Abstract
Rationale
Early-life adverse events, like maternal deprivation (MD), have been associated with the later development of mood and anxiety disorders. Scarce data are available describing behavioural and endocrine alterations in maternally deprived (DEP) animals during the periadolescent period. We hypothesize that a single episode of MD early in life would alter reward function and lead to a long-lasting behavioural and neuroendocrine changes during adolescence.
Objectives
Our aim was to evaluate the effects of a single episode of MD in CD1 adolescent mice (postnatal day 35) on a range of tests for anxiety- and depression-related behaviours (open field, elevated plus maze and tail suspension test). We further assess whether these effects could affect cocaine self-administration behaviour. In order to correlate behavioural and neuroendocrine responses to stress, brain-derived neurotrophic factor (BDNF) levels were assessed in brain structures related to emotional and cognitive processes.
Results
During the cocaine self-administration, the time required for achieving the acquisition criteria was significantly increased and the breaking point values in progressive schedule were significantly reduced in DEP adolescent mice, suggesting impairment in rewarding functions. The behavioural tests also confirm an increase in anxiety- and depression-related behaviours in DEP adolescent mice. The results on BDNF level indicated a decrease in response to MD in amygdala and hippocampus, confirming the behavioural data.
Conclusions
Our findings demonstrated for the first time that a single episode of early MD can impair the motivation for cocaine consumption in adolescent mice and can be associated with anxiety- and depressive-like behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early life represents a critically vulnerable period during which exposure to traumatic experiences can increase later risk for a variety of neuropsychiatric illness, including anxiety- and mood-related disorders (Heim and Nemeroff 2001; Morgan et al. 2007).
Animal models of early life stress, such as maternal deprivation (MD), have been developed in an attempt to elucidate the neurochemical and behavioural alterations resulting from exposure to the early-life stressor. During the early postnatal period, rats and mice are dependent upon the mother for warmth, food and bodily waste excretion (Gubernick and Alberts 1983). Subjecting rodents to MD during this period results in lasting changes in various measures of emotion-related behaviour and stress reactivity during adultness, such as anxiety-like and depression-related behaviours and exaggerated hypothalamic–pituitary–adrenal (HPA) axis response to stress (for reviews, see Levine 2000; Pryce and Feldon 2003; de Kloet et al. 2005).
In rodents, a 24-h single episode of MD during the first 2 weeks of postnatal life at different time windows appears to provide a useful model to investigate the impact of early life stress on diverse behavioural and neuroendocrine parameters and to analyze neurobiological changes during development (for a review, see Millstein and Holmes 2007).
There are numerous studies describing behavioural and endocrine alterations in maternally deprived (DEP) adult animals (Ellenbroek et al. 1998; Ellenbroek and Cools 2002; Millstein and Holmes 2007). However, scarce data are available regarding psychoneuroendocrine anomalies at the periadolescent period, a critical developmental stage during which some major neuropsychiatric disorders may become evident. More precisely, the term “periadolescence” defines an ontogenetic period that encompasses the 7–10 days preceding the onset of puberty (at about 40 days in rats and mice) and the few days thereafter (Spear and Brake 1983). Furthermore, an adolescent rodent has been classified by the use of three age intervals, namely early adolescence (prepubescent or juvenile, postnatal day (PND) 21–34), middle adolescence (periadolescent, PND 34–46) and late adolescence (young adult, PND 46–59) (see Laviola et al. 1999, 2003).
Behavioural and neuroendocrine consequences of MD during adolescence have not been deeply studied in rodents (see, however, Macri and Laviola 2004; Llorente et al. 2007; Marco et al. 2009) and sometimes the different studies exhibit contradictory findings, probably because of differences in postnatal time of MD episode.
Moreover, adverse events early in life have also been reported to alter brain parameters of relevance to the pathogenesis of both schizophrenia and affective disorders (Heim et al. 1997; Lipska and Weinberger 2000; Laviola et al. 2009). Within this framework, MD has been shown to induce, in adult male rats, a significant reduction in brain-derived neurotrophic factor (BDNF) levels in the hippocampus (Roceri et al. 2002), as well as a decrement in neuropeptide Y and calcitonin gene-related peptide levels in the same brain area (Husum et al. 2002). These changes at the hippocampal level have been related to an aberrant regulation of neuronal plasticity that may influence the neuroendocrine response to stress and, thus, underlie some of the detrimental neurobehavioural outcomes of MD. Thus, all these data point out that early MD reduces the ability of animals to cope with a stressful situation and suggest that the emergence of the depressive-like symptoms associated to the neonatal exposure to a stressful event might already be evident during adolescence (Macri and Laviola 2004; Marco et al. 2009).
Early-life stress models such as MD have been developed in an attempt to model early adverse events in humans. A history of early-life abuse or neglect appears to increase the risk of psychopathology development (Heim and Nemeroff 2001; Teicher et al. 2006), and these changes in mood concerns are also frequently reported in human adolescents (Arnett 1999). Epidemiological analyses indicate that adolescence is associated with an increased risk of drug-related problems (Compas et al. 1995). Similarly, periadolescent rodents present a characteristic behavioural profile and are highly sensitive to the administration of psychostimulant agents. Thus, these animals are considered a useful model for the study of the risk factors associated with vulnerability to behavioural disorders in human adolescents (Laviola et al. 1999; Adriani and Laviola 2004).
MD stress, like other stressful life events, has been proposed to significantly affect behavioural response to various drugs of abuse (Matthews et al. 1999; Sinha 2001; Miczek et al. 2008). Several neurochemical alterations in the dopamine mesolimbic system and the serotonergic system may underlie some of the observed behavioural changes (Hall et al. 1999; Meaney et al. 2002; Brake et al. 2004; Vicentic et al. 2006).
The exact mechanisms by which MD affects the behavioural and neurochemical profiles of adolescent mice are not yet fully understood, but they include the long-term alteration of various neurotransmitter and hormone systems, with potentially crucial implications for emotional and cognitive processes. We hypothesize that a single episode of MD early in life would alter reward function and lead to a long-lasting behavioural and neuroendocrine changes during adolescence. Thus, the aim of the present study was to evaluate the effects of a single episode of MD in adolescent mice on a range of tests for anxiety- and depression-related behaviours (open field, elevated plus maze (EPM) and tail suspension test). Drug self-administration in animals is a robust procedure that is highly predictive of drug-abuse liability in humans, so we further assess whether these effects could affect cocaine self-administration. In order to correlate behavioural and neuroendocrine responses to stress, BDNF levels were assessed in brain structures related to emotional and cognitive processes.
Materials and methods
Animals
Outbred CD-1 male and female mice purchased from Charles River (France) were housed in acclimatized room (temperature 21 ± 1°C, relative humidity 55 ± 10%, lights on from 08:00 to 20:00 hours) with water and pellet food available ad libitum. On arrival, breeding pairs were formed and housed in 45 × 25 × 15cm plexiglas boxes. After approximately 2 weeks, the male was removed, and pregnant females were housed individually.
Weaning procedure
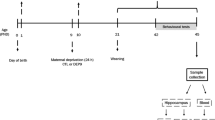
Pregnant female mice were monitored daily until parturition. For each litter, the date of birth was designed postnatal day 0 (PD0). Within 12 h from birth, litters were sexed and culled to six male pups to standardize litter size and to reduce some of the variability between litters. The offspring were weaned on PD21 and housed in groups of three to five males per cage in a temperature (21 ± 1°C), humidity (55 ± 10%) and light cycle controlled room. Light was on between 08:00 and 20:00 hours, and the experiments took place during the light phase. Food and water were available ad libitum during all experiments, except during the exposure to the different behavioural paradigms. The experiments take place at PD35 and mice were handled for 1 week before starting the experiments. For the self-administration studies, mice were exposed to a reversed cycle (lights on between 20:00 and 08:00 hours), and the experiments took place during the dark phase. A different cohort of male mice was used in each experiment.
All animal care and experimental procedures were conducted according to standard ethical guidelines (European Communities Council Directive 86/609/EEC) and were approved by the local ethical committee (CEEA-PRBB).
Maternal deprivation
Between PD12 and PD13, half of the litters (three male pups) were DEP for 24 h as described by Van Oers et al. (1997). Briefly, on PD12, the dam and half of the pups were removed and the remaining pups within their breeding cage were transferred to a different room for a 24-h period. The cages were placed on a heating pad set at nest temperature (32.5°C) to prevent any additional stress due to hypothermia (Zimmerberg and Shartrand 1992). Pups from the remaining litters (non-deprived control litters—NDEP, N = 3) were left undisturbed with their mother.
Behavioural tests
Elevated plus maze
EPM consisted of a black plastic apparatus with four arms (16 × 5 cm) set in a cross from a neutral central square (5 × 5 cm). Two opposite arms were delimited by vertical walls (closed arms), whereas the two other opposite arms had unprotected edges (open arms). The maze was elevated 30 cm above the ground and illuminated from the top (100 lx). The experiments were conducted as previously reported (Simonin et al. 1998). At the beginning of the 5-min observation session, each mouse was placed in the central neutral area, facing one of the open arms. The total number of visits to the open arms and the cumulative time spent in the open arms were then observed on a monitor through a videocamera system (ViewPoint, Lyon, France). Time spent in the open arms were recorded when the mouse moved two forepaws and head into the arm. We calculated the percentage of time spent in the open arms of the EPM as a measure of anxiety-like behaviour in this task. The number of entries made to the open arms was considered as a measure of motor activity of mice in this task.
Open-field test
Each animal was placed in an OF apparatus (Simonin et al. 1998) consisting of a rectangular area (70 cm wide × 90 cm long × 60 cm high) under bright illumination (500 lx). A total of 63 squares (10 × 10 cm) were drawn with black lines on the white floor dividing the field into central and peripheral areas. To start the experiment, each mouse was placed in the central area of the field. The parameters measured during a 5-min observation were the latency to go out from the central area and the time spent in the central area of the field, as indicators of anxiety-like behaviour.
Tail suspension test
Animals were exposed to the tail suspension test (TST). Mice were individually suspended by adhesive tape 1 cm from the tip of the tail, 50 cm above a bench top for a 6-min period as described by Steru et al. (1985) and Aso et al. (2008). The time that the animal was totally inactive during this period was recorded.
Operant cocaine self-administration
Drug
Cocaine hydrochloride was obtained from Ministerio de Sanidad (Spain) and was dissolved in sterile 0.9% physiological saline and administered by intravenous (i.v.) route.
Apparatus
The self-administration experiments were conducted in mouse operant chambers (Model ENV-307A-CT, Medical Associates, Georgia, VT, USA) equipped with two holes: one was selected as active hole for delivering the reinforcer and the other as inactive hole. Nose-poking on the active hole resulted in a reinforcer (cocaine infusion), while nose-poking on the inactive hole had no consequences. The chambers were housed in sound- and light-attenuated boxes equipped with fans to provide ventilation and white noise. A stimulus light, located above the active hole, was paired contingently with the delivery of the reinforcer.
Surgery
Mice were anaesthetized with ketamine/xylazine mixture (5:1; 0.10 ml/10 g body weight, i.p.) and then implanted with an indwelling i.v. silastic catheter (Soria et al. 2005). Briefly, a 6-cm length of silastic tubing (0.3 mm inner diameter, 0.6 mm outer diameter; Silastic®, Dow Corning, Houndeng-Goegnies, Belgium) was fitted to a 22-G steel cannula (Semat, Herts, UK) that was bent at a right angle and then embedded in a cement disc (Dentalon Plus, Heraeus Kulzer, Wehrheim, Germany) with an underlying nylon mesh. The catheter tubing was inserted 1.3 cm into the right jugular vein and anchored with suture. The remaining tubing ran subcutaneously to the cannula, which exited at the midscapular region. All incisions were sutured and coated with antibiotic ointment (Bactroban, GlaxoSmithKline, Madrid, Spain). After surgery, animals were allowed to recover for 3 days prior to initiation of self-administration sessions. The catheter was flushed daily with a saline solution. The patency of intravenous catheters was evaluated periodically (approximately every 5 to 6 days) and whenever drug self-administration behaviour appeared to deviate dramatically from that observed previously by infusion of 0.1 ml of thiopental (5 mg/ml) through the catheter. If prominent signs of anaesthesia were not apparent within 3 s of the infusion, the mouse was removed from the experiment.
Procedure
The self-administration experiment was conducted as previously described (Soria et al. 2006, 2008), with minor modifications. Briefly, responding was maintained by cocaine (1 mg/kg per injection) delivered in 58.75 μl over 4 s. Daily self-administration started with a priming injection of the drug, which lasted for 60 min and was conducted during 10 days. The house light was on at the beginning of the session for 3 s and off during the remaining time of the session. Each daily session started with the presentation of active and inactive holes, a priming injection of the drug and a 4-s presentation of the light cue (located above the active hole). Nose-poking on the active hole led to cocaine infusions and the presentation of the light cue for 4 s. Mice were trained to nose-poke for cocaine under a fixed ratio 1 (FR1) schedule of reinforcement. A 30-s time-out period was established after obtaining a new reinforcer. During this 30-s period, the cue light was off and no drug infusions were provided after nose-poking the active hole. Nose-poking on the inactive hole and all the responses performed during the 30-s time-out period were also recorded. The session was terminated after 50 reinforcements were delivered or after 1 h, whichever occurred first. The criteria for the acquisition was achieved when mice maintained a stable responding with less than 20% deviation from the mean of the total number of cocaine infusions earned in three consecutive sessions (80% of stability), with at least 75% responding on the active hole, and a minimum of three reinforcements per session. After mice achieved the acquisition criteria, the effects of MD on the motivational strength of cocaine as a reinforcer were evaluated by using a progressive-ratio (PR) schedule in which the response requirement to earn an injection escalates according to the following series: 1–2–3–5–12–18–27–40–60–90–135–200–300–450–675–1,000 (Richardson and Roberts 1996; Soria et al. 2005, 2006; Touriño et al. 2008). The PR session lasted for 2 h or until the mice did not complete the ratio for delivery of one reinforcer within 1 h and was performed only once. The breaking point to extinguish self-administration behaviour was determined in each animal.
BDNF protein quantification (ELISA)
BDNF was analyzed in the frontal cortex, hippocampus, amygdala and hypothalamus of a separate cohort of DEP and NDEP adolescent mice (PD35) with ELISA kits. Animals were sacrificed by rapid decapitation, and the brain was quickly removed. Brain areas were dissected according to the Mouse Brain Atlas (Franklin and Paxinos 1997) immediately frozen in dry ice and stored at −80°C until processed.
Brain areas processing
Frozen brain areas were dounce-homogenized in lysis buffer (137 mmol/L NaCl; 20 mmol/L Tris–HCl, pH 8.0; 1% NP-40; 10% glycerol; 1 mmol/L sodium vanadate; 5 mmol/L sodium pyrophosphate; 100 mmol/L NaF; 40 mmol/L glycerol phosphate; 1 mmol/L phenylmethylsulfonyl fluoride; 0.15 μmol/L aprotinine; 11 μmol/L leupeptine and 1.5 μmol/L pepstatine) to prepare protein extract. Hippocampus and frontal cortex were homogenized in 30 μl lysis buffer per milligram wet weight, whereas amygdala and hypothalamus was homogenized in 50 μl lysis buffer per milligram wet weight. After 20 min of incubation in agitation at 4°C, samples were centrifuged during 30 min at 16,000×g, and the supernatant was recovered and stored at −80°C. Protein content was determined by using the DC Protein Assay (Bio-Rad, Barcelona, Spain) following manufacturer's instructions.
BDNF determination
The BDNF Emax™ Immunoassay System (Promega Corporation®) was used to quantify the levels of BDNF protein. Prior to each assay, samples were diluted and acid treated to adjust the amount of BDNF to the standard curve and to increase the detectable amount of free BDNF in solution by dissociating it from its proforms or receptors (Okragly and Haak-Frendscho 1997). Samples from hippocampus, frontal cortex and hypothalamus were diluted 1:80 (v/v) and amygdala in 1:40 (v/v) in lysis buffer before the assay. All the samples were acidified with 1 μl 1 mol/L HCl/50-μl sample, and after 15 min of incubation at 21 ± 1°C, samples were neutralized with the same amount of NaOH 1 mol/L. MaxiSorp™ 96-well plates (Nunc™, Roskilde, Denmark) were used for antibody coating and ELISA was carried out according to manufacturer's instructions. BDNF levels were normalized to the total amount of protein from each individual sample.
Statistical analysis
All the analyses were conducted using the statistical package Statistical Package for the Social Sciences (SPSS) 15.0 for Windows (SPSS, Chicago, IL, USA). Differences were considered significant if the probability of error was less than 5%. Data are represented as mean±SEM. Effects of MD on motor activity and anxiety-like behaviour and BDNF level were compared using one-way analysis of variance (ANOVA).
Two-way repeated measures ANOVA were used to analyze the differences in number of responses for acquisition of cocaine self-administration between the two groups of animals (DEP and NDEP), followed by one-way ANOVA for comparisons between holes. The breaking point values obtained on the PR schedule were compared between conditions by calculating one-way ANOVA.
Results
Effects induced by the exposure to MD on the elevated plus maze
Mice exposed to MD exhibited a significant increase to anxiety-like behaviour evaluated in the EPM. Thus, one-way ANOVA calculated for the percentage of time spent in the open arms revealed a significant reduction in the percentage of the time spent in open arms in DEP adolescent mice when compared to the NDEP mice (F (1,22) = 44.935; p < 0.001; Fig. 1a).
We also detected a significant decrease in the percentage of entries in the open arms in DEP adolescent mice when compared to the NDEP mice (F (1,22) = 32.913, p < 0.001; Fig. 1b).
Effects of mice exposed to MD in the open-field test
Different responses were evaluated in the open-field test (OFT). Thus, the latency time to go out from the central area and time spent in the centre of the arena were considered as a measure of the anxiety-like behaviour. In adolescent DEP mice, one-way ANOVA revealed a significant decrease of the time latency to go out from the central area (F (1,22) = 4.776; p < 0.05; Fig. 2a) and of the time spent in the centre of the arena (F (1,22) = 4.793; p < 0.05; Fig. 2b).
Effects observed in response to MD in the tail suspension test
Mice exposed to MD exhibited a significant increase in depression-like profile evaluated in the TST. One-way ANOVA calculated for the time that the animal was totally inactive during the recorded period revealed a significant increase of the immobility time in DEP mice compared to NDEP mice (F (1,22) = 9.575; p < 0.01; Fig. 3).
Effects induced by MD on brain-derived neurotrophic factor protein
BDNF protein quantified with ELISA technique exhibited a level impairment in both amygdala and hippocampus in response to MD. Thus, one-way ANOVA indicated a decrease of BDNF levels in response to MD both in amygdala (F (1,12) = 4.847; p < 0.05) and in hippocampus (F (1,12) = 6.760; p < 0.05) of DEP adolescent mice (Fig. 4a, b).
The analysis of BDNF content in hypothalamus and prefrontal cortex revealed no differences between groups (F (1,12) = 2.150; p = n.s. and F (1,12) = 0.002; p = n.s., respectively), suggesting specific brain area BDNF impairment in DEP adolescent mice.
Cocaine self-administration
The effects of MD procedure in adolescent mice on the reinforcing properties of cocaine were evaluated by using the operant self-administration procedure. First, both DEP and NDEP adolescent mice were trained to self-administer cocaine (1 mg/kg per infusion) during 10 days under an FR1 schedule of reinforcement. The percentage of mice that reached the acquisition criteria was 42% for NDEP and 43% for DEP. Two-way repeated measures ANOVA revealed no significant differences in the nose-poke responses between DEP and NDEP mice during the self-administration sessions (F (1,16) = 0.012; p = n.s.), but significant differences were found for the days (F (1,16) = 4.397; p < 0.001) with interaction between these two factos (F (1,16) = 6.827, p < 0.001). Subsequent one-way ANOVA revealed that only NDEP mice discriminated between the active and the inactive holes from the first session, whereas DEP mice discriminated after the eighth session (Fig. 5a, b). The dose of 1 mg/kg per infusion of cocaine was chosen because accordingly to previous studies (Soria et al. 2005). This dose was the same used to train mice for the PR schedule. In this case, the breaking point achieved were significantly reduced in DEP adolescent mice when compared to NDEP littermates (F (1,16) = 20.742; p < 0.001; Fig. 6).
Discussion
In this study, adolescent mice, exposed as pups to a single prolonged (24-h long) episode of MD, were examined in cocaine self-administration and measured for depression- and anxiety-related behaviours, two behavioural dimensions that are frequently associated with human drug abuse (Brady et al. 2007). In addition, in order to correlate behavioural and neuroendocrine responses to stress, BDNF protein levels were assessed in brain structures related to emotional and cognitive processes.
Early MD in rodents has been proposed as an interesting animal model for certain aspects of schizophrenic and affective psychopathologies (Ellenbroek 2003; Price et al. 2005), and some major neuropsychiatric disorders may become evident during adolescence (Adriani and Laviola 2004; Andersen 2003). Thus, the psychoneuroendocrine characterization of MD animal model during this critical developmental period is of relevance. Previous works on adolescent and adult mice characterized the long-lasting emotional alterations induced by MD by means of a battery of behavioural tests aimed to assess motor and emotional behavioural profiles (Macri and Laviola 2004; Marco et al. 2009). In particular, DEP adolescent mice showed a significantly reduced latency to reach a passive floating posture and a significant increase in floating behaviour during forced swimming test (FST) (Macri and Laviola 2004). Our data provide an extension of previous studies demonstrating differences in depression- and anxiety-related behaviours between DEP and NDEP adolescent mice. In fact, previous studies in mice focussed their attention on the FST to assess the depressive profile of DEP mice. Instead, we pointed our attention on evaluation of anxiety behaviour, using the EPM and the OFT; furthermore, to verify if our results were in agreement with literature data, we also evaluated the despair behaviour with the TST. We demonstrated that DEP adolescent mice exhibited the lowest levels of open arms exploration in the EPM as well as the lowest percentage of entries in open arms. DEP mice also displayed the lowest levels of immobility in TST and a significant decrease of the time latency to go out from the central area and of the time spent in the central area in the OFT. Taken together, the behavioural profiles in these tests are indicative of an increased anxiety- and depression-related behaviour in MD adolescent mice. Moreover, our findings suggest that MD might precipitate the appearance of certain depressive- and anxiogenic-like latent symptoms in adolescent mice and that this trend persists until maturity (Macri and Laviola 2004).
Moreover, our study assessed the effects of a single episode of MD on maintenance of cocaine-reinforced responding under FR and PR schedules.
We demonstrated for the first time that a single episode of early MD can impair the motivation for cocaine in adolescent mice. Interestingly, the present findings suggest a lack of motivation to seek the drug in DEP adolescent mice since (1) the time required for achieving the acquisition criteria was significantly increased in DEP mice, and (2) the maximal effort required to obtain a cocaine infusion was significantly reduced in DEP mice. Previous studies reported an association between psychotropic drugs consumption and the presence of primary psychiatric disorders (Wurmser 1974; Khantzian 1985). Accordingly, certain individual takes drugs of abuse in an attempt to self-medicate intolerable affective states (Weiss et al. 1992). In our study, in PR reinforcement schedule, adolescent mice that have experienced early MD exhibited reduced motivation to obtain cocaine infusion and an increase in depression-related behaviour than NDEP subject, suggesting an opposite relation between a depression-related behaviour and the attenuation of reinforcing efficacy of cocaine intake. One explanation for decreased performance in PR schedule is that MD leads to the development of anhedonia, one of the core symptoms related to depression, that is described as the incapability to get pleasure and well-being. Moreover, increased anxiety observed during the OFT and EPM and increased depressive state in the TST could be other factors related to MD supporting the anhedonic interpretation of reduced PR-schedule responding. Some previous studies support our interpretation of the above data. Thus, a decreased sensitivity to reward was found in animals in which a depressive-like behaviour was induced either by exposure to chronic mild stress (CMS) (Willner 2005) or olfactory bulbectomy (Willner and Mitchell 2002). Furthermore, in rats, CMS induces an increased immobility in the FST associated with a decrease in reward sensitivity as measured by increased intracranial self-stimulation threshold, attenuated sucrose consumption, decreased preference for alcohol, impaired sexual behaviour and decreased amphetamine and morphine rewarding effects (Willner 2005). Similarly, olfactory bulbectomy that exhibits a high degree of neurochemical similarity to depression induces a decreased sensitivity to reward as shown by a decreased sexual behaviour (Lumia et al. 1992), an increased intracranial self-stimulation threshold (Slattery et al. 2007) and a reduced cocaine place preference (Calcagnetti et al. 1996).
In the last years, many studies have demonstrated that neurotrophic factors might be involved in the pathophysiology of mood disorders. BDNF is the most abundant neurotrophic factors and has an important role in depression (Duman 2004). In order to correlate behavioural and neuroendocrine responses to stress, we analyzed BDNF levels in discrete brain areas. DEP animals, which had higher anxiety and depressive behaviour, showed a lower BDNF protein levels in the hippocampus and amygdala. It is well-recognized that the hippocampus is a limbic structure involved in the regulation of learning and memory and also controls the activity of the HPA axis. Moreover, the hippocampus has connections with amygdala, and an impairment in its function have been related to cognitive deficits related to mood disorders (Duman and Monteggia 2006). The amygdala, which is involved in fear and anxiety, plays a role in emotion and reward processing (Murray 2007). Thus, it is possible that altered behaviour in DEP adolescent mice may give rise to BDNF changes in these brain areas. In addition, the hippocampal alterations discovered in this study can reflect the memory and learning deficits, which were reported in the maternal DEP animals (Fabricius et al. 2008) and could be a plausible explanation for the increasing time required for achieving the acquisition criteria during the cocaine self-administration. In fact, only NDEP mice discriminated between the active and the inactive holes from the first session, and DEP mice only after the eighth session (see Fig. 5a, b). These findings support the hypothesis that BDNF acts on these regions and ameliorates anxiety- and depressive-related behaviour.
In conclusion, we demonstrated for the first time that a single episode of MD during the early life period can impair motivation for cocaine self-administration in mice during the adolescence period. We further demonstrated an association with an anxiety- and depression-related behaviour, since adolescent mice belonging to the DEP group, in fact, showed a reduced ability to cope with stressful situations. Our findings let us emphasize the relevance of early periods of life in the presentation and development of some psychiatric disorders such as mood disorders and drug addiction.
References
Adriani W, Laviola G (2004) Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15(5–6):341–352
Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18
Arnett JJ (1999) Adolescent storm and stress, reconsidered. Am Psychol 54:317–326
Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, Valverde O (2008) BDNF impairment in the hippocampus is related to enhanced despair behaviour in CB1 knockout mice. J Neurochem 105:565–572
Brady KT, Verduin ML, Tolliver BK (2007) Treatment of patients comorbid for addiction and other psychiatric disorders. Curr Psychiatry Rep 9:374–380
Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19:1863–1874
Calcagnetti DJ, Quatrella LA, Schechter MD (1996) Olfactory bulbectomy disrupts the expression of cocaine-induced conditioned place preference. Physiol Behav 59:597–604
Compas BE, Hinden BR, Gerhardt CA (1995) Adolescent development: pathways and processes of risk and resilience. Annu Rev Psychol 46:265–293
de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV (2005) Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev 29:271–281
Duman RS (2004) Role of neurotrophic factors in the etiology and treatment of mood disorders. NeuroMolecular Medicine 5:11–25
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatr 59:1116–1127
Ellenbroek BA (2003) Animal models in the genomic era: possibilities and limitations with special emphasis on schizophrenia. Behav Pharmacol 14:409–417
Ellenbroek BA, Cools AR (2002) Early maternal deprivation and prepulse inhibition: the role of the postdeprivation environment. Pharmacol Biochem Behav 73:177–184
Ellenbroek BA, van den Kroonenberg PT, Cools AR (1998) The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res 30:251–260
Fabricius K, Wörtwein G, Pakkenberg B (2008) The impact of maternal separation on adult mouse behaviour and on the total neuron number in the mouse hippocampus. Brain Struct Funct 212:403–416
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic, New York
Gubernick DJ, Alberts JR (1983) Maternal licking of young: resource exchange and proximate controls. Physiol Behav 31:593–601
Hall FS, Wilkinson LS, Humby T, Robbins TW (1999) Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 32:37–43
Heim C, Nemeroff CB (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatr 49:1023–1039
Heim C, Owens MJ, Plotsky PM, Nemeroff CB (1997) Persistent changes in corticotropin-releasing factor systems due to early life stress: relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacol Bull 33:185–192
Husum H, Termeer E, Mathe AA, Bolwig TG, Ellenbroek BA (2002) Early maternal deprivation alters hippocampal levels of neuropeptide Y and calcitonin-gene related peptide in adult rats. Neuropharmacology 42:798–806
Khantzian EJ (1985) The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatr 142:1259–1264
Laviola G, Adriani W, Terranova ML, Gerra G (1999) Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev 23:993–1010
Laviola G, Macrì S, Morley-Fletcher S, Adriani W (2003) Risk-taking behaviour in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31
Laviola G, Ognibene E, Romano E, Adriani W, Keller F (2009) Gene-environment interaction during early development in the heterozygous reeler mouse: clues for modelling of major neurobehavioural syndromes. Neurosci Biobehav Rev 33:560–572
Levine S (2000) Influence of psychological variables on the activity of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol 405:149–160
Lipska BK, Weinberger DR (2000) To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology 23:223–239
Llorente R, Arranz L, Marco EM, Moreno E, Puerto M, Guaza C, De la Fuente M, Viveros MP (2007) Early maternal deprivation and neonatal single administration with a cannabinoid agonist induce long-term sex-dependent psychoimmunoendocrine effects in adolescent rats. Psychoneuroendocrinology 32:636–650
Lumia AR, Teicher MH, Salchli F, Ayers E, Possidente B (1992) Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res 587:181–185
Macri S, Laviola G (2004) Single episode of maternal deprivation and adult depressive profile in mice: interaction with cannabinoid exposureduring adolescence. Behav Brain Res 154:231–238
Marco EM, Adriani W, Llorente R, Laviola G, Viveros MP (2009) Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: altered responses to cannabinoid exposure. Neurosci Biobehav Rev 33:498–507
Matthews K, Robbins TW, Everitt BJ, Caine SB (1999) Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 141:123–134
Meaney MJ, Brake W, Gratton A (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27:127–138
Miczek KA, Yap JJ, Covington HE 3d (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128
Millstein RA, Holmes A (2007) Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev 31:3–17
Morgan C, Kirkbride J, Leff J, Craig T, Hutchinson G, McKenzie K, Morgan K, Dazzan P, Doody GA, Jones P, Murray R, Fearon P (2007) Parental separation, loss and psychosis in different ethnic groups: a case–control study. Psychol Med 37:495–503
Murray EA (2007) The amygdala, reward and emotion. Trends Cognit Sci 11:489–497
Okragly AJ, Haak-Frendscho M (1997) An acid-treatment method for the enhanced detection of GDNF in biological samples. Exp Neurol 145:592–596
Price CR, Rüedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B, Feldon J (2005) Long-term effects of early life environmental manipulations in rodents and primates: Potential animal models in depression research. Neurosci Biobehav Rev 29:649–674
Pryce CR, Feldon J (2003) Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 27:57–71
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth 66:1–11
Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA (2002) Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatr 7:609–616
Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL (1998) Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J 17:886–897
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 158:343–359
Slattery DA, Markou A, Cryan JF (2007) Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 190:555–568
Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M, Maldonado R, Valverde O (2005) Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30:1670–1680
Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O (2006) The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology 31:978–987
Soria G, Barbano MF, Maldonado R, Valverde O (2008) A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology (Berl) 199:593–603
Spear LP, Brake SC (1983) Periadolescence: age-dependent behaviour and psychopharmacological responsivity in rats. Dev Psychobiol 16:83–109
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85:367–370
Teicher MH, Tomoda A, Andersen SL (2006) Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci 1071:313–323
Touriño C, Ledent C, Maldonado R, Valverde O (2008) CB1 cannabinoid receptor modulates 3,4-methylenedioxymethamphetamine acute responses and reinforcement. Biol Psychiatr 63(11):1030–1038
Van Oers HJ, de Kloet ER, Levine S (1997) Persistent, but paradoxical, effects on HPA regulation of infants maternally deprived at different ages. Stress 1:249–262
Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ (2006) Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience 140:355–365
Weiss RD, Griffin ML, Mirin SM (1992) Drug abuse as self-medication for depression: an empirical study. Am J Drug Alcohol Abuse 18:121–129
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110
Willner P, Mitchell PJ (2002) The validity of animal models of predisposition to depression. Behav Pharmacol 13:169–188
Wurmser L (1974) Psychoanalytic considerations of the etiology of compulsive drug use. J Am Psychoanal Assoc 22:820–843
Zimmerberg B, Shartrand AM (1992) Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol 25:213–226
Acknowledgments
The authors wish to thank Neus Toro Ortiz and Raquel Martin Garcia for the technical support. This work was supported by the Spanish Ministry of Science and Innovation (SAF 2010/15793), the Spanish Ministry of Health (PNSD conv-2010 and RD06/0001/1001) and the Generalitat de Catalunya (SGR 2009/684).
Conflict of interest statement
The authors report no biomedical financial interest or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martini, M., Valverde, O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology 219, 149–158 (2012). https://doi.org/10.1007/s00213-011-2385-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-011-2385-2