Abstract
Rationale
Besides early drug initiation during adolescence, another vulnerability factor associated with increased risk for substance abuse later in life is early-life stress. One way of assessing such combined risk is by evaluating the emergence of increased negative affect during withdrawal (i.e., linked to persistence in drug seeking).
Objectives
To compare the impact of maternal deprivation with cocaine exposure at different ages on affective-like behavior and hippocampal neuroplasticity regulation.
Methods
Maternal deprivation was performed in whole-litters of Sprague-Dawley rats (24 h, PND 9-10). Cocaine (15 mg/kg, 7 days, i.p.) was administered in adolescence (PND 33–39) or adulthood (PND 64–70). Changes in affective-like behavior were assessed by diverse tests across time (forced-swim, open field, novelty-suppressed feeding, sucrose preference). Hippocampal multifunctional FADD protein (balance between cell death and plasticity) was evaluated by Western blot.
Results
Exposing rats to either maternal deprivation or adolescent cocaine did not modulate affective-like behavior immediately during adolescence, but increased negative affect in adulthood. Maternal deprivation combined with adolescent cocaine advanced the negative impact to adolescence. Adult cocaine exposure alone and/or in combination with maternal deprivation did not induce any behavioral changes at the time-points analyzed. FADD regulation might participate in the neural adaptations taking place in the hippocampus in relation to the observed behavioral changes.
Conclusions
Adolescence is a more vulnerable period, as compared to adulthood, to the combined impact of cocaine and early maternal deprivation, thus suggesting that the accumulation of stress early in life can anticipate the negative behavioral outcome associated with drug consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have investigated vulnerability factors that could lead to cocaine addiction, including early initiation during adolescence (e.g., Spear 2000), which is a critical period for neurobehavioral plasticity and vulnerability to drug use (e.g., Kelley et al. 2004; Spear 2011; Stanis and Andersen 2014). Prior studies from our research group demonstrated that treating rats with cocaine during a specific age-window in adolescence (post-natal day, PND 33–39) was more vulnerable than other time-periods, since immediate specific neurotoxic effects emerged in particular brain regions (see García-Cabrerizo et al. 2015), together with long-term consequences on addictive-like behavior (i.e., increased behavioral psychomotor sensitization, Parsegian et al. 2016; enhanced goal-tracking behavior in adult bred-low responder rats, Garcia-Fuster et al. 2017) and enhanced negative affect (i.e., increased immobility in the forced-swim test following cocaine re-exposure in adult rats, García-Cabrerizo and García-Fuster 2019a).
Besides adolescent drug exposure, another predisposing factor that could lead to drug consumption is having a prior psychiatric vulnerability (i.e., self-medication to cope with negative affect), which could be modeled in rodents (Koob 2012) by inducing adverse early-life experiences (Pryce et al. 2005; Schmidt et al. 2011; Gururajan et al. 2019). One such model, that has been shown to interfere with brain developmental trajectories and capable of modifying behavioral and neurochemical outcomes (see revision in Marco et al. 2015), applies a single episode (24 h) of early maternal deprivation on PND 9 (Ellenbroek et al. 1998). In the present study, we utilized this approach to evaluate whether the accumulation of stress early in life (maternal deprivation and cocaine exposure) could anticipate the negative behavioral outcome induced by adolescent cocaine alone, and to compare this outcome with the one obtained when cocaine was given in adulthood, and therefore corroborate possible age vulnerabilities of drug exposure.
Finally, the consequences of maternal deprivation on brain plasticity have been studied, with several molecules regulating hippocampal plasticity greatly affected (e.g., Marco et al. 2015). In line with this, and since the hippocampus participates in mediating affective and/or emotional-like responses, we selected FADD (Fas-associated protein with death domain) as a key neuroplasticity marker shown to be down-regulated in the brain of rodents during withdrawal from cocaine (García-Fuster et al. 2009) and other psychostimulants (García-Cabrerizo and García-Fuster 2015, 2019b) to evaluate how it is impacted by the combination of early life stress and cocaine exposure. Although FADD was initially described as a cell death signaling molecule, in the last years, it has been proven to be a key molecule balancing both pro- and anti-apoptotic signaling in response to the stimuli received (see revision in the context of FADD regulation by cocaine in García-Fuster et al. 2016).
Materials and methods
Animals
The present study is comprised by three independent and sequential experiments performed over time with Sprague-Dawley rats bred in the animal facility at the University of the Balearic Islands (see Fig. 1). Rats were housed in standard cages under precise environmental conditions (22 °C, 70% humidity, and 12 h light/dark cycle, lights on at 8:00 AM) with ad libitum access to a standard diet and tap water. All animal experiments complied with the ARRIVE guidelines (McGrath and Lilley 2015), and all rats were treated according to standard ethical guidelines (European Parliament and the Council of the European Union 2010; Louhimies 2003; National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research 2003). As stated in the Spanish Royal Decree 53/2013, all experimental procedures were awarded ethical approval by the Local Bioethical Committee (University of the Balearic Islands) and by the regional Government (Conselleria Medi Ambient, Agricultura i Pesca, Direcció General Agricultura i Ramaderia, Govern de les Illes Balears). The number of rats used and their suffering was minimized when possible and all procedures were performed during the light period (between 8:30 and 15:00 h).
Maternal deprivation early in life
For all experiments, a single episode of early maternal deprivation on PND 9 was carried out as previously described (Ellenbroek et al. 1998, 2005; Marco et al. 2009; also see revision in Marco et al. 2015) since this protocol is capable of inducing detrimental psychophysiological effects on rodents. For each individual experiment, a number of litters were used (6, 6, and 5, respectively) and randomly assigned to control or maternal deprivation groups (see Figs. 1, 2, 3). Briefly, the application of maternal deprivation was performed in whole litters (4, 3, and 3 for each individual experiment) for a set period of time (i.e., for 24 h, from PND 9 to 10). All pups were weighted right before maternal separation on PND 9 and at the end of the separation period on PND 10. During maternal deprivation, the mother was placed in an adjacent separate cage in the same room while pups were kept in their home cage with no nutritional supplements. Litters from the control groups (2, 3, and 2 for each experiment respectively, see Fig. 1) received the same amount of handling, since they were also weighted on PND 9 and 10, but were kept with the dam the whole duration of the procedure. At weaning (PND 22), only male rats (a total of 102, housed in groups of 2–4 rats; females were utilized in another unrelated studies) were selected for this study (n = 36, 33 and 33 respectively, see further details in Figs. 1, 2, 3), and were weighted on PND 24 (note that control or MD rats from different litters showed no significant differences in body weight, suggesting no litter effects; data not shown).
Effects of maternal deprivation (MD) and cocaine exposure on body weight. Body weight (g) on PND 24 post-weaning (control vs. MD rats) and a across days (PND 33–98) following adolescent cocaine or b across days (PND 64–98) following adult cocaine exposure. Data represents mean ± SEM of body weight (g). t tests (for PND 24) or three-way repeated measures ANOVAs followed by Sidak’s multiple comparisons test were performed: *p < 0.05 or ***p < 0.001 when comparing the effects of Early-Life Condition (Control vs. MD)
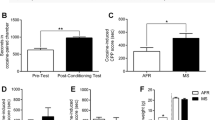
Effects of maternal deprivation (MD) and cocaine exposure on affective-like behavior. Affective-like behavior: time spent immobile (sec) in the FST on PND 26 (Control vs. MD prior to any pharmacological treatment), and a post-adolescent treatment on PND 40, or during withdrawal 45 min post-cocaine exposure on PND 71 and 98 or b post-adulthood treatment on PND 71, and 45 min post-cocaine exposure on PND 98. c Depressive- or anxiety-like behavior as measured by latency to center (sec) in the NSF on PND 43 and 74 post-adolescent treatment or d on PND 74 post-adulthood treatment. e Hedonic-like responses as measured by 1% sucrose preference in the two-bottle test on PND 47 and 78 post-adolescent treatment or f on PND 78 post-adulthood treatment. Data represents mean ± SEM of each measurement for each treatment group. A t test (for pair comparisons, e.g., FST on PND 26) or two- or three-way repeated measures ANOVAs followed by Sidak’s multiple comparison test: **p < 0.01 or *p < 0.05 when comparing the effects of Early-Life Condition (Control vs. MD); ψp < 0.05 vs. the corresponding Saline treated group
Cocaine exposure during adolescence or adulthood
To balance the experimental groups and avoid a possible litter effect, rats from each litter and early-life condition were randomly allocated to the different experimental groups and so each group had a representative number of animals from each litter (e.g., MD-Saline, n = 9, three rats from each one of the initial litters, see Fig. 1b). Groups of control or maternal-deprived rats were treated for 7 days with saline (0.9% NaCl, 1 ml/kg, i.p.) or cocaine HCl (15 mg/kg, i.p.) during adolescence (PND 33–39, see Fig. 1b for experimental groups; the adolescent age window for drug exposure was selected based on prior studies from our group, see García-Cabrerizo et al. 2015) or adulthood (PND 64–70, see Fig. 1c for experimental groups). Then, during drug withdrawal, rats were challenged with a single dose of cocaine (15 mg/kg, i.p., 45 min, PND 71 and/or PND 98, Fig. 1; see similar scheduling procedures followed by our group in García-Cabrerizo and García-Fuster 2019a) prior to exposing them to the forced-swim test (Fig. 1b–c).
Behavioral testing during adolescence and adulthood
Affective-like responses in rats (Gururajan et al. 2019) were assessed during adolescence and/or in adulthood by diverse tests across time (i.e., forced-swim test, open field, novelty-suppressed feeding, sucrose preference; see Figs. 1, 2, 3) that measure variations in the time spent immobile under the stress of being forced to swim in a water tank, differences in sensitivity to novelty (or decreased appetite or a combination thereof), and absolute sucrose consumption or reward sensitivity, respectively (see prior publications from our group at García-Cabrerizo and García-Fuster 2019a, b, Jiménez-Romero et al. 2020, Bis-Humbert et al. 2020).
Forced-swim test
All rats were handled for 2 days (PND 23–24, see Fig. 1) and then exposed to the stress of the forced-swim test (a 15-min pre-test) on PND 25, followed on PND 26 by a 5-min test that was videotaped (Barr et al. 2002; Slattery and Cryan 2017). For both sessions, each rat was placed in an individual tank (41 cm high × 32 cm diameter, water at 25 ± 1 °C, 25 cm depth; see for further details García-Cabrerizo et al. 2015). Videos recorded on PND 26 were then analyzed blinded to the experimental groups to evaluate the impact of early-life stress (maternal deprivation on PND 9 vs. normal control conditions) on immobility time for each rat (Behavioral Tracker software, CA, USA). Then, for some rats (Fig. 1b–c), the test was repeated to evaluate the combined impact of early-life stress and cocaine exposure (in adolescence or adulthood) at different time points. First, we wanted to test the immediate effects observed right after cocaine exposure (on PND 40 for adolescence, Fig. 1b, and PND 71 for adulthood, Fig. 1c), and then we evaluated the persistent effects emerging during drug withdrawal and following acute drug re-exposure (i.e., a drug paradigm known to induce psychomotor behavioral sensitization on PND 71 and/or PND 98; see Fig. 1b–c).
Open field test
Rats from the first experiment (on PND 28; see Fig. 1a) were exposed to the open field test (Walsh and Cummins 1976). This is a 5-min test in which rats are videotaped while being placed in a wall-enclosed square arena (60 × 60 cm) with a height of 40 cm, and evaluates exploratory-like behavior in an anxiogenic environment (i.e., latency to center of the arena, Hernández-Hernández et al. 2018; García-Cabrerizo and García-Fuster 2019a). Videos were analyzed (blind to the experimental groups) using a digital video tracking system (Smart Video Tracking software, Version 3.0.03, Panlab SL, Barcelona, Spain).
Novelty-suppressed feeding test
Rats were exposed to the novelty-suppressed feeding right after cocaine exposure (on PND 43 for adolescence, Fig. 1b, and on PND 74 for adulthood, Fig. 1c) or during prolonged withdrawal following adolescent cocaine exposure (PND 74, see Fig. 1b). This is a 5-min test that requires motivation for food and thus rats were food deprived for 48 h (see Fig. 1b–c). Sessions were videotaped while performed in a wall-enclosed square arena (60 × 60 cm) with a height of 40 cm, in which three food pellets are placed in the center of the arena. This test measures differences in sensitivity to novelty (or decreased appetite or a combination thereof) in an anxiogenic environment (e.g., Bodnoff et al. 1988; Blasco-Serra et al. 2017), by evaluating the latency to center (sec) and the time spent feeding (sec). The recorded videos were analyzed blind to the experimental groups (e.g., Turner et al. 2008).
Sucrose intake in a two-bottle choice test
Sucrose preference was evaluated following maternal deprivation (on PND 30–31, Fig. 1a) and following cocaine exposure (on PND 46–47 for adolescence, Fig. 1b, and on PND 77–78 for adulthood, Fig. 1c) or during prolonged withdrawal following adolescent cocaine exposure (PND 77–78, see Fig. 1b). Rats were single-housed 2 days prior to testing (see Fig. 1) and the preference for 1% sucrose was compared to water with a two-bottle choice test during 48 h (Slattery et al. 2007; see further details in García-Cabrerizo and García-Fuster 2019a, b; Jiménez-Romero et al. 2020). Bottles were placed in alternating positions each day to avoid bias towards any side of the cage, and were weighted daily to evaluate sucrose preference. The day before and after testing for sucrose intake, the two bottles were filled with water to ensure and confirm that rats drank evenly from both bottles. Results are expressed as preference on PND 31, PND 47, or PND 78.
Tissue collection and Western blot analyses
Rats were sacrificed by decapitation at the indicated times for each experiment (PND 33 or 99, see Fig. 1) and the right hippocampus was fast frozen in liquid nitrogen, and kept at − 80 °C until the cell fate adaptor FADD was analyzed by Western blot experiments (García-Fuster et al. 2007). Briefly, hippocampal proteins (40 μg of total proteins) were resolved by electrophoresis on 10% SDS–PAGE minigels (Bio-Rad Laboratories, Hercules, CA, USA), transferred to nitrocellulose membranes and incubated overnight at 4 °C with the appropriate primary antibody: (1) Santa Cruz Biotechnology (CA, USA): anti-FADD (H-181) (1:5000; sc-5559) and (2) Sigma-Aldrich (MO, USA): anti-β-actin (1:10000; clone AC-15). The corresponding secondary antibodies (anti-rabbit or anti-mouse IgG linked to horseradish peroxidase) were incubated for 1 h at room temperature (1:5000 dilution; Cell Signaling). Finally, immunoreactivity of FADD protein was detected with ECL chemicals (Amersham, Buckinghamshire, UK) and signal of bound antibody was transferred to an autoradiographic film (Amersham ECL Hyperfilm) for 1 to 60 min, which was later quantified by densitometric scanning (GS-800 Imaging Calibrated Densitometer, Bio-Rad). Percent changes in FADD immunoreactivity were calculated for each rat in each gel with respect to the corresponding control samples for each study (100%). Each rat sample was evaluated at least 2–3 times in different gels, and the mean value was used as a final estimate. The content of β-actin was measured following the same procedures and its immunoreactivity was used for each sample as a loading control (i.e., graphs represent the ratio FADD/β-actin for each animal), since β-actin was not modulated by any of the experimental procedures.
Statistical analyses
Data analysis was performed with GraphPad Prism, Version 8 (GraphPad Software, Inc., CA, USA) and results are reported as mean values ± standard error of the mean (SEM) following the guidelines for displaying data and statistical methods in experimental pharmacology (e.g., Curtis et al. 2018; Michel et al. 2020). Depending on the number of independent variables (Early-Life Condition, Treatment, Day) two- or three-way ANOVAs (with or without repeated measures), or two-tail Student’s t tests were utilized to evaluate behavioral and/or neurochemical changes. To note, that day was used as an independent variable to evaluate the progression in the behavioral response, since tests were repeated across time, and at a given day, animals from all groups (for each individual experiment) were exposed to the same conditions. Sidak’s multiple comparison tests were used for post-hoc analysis when appropriate. The level of significance was set at p ≤ 0.05.
Results
Effects of maternal deprivation and cocaine exposure on body weight
For all three independent experiments, a single exposure of maternal deprivation reduced body weight gain post-weaning as measured on PND 24 (− 8.9 ± 0.8 g, ***p < 0.001 vs. control rats, data not shown in graphs; − 17.4 ± 1.7 g, t = 10.40, df = 32, *p < 0.001 vs. control rats, Fig. 2a; and − 6.3 ± 1.4 g, t = 4.491, df = 31, *p < 0.001 vs. control rats; Fig. 2c), and this effect was independent of the litter used (data not shown).
Interestingly, these results persisted over time when further manipulations were performed in adolescence (Fig. 2a) but not in adulthood (Fig. 2b). In particular, Fig. 2a shows a significant effect of Early-Life Condition (F1,30 = 52.28, p < 0.001; represented in Fig. 2a by ***), of Day (F4.17,125.1 = 2213, p < 0.001), and a significant Early-Life Condition × Day interaction (F22,660 = 3.49, p < 0.001). Since no significant effect of Treatment was observed (F1,30 = 0.32, p = 0.577), rats exposed to maternal deprivation early in life showed reduced normal weight gain across time, independently of the adolescent drug treatment (saline vs. cocaine). However, when rats were left undisturbed until adulthood, at which point weight was measured again across days, the results showed the expected effect of Day in normal body weight increase (F1.83,53.15 = 627.8, p < 0.001), but no effect of treatment (F1,29 = 0.01, p = 0.942) nor of Early-Life Condition (F1,29 = 0.02, p = 0.896; Fig. 2b).
Effects of maternal deprivation and cocaine exposure on affective-like behavior
A single exposure of maternal deprivation early in life did not increase negative affect during adolescence (see Fig. 1a), since no significant changes were observed in the forced-swim test (time spent immobile: t = 0.813, df = 34, p = 0.422), open field (latency to center: t = 1.301, df = 32, p = 0.203), or two-bottle choice test (sucrose preference: t = 0.192, df = 34, p = 0.849) when compared to control rats (data not shown in graphs). The negative results detected on PND 26 in the FST were also replicated in the other two experiments, which also showed no significant changes in immobility time by maternal deprivation (t = 1.611, df = 31, p = 0.117, Fig. 3a; t = 1.955, df = 31, p = 0.060, Fig. 3b) as compared to controls (Fig. 3a–b). In fact, if data from all three independent tests were combined (with a total of n = 40 controls and n = 62 maternal deprived rats), maternal deprivation early in life did not significantly alter immobility in the forced-swim test (controls: 206 ± 12 s; maternal deprivation: 190 ± 8 s; t = 1.09, df = 100 p = 0.277).
Then, we evaluated the combined impact of early-life stress followed by cocaine exposure (either during adolescence or adulthood) in the forced-swim test. When cocaine was administered in adolescence, the results showed a significant effect of Early-Life Condition (F1,29 = 10.39, p < 0.01; increased immobility in maternal deprived rats as compared to controls, represented in Fig. 3a by **), of Day (F1.94,56.18 = 123.3, p < 0.001), and a significant Early-Life Condition × Day interaction (F2,58 = 7.88, p < 0.001; driven by the smaller psychomotor response induced by acute cocaine re-exposure observed on PND 71 and 98 in maternal deprived vs. control rats). However, no significant effect of Treatment was observed (F1,29 = 0.44, p = 0.513). Therefore, independently of treatment (a prior history of cocaine or saline during adolescence did not alter the outcome), maternal deprivation early in life did not induce changes in immobility in the forced-swim test during adolescence (PND 40) but increased immobility later on in adulthood (i.e., increased negative affect), since the expected psychomotor activating effects of acute cocaine on PND 71 and 98 were dampened. However, when cocaine was administered in adulthood, the results showed a significant effect of Day (F1,27 = 27.26, p < 0.001), but no effect of Treatment (F1,27 = 1.12, p = 0.299) nor of Early-Life Condition (F1,27 = 1.43, p = 0.243; Fig. 3b).
Next, we evaluated the combined impact of early-life stress followed by cocaine exposure in the novelty-suppressed feeding test. When cocaine was administered in adolescence, although the latency to center was not impacted by Early-Life Condition (F1,30 = 0.933, p = 0.342), there was a significant Early-Life Condition × Treatment × Day interaction (F1,30 = 6.07, p < 0.05), which was mainly driven by an effect of Treatment (F1,30 = 8.00, p < 0.01). In control rats, although no changes were observed in adolescence, later on in adulthood (PND 74) following adolescent cocaine exposure, some changes emerged, such as a higher latency to approach the center (+ 194 ± 55 s, Ψp < 0.05 when compared to saline-treated control rats; Fig. 3c). However, for rats exposed to maternal deprivation early in life, cocaine increased the latency to approach the center, an effect that was apparent earlier on during adolescence and was significant overall when comparing the effects of cocaine vs. saline independently of when the test was performed (+ 66 ± 30 s, Ψp = 0.05 when compared to saline-treated maternal deprived rats; combined data for maternal deprived rats on PND 43 and 74, Fig. 3c). Similarly, when analyzing the time spent feeding (data not shown in figures), there was a significant effect of Treatment (F1,30 = 4.93, p < 0.05) and Day (F1,30 = 34.69, p < 0.001). In particular, all rats showed lower time feeding on PND 74 as compared to PND 43, suggesting an anxiogenic-like effect developed long-term. Interestingly, cocaine induced an overall negative impact that was only significant in maternal-deprived rats and was observed as early as during adolescence (– 42 ± 21 s feeding; p = 0.05 when compared to saline-treated maternal deprived rats; combined data for maternal deprived rats on PND 43 and 74, data not shown in figures). However, when cocaine was administered in adulthood, the results showed no effect of Early-Life Condition (latency to center: F1,29 = 0.64, p = 0.430; time feeding: F1,29 = 0.27, p = 0.605), Treatment (latency to center: F1,29 = 0.53, p = 0.472; time feeding: F1,29 = 1.61, p = 0.214), or Early-Life Condition × Treatment interaction (latency to center: F1,29 = 0.32, p = 0.573; time feeding: F1,29 = 0.01, p = 0.930, Fig. 3d).
Finally, we assessed the combined impact of early-life stress followed by cocaine exposure over sucrose preference in the two-bottle choice test. When cocaine was administered in adolescence, the results showed a significant effect of Early-Life Condition (F1,29 = 4.89, p < 0.05; decreased sucrose preference in maternal deprived rats as compared to controls, represented in Fig. 3e by *), but no effect or Treatment (F1,29 = 0.30, p = 0.589) nor of Day (F1,29 = 1.60, p = 0.217). However, when cocaine was administered in adulthood, no significant effects of Early-Life Condition (F1,27 = 2.14, p = 0.155) or Treatment (F1,27 = 0.01, p = 0.963) were observed (see Fig. 3f). The absolute values of water consumption were not altered when comparing experimental groups (data not shown).
Effects of maternal deprivation and cocaine exposure on hippocampal FADD
Although maternal deprivation early in life did not induce any behavioral changes during early adolescence (see Fig. 2), it increased the content of hippocampal FADD protein as compared to control rats on PND 33 (FADD/β-actin: t = 2.808, df = 30, **p < 0.01; Fig. 4a). Interestingly, when evaluating the combined impact of early-life stress followed by adolescent cocaine exposure, the results showed a significant effect of Early-Life Condition (F1,30 = 5.21, p < 0.05, this effect observed on PND 99 is represented in Fig. 4b by *) and a significant Early-Life Condition × Treatment interaction (F1,30 = 9.97, p < 0.01). Post-hoc analysis revealed a significant decrease in FADD protein content in control rats treated with cocaine during adolescence and exposed to prolonged forced withdrawal and cocaine re-exposure (34 ± 11% vs. control-saline rats, Fig. 4b), while showed no effects of maternal deprivation (with or without cocaine; Fig. 4b). When cocaine was administered during adulthood following early-life stress, the results for hippocampal FADD regulation on PND 99 showed no effect of Early-Life Condition (F1,29 = 0.02, p = 0.882) nor of Treatment (F1,29 = 0.489, p = 0.490; Fig. 4c).
Effects of maternal deprivation (MD) and cocaine exposure on hippocampal FADD. FADD protein content. Data represents mean ± SEM of FADD/β-actin content expressed as % change vs. a Control or vs. (b–c) Control-Saline. Symbols represent individual rat values within each experimental group. Student’s t test or two-way ANOVAs followed by Sidak’s post-hoc were used for statistical analysis: **p < 0.01 or *p < 0.05 when compared to Control, ψψp < 0.01 vs. Control-Saline group. Representative immunoblots are shown depicting labeling of FADD and β-actin (as a loading control).
Discussion
In this manuscript, we present a sequential set of experiments that compared the combined impact of early-life stress and cocaine exposure at different age windows (adolescence or adulthood) on inducing negative affect in rats and modulating a key neuroplasticity marker in the hippocampus.
Maternal deprivation induced a drop in normal weight gain as observed post-weaning (PND 24) for all experiments performed. This effect was observed during adolescence and persisted until adulthood when further manipulations were performed during adolescence (body weight measurements in adolescence; Fig. 2a), but was not apparent when rats were left undisturbed until adulthood and were weighted again starting on PND 64 and onwards (Fig. 2b). The short- and long-term effects of maternal deprivation on body weight are well known (effects in adolescence: e.g., Ellenbroek et al. 2005; persistent effects in adulthood: e.g., Viveros et al. 2010), and since outside of the main scope of this manuscript, a revision discussing its implications could be found in Marco et al. (2015) and the references within.
When characterizing the impact of early-life stress (24 h of maternal deprivation on PND 9) on affective-like behavior in adolescent rats, as described earlier, no changes were observed in depressive- (PND 26), anxiety- (PND 28), and anhedonic-like behaviors (PND 31) during early adolescence. Contrarily, some prior studies have shown that maternal deprivation early in life facilitated the emergence of depressive- (as measured in the forced-swim test), but not of anxiety-like symptoms (as measured in the elevated plus maze) during adolescence (Llorente et al. 2007; Marco et al. 2009). However, some contradictory data has led to the conclusions that early-life stress does not consistently induce a depressive-phenotype, and that these discrepancies might be mainly due to variations in the methodology followed (i.e., duration of separation, age of stress exposure and age of behavioral testing, sex, species) among research groups (Schmidt et al. 2011) or might depend on the nature of stress (He et al. 2020), and thus our conclusions could be drawn particularly for male Sprague-Dawley rats and a single episode of separation. For example, a recent study reported that early-life stress induced negative affect only in female mice, as observed by an increase in depressive-like behavior that started in adolescence but amplified its severity in adulthood (Goodwill et al. 2019). In this context, the negative behavioral consequences of maternal separation did emerge with age, since immobility increased in the forced-swim test (Fig. 3a) and sucrose preference decreased (i.e., increased anhedonic-like behavior, Fig. 3e) during adulthood. Similarly, prior studies have shown that maternal deprivation early in life induced a depressive-like phenotype during adulthood (see revisions in Marco et al. 2009, 2015).
When cocaine was administered in adolescent rats, with no early-life stress (control group), an anxiogenic-like phenotype emerged later on in adulthood (PND 74, Fig. 3c), suggesting with prior data from other groups (e.g., Perrine et al. 2008; Zilkha et al. 2014) and our own (García-Cabrerizo and García-Fuster 2019a), that cocaine administration during this particular window of adolescence (PND 33–39) induces long-term and enduring negative behavioral effects on affect. Interestingly, combining early-life stress with adolescent cocaine advanced the development of an anxiety-like phenotype to adolescence (i.e., overall increase in latency to center and decrease in feeding time; Fig. 3c and data not shown). These results, in line with the prior literature (e.g., Zhu et al. 2016; adolescent exposure to cocaine increases anxiety-like behavior in adult rats) suggest that exposing rats to both early-life stress and adolescent cocaine increases the vulnerability to enhanced negative affect (i.e., worse behavioral response in the novelty-suppressed feeding test). In fact, when cocaine was administered during adulthood (PND 64–70) following early-life stress, no changes were observed in affective-like behavior, reinforcing the idea that early initiation in drug use during adolescence, and in particular, during this window of vulnerability (PND 33-39), induced a higher impact on negative affect during drug withdrawal (e.g., García-Cabrerizo and García-Fuster 2019a; adolescent cocaine exposure enhanced immobility in the forced-swim test in adult rats), likely leading to higher relapse rates and higher vulnerability to develop addictive-like responses (e.g., Parsegian et al. 2016 for increased cocaine sensitization when cocaine was administered at the same age-window during adolescence).
As one potential down-stream signaling molecule that regulates plasticity in the hippocampus and that could somehow be involved in the behavioral effects observed (i.e., modulation during drug withdrawal: García-Fuster et al. 2009; García-Cabrerizo and García-Fuster 2019b; modulation in depressive-like phenotypes: García-Fuster et al. 2014; modulation by antidepressant drugs: García-Fuster and García-Sevilla 2016), we evaluated multifunctional FADD protein content. The main results showed that maternal deprivation early in life increased FADD content in adolescence (PND 33), suggesting that although no behavioral changes were observed, maternal deprivation had a negative impact in brain plasticity (i.e., a possible neurotoxic effect), that might participate in the long-term effects observed later on in adulthood. On the other hand, FADD was decreased in adult control rats treated with adolescent cocaine (effects observed on PND 99 during drug withdrawal and following cocaine re-exposure 24 h earlier), supporting prior data from our lab showing decreased FADD protein content during withdrawal from several psychostimulants (García-Fuster et al. 2009; García-Cabrerizo and García-Fuster 2015, 2019b) in rat hippocampus, and suggesting a role for FADD as one of the possible strategic neuroplasticity markers mediating some of the repair mechanisms emerging following an earlier drug exposure (i.e., neurochemical adaptations; see García-Cabrerizo and García-Fuster 2019b for further discussion). Interestingly and in parallel to the behavioral effects, when cocaine was administered during adulthood (PND 64–70) following early-life stress, no changes were observed in FADD regulation, again suggesting that early drug initiation during adolescence has a broader impact than when the first exposure occurred in adulthood. Moreover, FADD was not regulated in adult rats that were exposed to maternal deprivation early in life with or without cocaine exposure. Since maternal deprivation early in life increased FADD during adolescence, the absence of significant effects in adulthood might suggest the induction of adaptative mechanisms throughout time. Overall, FADD regulation in adolescence by early-life stress and in adulthood by prior adolescent cocaine exposure might contribute to the neuroadaptations taking place in the hippocampus with some relevance to the induced depressive-like symptomatology (e.g., Markou and Kenny 2002). In any case, other brain regions as well as other neuroplasticity markers may be involved in such regulations and deserve future studies.
In summary, although maternal deprivation early in life did not induce changes in affective-like behavior in adolescence, its negative impact emerged during adulthood (i.e., increased depressive- and anhedonic-like phenotype). Similarly, adolescent cocaine in control rats did not induce negative affect during adolescence but triggered persistent effects in affective-like behavior in adulthood (i.e., an anxiogenic-like phenotype). Interestingly, when early-life stress was combined with adolescent cocaine, the anxiogenic-like effect advanced to adolescence demonstrating that the accumulation of stress can anticipate negative affect at the behavioral level. Future studies should center in evaluating experimental settings that better translate to the human literature, both in terms of the affective-like behavioral measurements and in evaluating a cocaine paradigm that reflects drug-seeking/-taking propensity (i.e., self-administration) to be able to characterize individual variabilities. Moreover, including female rats would allow to evaluate potential sexually dimorphic effects emerging following the combination of early-life stress and adolescent cocaine on affective-like behavioral phenotypes.
References
Barr AM, Markou A, Phillips AG (2002) A ‘crash’ on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci 23:475–482
Bis-Humbert C, García-Cabrerizo R, García-Fuster MJ (2020) Decreased sensitivity in adolescent versus adult rats to the antidepressant-like effects of cannabidiol. Psychopharmacology 237:1621–1631
Blasco-Serra A, González-Soler EM, Cervera-Ferri A, Teruel-Martí V, Valverde-Navarro AA (2017) A standardization of the Novelty-Suppressed Feeding Test protocol in rats. Neurosci Lett 658:73–78
Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ (1988) The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology 95:298–302
Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, Hoyer D, Insel PA, Izzo AA, Ji Y, MacEwan DJ, Sobey CG, Stanford SC, Teixeira MM, Wonnacott S, Ahluwalia A (2018) Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Br J Pharmacol 175:987–993
Ellenbroek BA, van den Kroonenberg PT, Cools AR (1998) The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res 30:251–260
Ellenbroek BA, Derks N, Park HJ (2005) Early maternal deprivation retards neurodevelopment in Wistar rats. Stress 8:247–257
European Parliament and the Council of the European Union (2010) Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2020 on the protection of animals used for scientific purposes. Off J Eur Union L276:33–79
García-Cabrerizo R, García-Fuster MJ (2015) Chronic MDMA induces neurochemical changes in the hippocampus of adolescent and young adult rats: down-regulation of apoptotic markers. Neurotoxicology 49:104–113
García-Cabrerizo R, García-Fuster MJ (2019a) Adolescent cocaine exposure enhanced negative affect following drug re-exposure in adult rats: attenuation of c-Fos activation. J Psychopharmacol 33:154–162
García-Cabrerizo R, García-Fuster MJ (2019b) Methamphetamine binge administration dose-dependently enhanced negative affect and voluntary drug consumption in rats following prolonged withdrawal: role of hippocampal FADD. Addict Biol 24:239–250
García-Cabrerizo R, Keller B, García-Fuster MJ (2015) Hippocampal cell fate regulation by chronic cocaine during periods of adolescence vulnerability: consequences of cocaine exposure during adolescence on behavioral despair in adulthood. Neuroscience 304:302–315
García-Fuster MJ, García-Sevilla JA (2016) Effects of anti-depressant treatments on FADD and p-FADD protein in rat brain cortex: enhanced anti-apoptotic p-FADD/FADD ratio after chronic desipramine and fluoxetine administration. Psychopharmacology 233:2955–2971
García-Fuster MJ, Miralles A, García-Sevilla JA (2007) Effects of opiate drugs on Fas-associated protein with death domain (FADD) and effector caspases in the rat brain: regulation by the ERK1/2 MAP kinase pathway. Neuropsychopharmacology 32:399–411
García-Fuster MJ, Clinton SM, Watson SJ, Akil H (2009) Effect of cocaine on Fas-associated protein with death domain in the rat brain: individual differences in a model of differential vulnerability to drug abuse. Neuropsychopharmacology 34:1123–1134
García-Fuster MJ, Díez-Alarcia R, Ferrer-Alcón M, La Harpe R, Meana JJ, García-Sevilla JA (2014) FADD adaptor and PEA-15/ERK1/2 partners in major depression and schizophrenia postmortem brains: basal contents and effects of psychotropic treatments. Neuroscience 277:541–551
García-Fuster MJ, Álvaro-Bartolomé M, García-Sevilla JA (2016) The Fas receptor/Fas-associated protein and cocaine. neuropathology of drug addictions and substance misuse, volume 2: stimulants, club and dissociative drugs, hallucinogens, steroids, inhalants and international aspects. Academic Press 2:63–73
Garcia-Fuster MJ, Parsegian A, Watson SJ et al (2017) Adolescent cocaine exposure enhances goal-tracking behavior and impairs hippocampal cell genesis selectively in adult bred low-responder rats. Psychopharmacology 234:1293–1305
Goodwill HL, Manzano-Nieves G, Gallo M, Lee HI, Oyerinde E, Serre T, Bath KG (2019) Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 44:711–720
Gururajan A, Reif A, Cryan JF, Slattery DA (2019) The future of rodent models in depression research. Nat Rev Neurosci 20:686–701
He T, Guo C, Wang C, Hu C, Chen H (2020) Effect of early life stress on anxiety and depressive behaviors in adolescent mice. Brain Behav 10:e01526
Hernández-Hernández E, Miralles A, Susana E, García-Fuster MJ (2018) Improved age-related deficits in cognitive performance and affective-like behavior following acute, but not repeated, 8-OH-DPAT treatments in rats: regulation of hippocampal FADD. Neurobiol Aging 71:115–126
Jiménez-Romero F, Bis-Humbert C, García-Fuster MJ (2020) Adolescent morphine induces emotional signs of withdrawal paired with neurotoxicity selectively in male rats: female resilience. Neurosci Lett 715:134625
Kelley AE, Schochet T, Landry CF (2004) Risk taking and novelty seeking in adolescence: introduction to part I. Ann N Y Acad Sci 1021:27–32
Koob GF (2012) Animal models of psychiatric disorders. Handb Clin Neurol 106:137–166
Llorente R, Arranz L, Marco EM, Moreno E, Puerto M, Guaza C, De la Fuente M, Viveros MP (2007) Early maternal deprivation and neonatal single administration with a cannabinoid agonist induce long-term sex-dependent psychoimmunoendocrine effects in adolescent rats. Psychoneuroendocrinology 32:636–650
Louhimies S (2003) Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes. Alternatives to Laboratory Animals: ATLA 30 Suppl 2(2_suppl):217–219
Marco EM, Adriani W, Llorente R, Laviola G, Viveros MP (2009) Detrimental psychophysiological effects of early maternal deprivation in adolescent and adult rodents: altered responses to cannabinoid exposure. Neurosci Biobehav Rev 33:498–507
Marco EM, Llorente R, López-Gallardo M, Mela V, Llorente-Berzal Á, Prada C, Viveros MP (2015) The maternal deprivation animal model revisited. Neurosci Biobehav Rev 51:151–163
Markou A, Kenny PJ (2002) Neuroadaptations to chronic exposure to drugs of abuse: relevance to depressive symptomatology seen across psychiatric diagnostic categories. Neurotox Res 4:297–313
McGrath JC, Lilley E (2015) Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172:3189–3193
Michel MC, Murphy TJ, Motulsky HJ (2020) New author guidelines for displaying data and reporting data analysis and statistical methods in experimental biology. J Pharmacol Exp Ther 372:136–147
National Research Council (US) Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press, Washington, DC
Parsegian A, García-Fuster MJ, Watson SJ, et al. (2016) Adolescent cocaine experience differentially augments psychomotor sensitization in adulthood and alters dopamine receptor and epigenetic profiles in the nucleus accumbens of selectively bred high- and low-responder rats. Society for Neuroscience Annual Meeting 2016-S-4910-SfN
Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM (2008) Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology 54:355–364
Pryce CR, Ruedi-Bettschen D, Dettling AC, Weston A, Russig H, Ferger B et al (2005) Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci Biobehav Rev 29:649–674
Schmidt MV, Wang XD, Meijer OC (2011) Early life stress paradigms in rodents: potential animal models of depression? Psychopharmacology 214:131–140
Slattery DA, Cryan JF (2017) Modelling depression in animals: at the interface of reward and stress pathways. Psychopharmacology 234:1451–1465
Slattery DA, Markou A, Cryan JF (2007) Evaluation of reward processes in an animal model of depression. Psychopharmacology 190:555–568
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463
Spear LP (2011) Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci 1:390–403
Stanis JJ, Andersen SL (2014) Reducing substance abuse during adolescence: a translational framework for prevention. Psychopharmacology 231:1437–1453
Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H (2008) Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res 1224:63–68
Viveros MP, Llorente R, Díaz F, Romero-Zerbo SY, Bermudez-Silva FJ, Rodríguez de Fonseca F, Argente J, Chowen JA (2010) Maternal deprivation has sexually dimorphic long-term effects on hypothalamic cell-turnover, body weight and circulating hormone levels. Horm Behav 58:808–819
Walsh RN, Cummins RA (1976) The open-field test: a critical review. Psychol Bull 83:482–504
Zhu W, Mao Z, Zhu C, Li M, Cao C, Guan Y, Yuan J, Xie G, Guan X (2016) Adolescent exposure to cocaine increases anxiety-like behavior and induces morphologic and neurochemical changes in the hippocampus of adult rats. Neuroscience 313:174–183
Zilkha N, Feigin E, Barnea-Ygael N, Zangen A (2014) Induction of depressive-like effects by subchronic exposure to cocaine or heroin in laboratory rats. J Neurochem 130:575–582
Acknowledgments
The authors would like to thank Fernando Jiménez-Romero for procedural assistance.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Delegación del Gobierno para el Plan Nacional sobre Drogas (grant 2016/002, Ministerio de Sanidad, Servicios Sociales e Igualdad, Spain) and by Fundación Alicia Koplowitz to MJG-F; CB-H was supported by the program “TECH” from project “TALENT PLUS Construint Salut, Generant Valor” (IdISBa, GOIB) and RG-C by a pre-doctoral fellowship (Consejería de Innovación, Investigación y Turismo del Gobierno de las Islas Baleares y del Fondo Social Europeo). MJG-F is a member of RETICS-RTA (RD16/0017/0010; Instituto de Salud Carlos III, MINECO/FEDER).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bis-Humbert, C., García-Cabrerizo, R. & García-Fuster, M.J. Increased negative affect when combining early-life maternal deprivation with adolescent, but not adult, cocaine exposure in male rats: regulation of hippocampal FADD. Psychopharmacology 238, 411–420 (2021). https://doi.org/10.1007/s00213-020-05689-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05689-4