with reduced miR-212 and increased Mecp2 and Bdnf exon IV mRNA levels in the PFC. Early life stress seems to disrupt such homeostatic mechanisms by blunting dynamic changes in miR-212 and Mecp2 expression and decreasing Bdnf gene expression in the PFC, which is associated with higher cocaine-induced CPP during periadolescence. This is relevant since the PFC has a protracted development that continues throughout periadolescence, which may render it increasingly vulnerable to the effects of stress and cocaine abuse.
References
Anand KJ, Scalzo FM (2000) Can adverse neonatal experiences alter brain development and subsequent behavior? Biol Neonate 77:69–82
Andersen SL, Teicher MH (2004) Delayed effects of early stress on hippocampal development. Neuropsychopharmacology 29:1988–1993
Andersen SL, Teicher MH (2009) Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev 33:516–524
Baker-Andresen D, Flavell CR, Li X, Bredy TW (2013) Activation of BDNF signaling prevents the return of fear in female mice. Learn Mem 20:237–240
Bali P, Kenny PJ (2013) MicroRNAs and drug addiction. Front Genet 4:43
Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, Bailly A, Benecke A, Tronche F (2010) Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biol Psychiatry 68:231–239
Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van Obberghen E (2007) MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem 282:19575–19588
Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Miller SW, McGinty JF (2007) A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci 26:757–766
Bhansali P, Dunning J, Singer SE, David L, Schmauss C (2007) Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci 27:1467–1473
Blaze J, Asok A, Roth TL (2015) Long-term effects of early-life caregiving experiences on brain-derived neurotrophic factor histone acetylation in the adult rat mPFC. Stress 18:607–615
Boku S, Toda H, Nakagawa S, Kato A, Inoue T, Koyama T, Hiroi N, Kusumi I (2015) Neonatal maternal separation alters the capacity of adult neural precursor cells to differentiate into neurons via methylation of retinoic acid receptor gene promoter. Biol Psychiatry 77:335–344
Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS (2011) MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem 96:89–94
Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, Barad M (2007) Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem 14:268–276
Calfa G, Li W, Rutherford JM, Pozzo-Miller L (2015) Excitation/inhibition imbalance and impaired synaptic inhibition in hippocampal area CA3 of Mecp2 knockout mice. Hippocampus 25:159–168
Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224–1229
Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME (2003) Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302:885–889
Choi DC, Gourley SL, Ressler KJ (2012) Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry 2:e205
Ciccocioppo R, Sanna PP, Weiss F (2001) Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci U S A 98:1976–1981
Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE (2005) Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex 15:341–348
Cunningham CL, Gremel CM, Groblewski PA (2006) Drug-induced conditioned place preference and aversion in mice. Nat Protoc 1:1662–1670
de Lima MN, Presti-Torres J, Vedana G, Alcalde LA, Stertz L, Fries GR, Roesler R, Andersen ML, Quevedo J, Kapczinski F, Schroder N (2011) Early life stress decreases hippocampal BDNF content and exacerbates recognition memory deficits induced by repeated D-amphetamine exposure. Behav Brain Res 224:100–106
Dong E, Dzitoyeva SG, Matrisciano F, Tueting P, Grayson DR, Guidotti A (2015) Brain-derived neurotrophic factor epigenetic modifications associated with schizophrenia-like phenotype induced by prenatal stress in mice. Biol Psychiatry 77:589–596
Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003) Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111:564–572
Eisener-Dorman AF, Grabowski-Boase L, Tarantino LM (2011) Cocaine locomotor activation, sensitization and place preference in six inbred strains of mice. Behav Brain Funct 7:29
Elton A, Smitherman S, Young J, Kilts CD (2015) Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict Biol 20:820–831
Francke ID, Viola TW, Tractenberg SG, Grassi-Oliveira R (2013) Childhood neglect and increased withdrawal and depressive severity in crack cocaine users during early abstinence. Child Abuse Negl 37:883–889
Fumagalli F, Caffino L, Racagni G, Riva MA (2009) Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Eur Neuropsychopharmacol 19: 402–408.
Fumagalli F, Di Pasquale L, Caffino L, Racagni G, Riva MA (2007) Repeated exposure to cocaine differently modulates BDNF mRNA and protein levels in rat striatum and prefrontal cortex. Eur J Neurosci 26:2756–2763
Hays SL, McPherson RJ, Juul SE, Wallace G, Schindler AG, Chavkin C, Gleason CA (2012) Long-term effects of neonatal stress on adult conditioned place preference (CPP) and hippocampal neurogenesis. Behav Brain Res 227:7–11
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ (2010) Striatal microRNA controls cocaine intake through CREB signalling. Nature 466:197–202
Im HI, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13:1120–1127
Kikusui T, Faccidomo S, Miczek KA (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology 178:202–210
Kundakovic M, Lim S, Gudsnuk K, Champagne FA (2013) Sex-specific and strain-dependent effects of early life adversity on behavioral and epigenetic outcomes. Front Psychiatry 4:78
Laviola G, Adriani W, Terranova ML, Gerra G (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010
Leussis MP, Andersen SL (2008) Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 62:22–30
Lewis CR, Staudinger K, Scheck L, Olive MF (2013) The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front Psychiatry 4:55
Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, D’Alessio A, Zhang Y, Bredy TW (2014) Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A 111:7120–7125
Li X, Wolf ME (2015) Multiple faces of BDNF in cocaine addiction. Behav Brain Res 279:240–254
Liu QR, Lu L, Zhu XG, Gong JP, Shaham Y, Uhl GR (2006) Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Res 1067:1–12
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lonetti G, Angelucci A, Morando L, Boggio EM, Giustetto M, Pizzorusso T (2010) Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol Psychiatry 67:657–665
Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM (2010) Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron 67:821–833
Martini M, Valverde O (2012) A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology 219:149–158
McEwen BS, Gray JD, Nasca C (2015) 60 Years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol 226:T67–T83
McGinty JF, Whitfield TW, Berglind WJ (2010) Brain-derived neurotrophic factor and cocaine addiction. Brain Res 1314:183–193
Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73:321–330
Munoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L (2013) Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci 16:1367–1369
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200
Quinn RK, Brown AL, Goldie BJ, Levi EM, Dickson PW, Smith DW, Cairns MJ, Dayas CV (2015) Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats. Transl Psychiatry 5:e503
Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS (2010) Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428:281–291
Roma PG, Huntsberry ME, Riley AL (2007) Separation stress, litter size, and the rewarding effects of low-dose morphine in the dams of maternally separated rats. Prog Neuro-Psychopharmacol Biol Psychiatry 31:429–433
Roth TL, Lubin FD, Funk AJ, Sweatt JD (2009) Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry 65:760–769
Rousseaud A, Delepine C, Nectoux J, Billuart P, Bienvenu T (2015) Differential expression and regulation of brain-derived neurotrophic factor (BDNF) mRNA isoforms in brain cells from Mecp2(308/y) mouse model. J Mol Neurosci 56:758–767
Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwilliger EF, Pierce RC, Cha JH (2010) Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci 30:11735–11744
Schechter MD, Calcagnetti DJ (1993) Trends in place preference conditioning with a cross-indexed bibliography; 1957-1991. Neurosci Biobehav Rev 17:21–41
Schechter MD, Calcagnetti DJ (1998) Continued trends in the conditioned place preference literature from 1992 to 1996, inclusive, with a cross-indexed bibliography. Neurosci Biobehav Rev 22:827–846
Sun H, Cocker PJ, Zeeb FD, Winstanley CA (2012) Chronic atomoxetine treatment during adolescence decreases impulsive choice, but not impulsive action, in adult rats and alters markers of synaptic plasticity in the orbitofrontal cortex. Psychopharmacology 219:285–301
Sun WL, Eisenstein SA, Zelek-Molik A, McGinty JF (2015) A single brain-derived neurotrophic factor infusion into the dorsomedial prefrontal cortex attenuates cocaine self-administration-induced phosphorylation of synapsin in the nucleus accumbens during early withdrawal. Int J Neuropsychopharmacol 18.
Wanet A, Tacheny A, Arnould T, Renard P (2012) miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40:4742–4753
Wang L, Jiao J, Dulawa SC (2011) Infant maternal separation impairs adult cognitive performance in BALB/cJ mice. Psychopharmacology 216:207–218
Whitfield TW, Shi X, Sun WL, McGinty JF (2011) The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J Neurosci 31:834–842
Xiao L, Kish VL, Benders KM, Wu ZX (2015) Prenatal and early postnatal exposure to cigarette smoke decreases BDNF/TrkB signaling and increases abnormal behaviors later in life. Int J Neuropsychopharmacol
Yap JJ, Miczek KA (2008) Stress and rodent models of drug addiction: role of VTA-accumbens-PFC-amygdala circuit. Drug Discov Today Dis Models 5:259–270
Zhou Z, Hong EJ, Cohen S, Zhao WN, Ho HY, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JA, Weitz CJ, Greenberg ME (2006) Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 52:255–269
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted in accordance with the NIH laboratory animal care guidelines and approved by the Ethical Committee on the Use of Animals of the Pontifical Catholic University of Rio Grande do Sul, Brazil.
Rights and permissions
About this article
Cite this article
Viola, T.W., Wearick-Silva, L.E., De Azeredo, L.A. et al. Increased cocaine-induced conditioned place preference during periadolescence in maternally separated male BALB/c mice: the role of cortical BDNF, microRNA-212, and MeCP2. Psychopharmacology 233, 3279–3288 (2016). https://doi.org/10.1007/s00213-016-4373-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4373-z