Abstract
Stress and high levels of glucocorticoids during pre- and early postnatal life seem to alter developmental programs that assure dopaminergic transmission in the mesolimbic, mesocortical, and nigrostriatal systems. The induced changes are likely to be determined by the ontogenetic state of development of these brain regions at the time of stress exposure and their stability is associated with increased lifetime susceptibility to psychiatric disorders, including drug addiction. This article is intended to serve as a starting point for future studies aimed at the attenuation or reversal of the effects of adverse early life events on dopamine-regulated behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The catecholaminergic neurotransmitter dopamine (DA; 4-[2-aminoethyl]benzene-1,2-diol) is prominently involved in a number of brain functions such as cognition, emotion, reward, and motor control (Nieoullon and Coquerel 2003; Wise 2008), as well as neuropsychiatric disorders such as schizophrenia, drug addiction, attention deficit hyperactivity disorder (ADHD), and Parkinson’s disease (Genro et al. 2010; Howes and Kapur 2009; Melis et al. 2005; Oades et al. 2005; Piazza and Le Moal 1996; Weiner 2002). DA is also implicated in the regulation of depression, social behavior and pain processing (Kapur and Mann 1992; Wood 2008). DAergic activity changes in a graded fashion over the lifespan, resulting in the manifestation of age-related behavioral profiles and neurological conditions. In rodents, DA-producing neurons begin to form during early mid-gestation (E10.5); at E12.5, these neurons start to express tyrosine hydroxylase, the rate-limiting enzyme in the conversion of l-tyrosine into l-DOPA (3,4-dihydroxyphenylalanine) and, subsequently, into DA. Thereafter, the generation of DAergic cells gradually declines, and importantly, DAergic neurons increasingly undergo two peaks of apoptosis: immediately after birth and again, during the second week of postnatal life (Burke 2004; Oo and Burke 1997). It is estimated that adult human and rat brains contain some 600,000 and 45,000 DAergic cells, respectively (German and Manaye 1993)—a relatively small proportion of the total population of neurons in the brain.
Knowledge of the various transcription factors that contribute to the ontogeny of DAergic neurons has grown considerably in the last decade (Prakash and Wurst 2006). On the other hand, besides knowing that increased levels of reactive oxygen species derived from neurotoxins and that, perhaps, some therapeutic agents can compromise the viability of DA neurons, our understanding of other environmental and physiological factors that are responsible for the survival and demise of these neurons is surprisingly limited. In light of the narrow window within which DAergic cells are born, and the fact that the fate of the developing nervous system is particularly sensitive to environmental influences (Bjorklund and Dunnett 2007), studying how early life events may sculpt DAergic circuits, and therefore predispose individuals, or indeed contribute to their resilience to DA-related disorders later in life, is particularly important.
This article focuses on how early life stress, implicated in a number of behavioral disorders associated with DAergic dysfunction, may exert its effects. Notably, a number of studies, mainly carried out in norepinephrine neurons of adult animals, have shown that glucocorticoids (GC), the primary humoral effectors of the physiological response to stress, can upregulate tyrosine hydroxylase (TH) synthesis and, therefore, as DA production is also under regulation of TH, it is admissible that GCs might also regulate DA production (Makino et al. 2002; Markey et al. 1982; Ortiz et al. 1995, 1996). While these effects are likely to reflect direct GC actions on TH neurons following their activation of GCs receptors (which have transcriptional properties), indirect regulation of TH synthesis through intersecting pathways cannot be excluded (Otten and Thoenen 1975). Administration of GCs significantly change DA and its metabolites levels in the striatum and prefrontal cortex (PFC), importantly, adrenalectomy seems to have an antagonist effect (Lindley et al. 1999; Lindley et al. 2002), although contradictory findings have also been published (Dunn 1988). Nevertheless, it has been shown that dopaminergic transmission in the nucleus accumbens (NAcc) seems to be GC-dependent, both in basal conditions and after stimulus (Barrot et al. 2000).
Programming of behavior by early life stress
Adversity during early life, including physical and emotional neglect and traumatic experiences, can induce persistent effects on physical and mental health (Heim and Nemeroff 2002; Teicher et al. 2003). Specifically, there is now well-documented evidence that adversity in childhood increases the risk for development of conduct disorders, personality disorders, ADHD, major depression, posttraumatic stress disorder, schizophrenia, anxiety, and addictive disorders (Agid et al. 1999; Bernet and Stein 1999; Chapman et al. 2004; Dube et al. 2003; Heim and Nemeroff 2001; Kendler et al. 2004; Weiss et al. 1999; Young et al. 1997). The clinical importance of these findings can be better appreciated when one considers that some 80% of adults who experienced abuse or neglect in early life are predicted to suffer at least one episode of a psychiatric disorder such as depression and anxiety or a behavioral disorder such as addiction (Edwards et al. 2003; Espejo et al. 2007; Gutman and Nemeroff 2003; Heim and Nemeroff 2001; McFarlane et al. 2005). In contrast, the predicted incidence of such disturbances is much lower in women abused as adults (Brown and Moran 1994; McCauley et al. 1997), a finding that points to the existence of critical time windows during which the organism is particularly sensitive to stress-induced pathology later in life.
Most of the above clinical conditions are linked to impaired DAergic transmission and are likely to be underpinned by structural alterations in the nervous tissue which, in turn, translate into a resetting of homeostatic mechanisms that promote either adaptation or pathology. Much attention has been recently focused on the ability of early life stress (ELS) to program the hypothalamic–pituitary–adrenocortical (HPA) axis (Heim et al. 2008; Tarullo and Gunnar 2006). Information about the physical and psychological environments converges on this axis, which, through its secretion of GCs, determines the organism’s physiological and behavioral response. In a simplistic way, physical or physiological stress activates the production of corticotrophin-releasing factor in the hypothalamus, which in turns binds to specific receptors in pituitary cells stimulating the production of adrenocorticotropic hormone (ACTH). ACTH is then transported to adrenal glands, culminating with the secretion of GCs (cortisol in humans and corticosterone in rodents). GCs have a series of metabolic effects for improving stress response and act through negative feedback to both the hypothalamus and the anterior pituitary, once the state of stress subsides. Yet, it should be noted that stress response involves far more than the elevation of GCs and, as a consequence, the stress effects cannot be confined to elevations of GCs. Indeed, it has been shown that severe forms of stress can also result in decreased levels of GCs release; as an example, insufficient GC signaling may lie beneath the pathophysiology of some stress-related disorders such as posttraumatic stress disorder (Raison and Miller 2003).
Importantly, in utero exposure to GC/stress has also been found to be associated with long-lasting deficits in cognitive, mood and affective, as well as addictive and affiliative behaviors in humans (French et al. 1999; Heim and Nemeroff 2001; MacArthur et al. 1982; Malaspina et al. 2008; Sinha 2001) and in animal models (Caldji et al. 1998; Liu et al. 1997; Oliveira et al. 2006; Rayburn et al. 1997). It is of interest to note that GC administration or separation of rodents from their mothers during the first week of postnatal life shifts the timing of a number of neurodevelopmental milestones. Such treatments delay the acquisition of neurological reflexes (e.g. righting and postural reflexes, negative geotaxis) that depend on vestibular and cerebellar function (Ellenbroek et al. 2005; Mesquita et al. 2007), while advancing eye and ear opening. On the other hand, prenatal stress advances the time of ear-flap and eye opening (Secoli and Teixeira 1998). While these neurodevelopmental changes may reflect delayed myelination (Ferguson and Holson 1999; Murphy et al. 2001; Valkama et al. 2000), there is strong evidence for a role of altered catecholaminergic transmission in the vestibular region, the ventral tegmental area (VTA) and raphe nuclei (Mesquita et al. 2007). Since these brainstem structures project to corticolimbic structures, it is plausible that their altered activity impacts on neuroendocrine (HPA axis activity) and behavioral functions.
In the majority of cases, the behavioral consequences of ELS are attributable to transient or persistent dysregulation of GC secretion which, in turn, is causally related to increased susceptibility to depression and anxiety disorders (Carroll et al. 1976; Heim et al. 2001; Heim et al. 2000; Holsboer 2001; Yehuda et al. 1991), impaired social behaviors (Rinne et al. 2002), ADHD (Sullivan and Brake 2003; Swanson et al. 2007), and drug abuse (Huizink et al. 2006; Prendergast and Little 2007), all of which appear to involve an altered DAergic tone. Yet, whereas severe stress is usually associated with HPA-mediated pathology, mild stressful experiences may be linked to “positive” effects and/or resilience in rodents (Catalani et al. 1993; Levine 1957; Macri et al. 2009).
Pioneering work by Meaney and colleagues showed that the HPA axis can be epigenetically programmed (McGowan et al. 2009; Weaver et al. 2004) and further, that epigenetic (methylation) marks may be transmitted across generations. Other studies have shown that ELS-induced alterations in the epigenetic control of the activity of the HPA axis are associated with enduring expression of impaired cognitive- and depressive-like behavior in rodents (Murgatroyd et al. 2009). It remains to be demonstrated whether drugs with the potential to reverse DNA methylation (e.g. 5-aza-2′-deoxycytidine, already approved for use in cancer chemotherapy), can reverse the central effects of ELS. It should be noted that stress also leads to transient epigenetic alterations by deacetylation of histones with concomitant changes in behavior; such changes are drug-reversible with inhibitors of histone decaetyltransferase which have also proved effective in reversing age-dependent cognitive decline in experimental animals (Peleg et al. 2010).
Linking ELS to DAergic activity
The developing postnatal and adolescent brain is characterized by high levels of neuroplasticity and reorganization. Given the evidence that prenatal, perinatal, and early postnatal life represent windows of susceptibility to the long-lasting effects of stress on brain pathologies related to DAergic dysfunction, it is reasonable to assume that DAergic circuits are direct or indirect targets of stress and stress hormones (GC). The clinical studies about ELS, DAergic transmission and psychiatric conditions are sparse. Nevertheless, it has been shown that low parental care is associated with higher cortisol and, consequently, ventral striatum dopamine levels in response to a psychosocial stress task (Pruessner et al. 2004). Moreover, it has been shown that a polymorphism in the DA enzyme COMT and childhood trauma may interact together to contribute to the risk of developing psychopathological personality traits (Savitz et al. 2010). COMT polymorphisms also seem relevant for the manifestation of depressive symptoms in children exposed to severe social deprivation (Drury et al. 2010) and for the modulation of emotionality in sexually abused children (Perroud et al. 2010). A functional polymorphism that leads to higher expression of the enzyme monoamine oxidase A (degrades DA), was found to be correlated with reduced propensity for anti-social behaviors in maltreated children (Caspi et al. 2002; Kim-Cohen et al. 2006). Altogether, these findings reveal that variations in DA metabolism may modulate the impact of early life adversity on behavior and suggest a close link between DA, stress and mental illness. Stress may influence DAergic (1) cell fate; (2) neuron metabolism (DA production and turnover); (3) neuron morphology; and/or (4) receptor expression and synaptic transmission. Its effects, whether transient or permanent, can thus be expected to have long-term consequences on the shaping and expression of DA-regulated behaviors. Notably, the consequences of ELS appear to be different upon the different DAergic circuits. Perinatal stress seems to decrease steady state levels of DA in the PFC and to increase it in both the NAcc and striatum (Boksa and El-Khodor 2003). While perinatal anoxia enhances stress-induced DA release in the NAcc, it seems to blunt it in the PFC (Brake et al. 1997; 2000), which strongly suggests different vulnerabilities of the mesocortical, mesolimbic, and nigrostriatal pathways to the deleterious effects of stress. A different timing of development and maturation of neurons of each circuit or different intrinsic sensibilities may explain these differences, although this needs to be further explored.
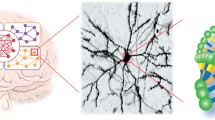
DAergic neurons show marked anatomical and functional heterogeneity. They are principally located in the diencephalon, mesencephalon, and olfactory bulb (Bjorklund and Dunnett 2007); the largest number (∼90%) is found in the ventral part of the mesencephalon. These mesencephalic neurons are the origin of the so-called mesocortical, mesolimbic, and nigrostriatal DAergic systems (Fig. 1); a fourth set of DAergic neurons, less relevant to this article, follow the tuberoinfundibular pathway to terminate in the hypothalamo–pituitary unit. Both the mesolimbic and mesocortical systems arise from the VTA. While the mesocortical pathway terminates in the cortex, where it is thought to control cognition and executive functioning, the mesolimbic projections innervate limbic areas such as the nucleus accumbens (NAcc), amygdala and hippocampus and serve in the regulation of memory, motivation, reward and addiction. Due to their common origins in the VTA, these two pathways are jointly referred to as the mesocorticolimbic system, although the activity of each is subject to regulation by distinct feedback loops. DAergic neurons that project from the substantia nigra to the striatum comprise the nigrostrial system; this pathway is mainly implicated in the initiation and maintenance of motor behavior. As already mentioned, these midbrain DAergic neurons are formed during early development, according to a rostrolateral to caudomedial gradient (Bayer et al. 1995) and their fibers project to terminal fields in the mesocortical and nigrostrial areas (Kawano et al. 1995). All these DAergic systems are thought to be fully mature and functional by the first few weeks of postnatal life in both rats (Voorn et al. 1988) and humans (Prakash and Wurst 2006), although some others have suggested that this maturation can occur until early adulthood in the PFC for example (Benes et al. 2000).
DAergic pathways of the brain. The mesolimbic and mesocortical pathways arise from the VTA, which lies close to the substantia nigra (sn). The mesolimbic pathway projects especially to the nucleus accumbens (NAcc), but also to the amygdala (amy). The mesocortical pathway projects to the prefrontal cortex (PFC). The tuberoinfundibular tract terminates in the hypothalamo–pituitary (pit) unit. The nigrostriatal pathway projects from sn to striatum (st). Altered dopaminergic tone in each of these circuits (either hypo- or hyperactivity) is associated with a particular pathological condition. ADHD attention deficit hyperactivity disorder
Indicating that the developing and maturing DAergic systems are highly sensitive to perturbations, including stress and high levels of GC, experiments from our laboratory found that GC administration during late gestation (E18–19) significantly increases the ratio of apoptotic to proliferative cells in the VTA, resulting in a sustained decrease in DAergic inputs to the NAcc (Leao et al. 2007). The same treatment altered a number of DA-regulated behaviors, including anxiety (Oliveira et al. 2006), prepulse inhibition and drug preference (Leão, Rodrigues et al., unpublished observations). Some of these behavioral changes might be additionally explained by prenatal stress-induced variations in DA turnover in the PFC (Fride and Weinstock 1988) and NAcc (Alonso et al. 1994), reflected in altered sensitivity to certain drugs of abuse. Remarkably, ELS also adjusts DAergic tone in response to certain drugs of abuse and to stress. For example, progeny from stressed dams display higher NAcc DA output under basal conditions and in response to amphetamine or cocaine exposure (Kippin et al. 2008; Silvagni et al. 2008). Similarly, maternal separation (MS) enhances DA release in the NAcc following amphetamine administration (Hall et al. 1999; Moffett et al. 2006). Variations in MS and handling cause changes in ethanol and cocaine self-administration with concomitant changes in DA receptors in the NAcc (Moffett et al. 2007). A short-term insult such as perinatal anoxia results in long-term alterations in the NAcc DAergic response to tail-pinch (Brake et al. 1997). ELS also affects DA transporter (DAT) and DA receptor expression, function and sensitivity. The role of DAT1 which regulates DAergic tone by clearing DA in the synaptic cleft may be significant in this respect; this is exemplified by the fact that drugs such as cocaine induce pleasurable feelings by inhibiting DAT1 activity. In this vein, it is interesting to note that MS decreases DAT levels in the NAcc (Brake et al. 2004; Meaney et al. 2002).
Besides their well-described ability to determine neuronal cell fate (Yu et al. 2010) and neuronal morphology in the hippocampus (Fujioka et al. 2006; Seidel et al. 2008; Sousa et al. 2000) and PFC (Bock et al. 2005; Cerqueira et al. 2007a; Cerqueira et al. 2007b; Michelsen et al. 2007; Murmu et al. 2006), stress (early or in adulthood) and GCs have been found to influence the morphology of neurons in the mesocorticolimbic circuitry. In the above-mentioned study by Leao et al. (2007), we observed that GC during late gestation results in a significant reduction in the volume of the NAcc with significant changes in spine density and morphology (Leão, Rodrigues et al., unpublished observations). These findings were extended by recent work from Martinez-Tellez et al. (2009) who demonstrated decreased spine densities in the NAcc and hippocampus of the progeny of rat dams subjected to restraint stress from mid-late gestation. Since spine density and morphology correlates with synaptic transmission and plasticity (Blanpied and Ehlers 2004; Luscher et al. 2000; Murthy et al. 2001), these findings indicate that ELS interferes with transmission at neuronal networks. Interestingly, however, prenatal stress has been shown to alter the relative number of mushroom spines in the PFC (Michelsen et al. 2007); as compared to other spine types, mushroom spines are relatively stable, i.e., do not show spontaneous appearance and disappearance, suggesting a mechanism through which early life manipulations of the GC milieu might leave a permanent trace within mesocorticolimbic pathways.
As mentioned earlier, there is a convincing correlation between adverse experience during early life and depression (Edwards et al. 2003; Felitti et al. 1998; McCauley et al. 1997). Given that the therapeutic efficacy of the antidepressant tricyclic drugs was based on their ability to inhibit norepinephrine (NE) and serotonin (5-HT) transporters, the role of dopamine in depression was less explored over the years. Yet, ELS has long-term effects not only on noradrenergic and serotonergic but also on DAergic circuits (Schneider et al. 1998; Takahashi et al. 1992). Research, based on measurements of DA metabolites, suggests that a hypo-DAergic state may be causally related to the depressed state; for example, depressed patients display reduced cerebrospinal fluid levels of homovanillic acid (Mendels et al. 1972) and levels of dihydroxyphenylacetic acid (DOPAC) are reduced in the caudate, putamen, and NAcc of depressed suicide victims (Bowden et al. 1997). Hypofunction of the mesocorticolimbic DA system is thought to underlie anhedonia, a cardinal symptom in depression, as well as the loss of motivation experienced by subjects suffering from cognitive and mood disturbances. Interestingly, boosting DA levels through administration of l-DOPA to Parkinsonian patients improves their depressive symptoms (Maricle et al. 1995), and antidepressant drugs that increase DAergic transmission (inhibitors of monoamine oxidase inhibitors, catechol-O-methyltransferase, DA reuptake, and DA receptor agonists) have mood-improving effects (Papakostas 2006). It should be noted, however, that other authors failed to observe any antidepressant actions of l-DOPA (Cools 2006; Shaw et al. 1980). Again, it is important to highlight that disruption of other monoamines transmission such as NE may underlie depression basic symptoms. In fact, drugs that act selectively to enhance either DA or NE transmission can produce a clear antidepressant action; moreover, DA is able to modulate noradrenergic transmission and vice-versa (El Mansari et al. 2010). Importantly, some strategies acting on both systems have been shown to be more effective, not only in drug naive patients, but also in treatment-resistant depression (El Mansari et al. 2010).
Schizophrenia, a neurodevelopmental disorder in which symptoms are first seen in teenagers and young adults, is clearly associated with disturbed DAergic tone. Childhood malnutrition and viral infection, as well as obstetric complications or genetic defects are thought to be triggers of the disease (Bayer et al. 1999; Cannon et al. 2003; Murray and Fearon 1999), although in the more recent “two-hit” hypothesis on the origins of schizophrenia, stress during young adulthood has been added to the list of aforementioned neurodevelopmental factors in disease causation (Bayer et al. 1999; Malaspina et al. 2008; Pantelis et al. 2003). Indeed, the role of stress in schizophrenia has recently received support from studies in humans (Weber et al. 2008) and animals (Choi et al. 2009). Currently, the leading hypothesis is that a deficit in DA activity at D1 receptors in the PFC is responsible for the cognitive impairment and negative symptoms of schizophrenia, while hyperstimulation of D2 receptors by subcortical (mesolimbic) DA is responsible for core (“positive”) disease symptoms (hallucinations, delusions) (Toda and Abi-Dargham 2007).
Early life adversity such as lead exposure, drug abuse (smoking, alcohol, cannabis), low birth weight or premature birth can increase the risk for developing ADHD, although genetic factors also play a substantial role on its etiology (Sullivan and Brake 2003; Swanson et al. 2007). A dysfunction of DAergic mesocortical (but also mesolimbic (Russell et al. 1995)) transmission is thought to underlie ADHD, though the involvement of other neurotransmitters such as noradrenaline has to be considered (Oades et al. 2005). Briefly, hypofunctioning (especially) of the DAergic transmission in the right PFC seems to occur in ADHD, and this is particularly interesting since ELS can induce lateralized changes on PFC DAergic function (Fride and Weinstock 1988). Other findings support the involvement of DA in ADHD: (1) changes in DAT expression were found in ADHD patients compared to controls (Dougherty et al. 1999); (2) genetic analysis identified an association between specific alleles of D4 receptor (Faraone et al. 2001; Rowe et al. 1998) and of DAT (Waldman et al. 1998) with ADHD, and (3) the use of methylphenidate which blocks DA reuptake into the cell by the DAT as the most common treatment for ADHD.
Besides its role in specific types of behavior, the DAergic mesocortical pathway seems to be particularly important in buffering HPA-response to stress. This circuit frequently shows functional hemispheric asymmetry that can be modulated by early life adversity. For example, DA metabolism is significantly higher in the right infralimbic cortex of handled pups (positive stress) than non-handled, and this has been suggested to underlie, in part, to their superior capacity to adapt to stress and restraint HPA activity (Sullivan and Dufresne 2006).
It emerges from the above brief overview that ELS may result in either hyper- or hypoactivity of DAergic systems. Thus, increased DA transmission in the mesolimbic system may result in schizophrenia and increased fear, respectively, whereas reduced DA activity in mesocorticolimbic circuits may lead to memory (hippocampus and frontal cortex) and mood (frontal cortex/ventral striatum) deficits (Fig. 1). Notably, hypoactivity in the hippocampus will likely result in increased GC secretion which, in turn will exacerbate neuronal dysfunction and behavioral anomalies. On the other hand, stress-induced hypoactivity in the mesocorticolimbic DAergic system is likely to enhance novelty-seeking and addictive behaviors, a subject that will be dealt with in greater detail in the following section.
ELS targets mesocorticolimbic DAergic circuits: impact on additive behavior
Despite their diverse chemical structures, cellular mechanisms of action and physiological and behavioral manifestations, all drugs of abuse share a common property: they all act as positive reinforcers and, as a consequence, induce addiction. Increased DA release in the NAcc characterizes drug reinforcement, but also other consumatory behaviors such as sex and food; thus the VTA-NAcc pathway is appropriately also known as the “reward pathway” (Piazza and Le Moal 1996). Subjective feelings of “pleasure” or hedonia after consummation are experienced as a result of parallel activation of mesocortical DAergic circuits. Though traditionally DA is seen as responsible for the “liking” part of a reward, more recently it has been suggested that DA is not essential/sufficient to mediate changes in hedonic behavior. In fact, DA seems to contribute substantially for incentive salience, i.e., the “wanting” part of the process rather than the “liking” part (Berridge 2007). Nevertheless, one way or another, DAergic transmission is certainly playing a vital role in the rewarding process. Perusal of the literature indicates that an apparently intricately-regulated balance between hypo- and hyper-DAergic states underlies an individual’s cycles of drug-seeking behavior and abuse. Thus, hyper-DAergic states seem to enhance the motivational or rewarding properties of drugs of abuse and hypo-DAergic states appear to enhance drug-seeking behavior in parallel with reductions in the perceived motivational impact of “natural” rewards such as food and sex (Diana et al. 1998; Diana et al. 1993; Melis et al. 2005; Parsons et al. 1991).
In the context of this review, it is interesting to note that stress or GC in adulthood enhance DA release in the NAcc (Kalivas and Duffy 1995; Rouge-Pont et al. 1998; Takahashi et al. 1998; Thierry et al. 1976) and increase the strength of excitatory synapses on mesencephalic DA neurons (Saal et al. 2003), while inducing similar patterns of dendritic organization in the NAcc (Liston et al. 2006; Robinson et al. 2001; Robinson and Kolb 1999). Drugs of abuse and stress display other common biobehavioral features: while repeated exposure to the same (Kalivas and Stewart 1991) or novel stressors (Dallman et al. 1994) leads to “facilitation” or “sensitization” of behavioral responses, stress as well as drugs of abuse (Robinson and Becker 1986; Sorg and Kalivas 1991; Stewart and Badiani 1993) are accompanied by augmented DA release in the NAcc (Doherty and Gratton 1992; Kalivas and Stewart 1991). Several other lines of evidence derived from animal studies suggest that stress and GC may act, like drugs of abuse, to induce positive reinforcement: (1) GC facilitate the psychomotor stimulant effects of cocaine, amphetamine and morphine (Cools 1991; Marinelli et al. 1994); (2) depletion of GC by adrenalectomy reduces drug and alcohol consumption (Fahlke et al. 1994; Marinelli and Piazza 2002; Marinelli et al. 1997a; 1997b); (3) GC levels before drug self-administration are positively correlated with the extent of low-dose self-administration of cocaine (Goeders and Guerin 1994; Piazza et al. 1991); and (4) naive rats self-administer GC in a dose-related manner (Piazza et al. 1993).
Addiction is determined by a number of factors other than the intrinsic properties of a given drug. In an interesting series of studies aimed at understanding individual differences in predisposition to drug abuse, Piazza and colleagues found that the liability of rats to self-administer drugs can be predicted by the response of mesolimbic DAergic neurons to stress; specifically, animals that were more sensitive to the DA-releasing actions of stress were more likely to display addictive behavior (Piazza and Le Moal 1996; Piazza et al. 1991). Polymorphisms in the human DA receptor 2 (Blum et al. 1990; Noble 2000) and DA receptor 1 (Batel et al. 2008; Huang et al. 2008) have been associated with increased propensity to alcohol and other substances of abuse, gambling, and compulsive shopping; however, there is no information available with respect to the physiological responses of the affected individuals to stressful stimuli. Val158Met polymorphism in catechol-O-methyltransferase gene, which is involved in DA degradation, has been associated with schizophrenia, bipolar disorder, and also with substance abuse, although some other studies have failed to prove so (Hosak 2007). Exposure to both, drugs with abuse potential and stress trigger neuroadaptative changes in DAergic circuits that ultimately determine neurochemical and behavioral responses. This indicates that the activities of addiction-related DAergic pathways are subject to programming by lifetime experiences, with the final neurochemical and behavioral phenotype reflecting both genetics and experiential history.
Early life adversity, i.e., during the ontogeny of mesocorticolimbic DAergic systems, has been repeatedly shown to induce addiction to a variety of drugs of abuse in adult animals; a few examples from the literature follow: (1) exposure of dams to restraint stress leads to persistent behavioral and neurobiological alterations that are associated with increased consumption of psychostimulants in the adult offspring (Kippin et al. 2008); (2) animals stressed during prenatal life display earlier sensitization to the behavioral effects of amphetamine, although their motor responses to the drug do not differ from those of non-stressed animals (Henry et al. 1995); (3) separation of pups from their mothers and/or littermates during the early postnatal period, a procedure that leads to hypersecretion of GC (Ladd et al. 2000; Liu et al. 1997; Mesquita et al. 2007), advances the time of acquisition of cocaine self-administration (Moffett et al. 2006) and enhances cocaine-induced locomotor activity as well as behavioral sensitization (Brake et al. 2004; Kikusui et al. 2005; Li et al. 2003); and (4) MS stress also increases alcohol and drug consumption during adulthood although handling or brief MS—a manipulation that results in reduced GC secretion and responses to stress (de Kloet et al. 1996; Levine 1967)—decreases voluntary ethanol intake (Huot et al. 2001; Ploj et al. 2003). Though human studies are sparse, it has been shown that childhood adversity is associated with blunted subjective responses to reward-predicting cues as well as dysfunction in left basal ganglia regions implicated in reward-related learning and motivation (Dillon et al. 2009), suggesting that in humans ELS can also change the impact of a reward.
The above examples illustrate the impact that ELS can have on the development of addictive behavior and reinforce the view that the neuronal circuits involved in the regulation of such behavior are particularly vulnerable to programming by stress and GC during the prenatal, perinatal, and early postnatal periods. Part of these effects are, as already mentioned, mediated by stress and GC participating in the regulation of the birth and maturation and DAergic cells in the mesolimbic system (Kawamura et al. 2006; Leao et al. 2007). We also noted that the adult progeny of dams stressed during gestation have significantly fewer TH-positive (DAergic) fibers of the NAcc (Leao et al. 2007). Interestingly, these presumably hypo-DAergic animals were recently found to have a greater propensity for developing drug-seeking behaviors (Leão, Rodrigues et al., unpublished observations). The above findings may be explained, at least partly, in terms of hypersensitivity to the DA-releasing effects of drugs of abuse, evidenced by the increased release of DA in response to amphetamine or cocaine in rats that have either experienced prenatal stress (Kippin et al. 2008; Silvagni et al. 2008) or maternal deprivation stress in the first postnatal days (Hall et al. 1999).
Finally, alterations in the thresholds required for activation of DA type-1 (D1) and type-2 (D2) receptors by DA (Volkow et al. 2004) could represent a potential mechanism through which ELS causes drug-seeking behavior and ultimately, addiction. One hypothetical model predicts that the ratio of D1 to D2 receptors in the NAcc determines the sensitivity to “natural rewards” vs. the proclivity to “seek for pleasure” through drug abuse (Volkow et al. 2004). Earlier studies in rats described late gestational stress-induced increases in the expression and ligand-binding capacity of D2 receptors in the frontal cortex, hippocampus, and core of the NAcc (Berger et al. 2002), with concomitant decreases in the number of D1 receptors in the NAcc. More recently, we observed that the offspring of mothers exposed to exogenous GC in the last trimester of gestation, display diminished DA levels in the NAcc and other mesolimbic structures, an altered D1/D2 ratio and, interestingly, proneness to addictive behaviors (Leão, Rodrigues et al., unpublished observations).
Together, the results summarized above demonstrate that ELS has sustained effects on the morphology and activity of mesolimbic and mesocortical DAergic circuits, accompanied by altered sensitivity and vulnerability to drugs of abuse. In the next section, we will consider the role of the nigrostriatal DAergic pathway which has received relatively little attention in the context of drug abuse. Considering the long-lasting changes in DA receptors expression in several models of early life stress, we may raise the hypothesis that these genes may be transcriptional targets of GCs/stress or that they may undergo epigenetic regulation in response to early life adversity.
A new player in addiction: the nigrostriatal DAergic pathway?
As recently reviewed by Wise (2009), the nigrostriatal DAergic system, best known for its role in motor control and Parkinson’s disease pathology, also seems to play an important role in addictive disorders. First hints were provided by the observations that electrical stimulation of nigrostriatal DAergic cells and terminal fields produced rewarding effects (Crow 1972; Prado-Alcala and Wise 1984; Wise 1981) and that selective lesions of the nigrostriatal pathway attenuated drug self-administration (Glick et al. 1975; Linseman 1976). Those early studies have been backed up by the results of further experimentation (Suto et al. 2004), including the demonstration that intra-nigral infusions of D1 receptor antagonists reduce drug self-administration (Quinlan et al. 2004).
Current views suggest that the contributions of the mesolimbic and nigrostriatal DAergic systems to the development of addiction are distinctly separated in time. Thus, whereas the mesolimbic pathway (especially the NAcc core) is responsible for the rewarding effects of drugs during the initial phases of addiction, the nigrostriatal system assumes an increasingly important role at later stages as drug consumption increases (Everitt et al. 2008; Everitt and Robbins 2005; Wise 2009). The NAcc core is important not only for the rewarding effect of drugs of abuse (Wise 2004) but also mediates the motivational drive or “wanting of a reward” that underlies drug-craving (Berridge 2007), and assures efficiency of response-outcome associative learning (Pavlovian conditioning; Yin and Knowlton 2006). However, second-order protocols of drug reinforcement and pharmacological experiments revealed that the dorsal striatum, rather than the NAcc, is essential for drug-seeking behavior after repetitive drug exposure (Ito et al. 2000). This interpretation is consistent with earlier work which showed that, while dorso-striatal lesions do not affect acquisition of Pavlovian responses (Taylor and Robbins 1986), infusion of DAergic antagonists into the dorsal striatum decreases drug-seeking under second-order drug reinforcement protocols (Vanderschuren et al. 2005). These findings have led to the concept that repetitive exposure to drugs of abuse evolve from being goal-directed behaviors into habit-based actions (Everitt et al. 2008; Everitt and Robbins 2005; Wise 2009). Self-administration protocols in monkeys have confirmed the progressive shift from goal-directed (Pavlovian) behaviors (facilitated by the NAcc in cooperation with associative cortico-basal ganglia networks) to habit-based (instrumental) actions that depend on the dorsal striatum (in particular, the dorso-lateral striatum, an integral component of the sensorimotor cortico-basal ganglia pathway (Porrino et al. 2004)).
The new knowledge concerning the contribution of the nigrostriatal DAergic pathway in drug addiction has been now extended to provide further new insights into how stress increases vulnerability to drug abuse behavior. Functional imaging studies in cocaine addicts have revealed a positive correlation between activation of the dorsal striatum by stress and the degree of cocaine craving (Sinha et al. 2005), and our own studies have demonstrated that stress promotes habit-based decisions in rats by increasing activation of the sensorimotor cortico-basal ganglia pathway (Dias-Ferreira et al. 2009); the latter results are reminiscent of the effects of repetitive drug administration.
Albeit several studies have shown that ELS can affect the mesolimbic circuit, the consequences in the nigrostriatal circuit remain poorly studied and understood. Prenatal DEX exposure increases TH + cell numbers in the substantia nigra, demonstrating that this region can be profoundly affected in terms of DAergic transmission (McArthur et al. 2005). Furthermore, it was shown that ELS can make dopamine neurons from the nigrostriatal pathway to become more susceptible to subsequent insults later in life (Pienaar et al. 2008). Nonetheless, due to the paucity of studies, the direct effect(s) of ELS in the development/maturation of this circuit and its relevance for addiction for example, remains to be determined.
Future perspectives
The available literature, in a rather fragmented way, suggests an association between ELS, DA transmission, and mental illness. Yet, it remains to be answer if the DAergic dysfunction is causal, or merely a consequence, of ELS and in several of the psychiatric conditions linked to ELS. Part of the problem relies on “snapshot approach” that is commonly used in the available studies that precludes the understanding of the dynamics of the insult-response-adaptation process. Thus, we believe that one of the priorities in the field should be to perform longitudinal studies that establish a direct link between altered DAergic transmission and specific endophenotypes for each of the pathological conditions in which ELS is implicated. In parallel, a longitudinal multimodal characterization of ELS exposure in the mesolimbic, mesocortical, or nigrostriatal DAergic pathways is needed. If this is achieved, ultimately, we could determine what the windows of vulnerability of each of these DAergic pathways are and which is more affected in each type of ELS. Furthermore, it could help us understand the long-term impact, and the adaptations, of the distinct DA pathways in neuropsychiatric conditions in which ELS is implicated. As an example, for addiction studies, this integrated approach would allow for a better insight on the role of different DA pathways throughout the different phases of addictive behavior. Moreover, this would give insights on how neurons in each of these pathways respond to drugs of abuse and/or stress in both animal models of ELS and human subjects and how these can be therapeutically modulated. Importantly, this approach is useful and applicable to many neuropsychiatric conditions.
Conclusions
Evidence for the persistent morphological, neurochemical and behavioral impact of elevated GC levels (pharmacologically or stress-induced) during development illustrates the importance of gene X environment (epigenetic) interactions in the etiology of psychiatric conditions. In light of the ontogenetic development of the mesocorticolimbic and nigrostriatal DAergic systems, reports that prenatal stress or manipulations of the maternal GC milieu and postnatal stress (ELS) may be causal to behavioral disorders ascribed to dysfunctional DAergic transmission (e.g., schizophrenia, drug addiction and possibly, depression) are not surprising. Having identified some of the neurobiological substrates that underpin the behavioral anomalies, the immediate challenge is to decipher the molecular and cellular mechanisms that underwrite these changes. Such studies will provide the conceptual basis for devising pharmacological interventions to ameliorate the undesired behavioral outcomes of mal-programmed DAergic circuits. Meanwhile, the existing literature suggests that serious psychiatric conditions in later life are preventable through the judicious use of GC in obstetrics and neonatal medicine, by avoiding stress during pregnancy and by placing emphasis on early parental care.
Abbreviations
- DA:
-
Dopamine
- DAergic:
-
Dopaminergic
- TH:
-
Tyrosine hydroxylase
- l-DOPA:
-
Levodopa
- ELS:
-
Early life stress
- ADHD:
-
Attention deficit hyperactivity disorder
- HPA:
-
Hypothalamus–pituitary–adrenal axis
- GC:
-
Glucocorticoids
- VTA:
-
Ventral tegmental area
- NAcc:
-
Nucleus accumbens
References
Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco-Levy U, Lerer B (1999) Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 4:163–172
Alonso SJ, Navarro E, Rodriguez M (1994) Permanent dopaminergic alterations in the n. accumbens after prenatal stress. Pharmacol Biochem Behav 49:353–358
Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV (2000) The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci 12:973–979
Batel P, Houchi H, Daoust M, Ramoz N, Naassila M, Gorwood P (2008) A haplotype of the DRD1 gene is associated with alcohol dependence. Alcohol Clin Exp Res 32:567–572
Bayer SA, Wills KV, Triarhou LC, Ghetti B (1995) Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res 105:191–199
Bayer TA, Falkai P, Maier W (1999) Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res 33:543–548
Benes FM, Taylor JB, Cunningham MC (2000) Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex 10:1014–1027
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC (2002) Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res 27:1525–1533
Bernet CZ, Stein MB (1999) Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depress Anxiety 9:169–174
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Bjorklund A, Dunnett SB (2007) Dopamine neuron systems in the brain: an update. Trends Neurosci 30:194–202
Blanpied TA, Ehlers MD (2004) Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry 55:1121–1127
Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB (1990) Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 263:2055–2060
Bock J, Gruss M, Becker S, Braun K (2005) Experience-induced changes of dendritic spine densities in the prefrontal and sensory cortex: correlation with developmental time windows. Cereb Cortex 15:802–808
Boksa P, El-Khodor BF (2003) Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev 27:91–101
Bowden C, Cheetham SC, Lowther S, Katona CL, Crompton MR, Horton RW (1997) Reduced dopamine turnover in the basal ganglia of depressed suicides. Brain Res 769:135–140
Brake WG, Noel MB, Boksa P, Gratton A (1997) Influence of perinatal factors on the nucleus accumbens dopamine response to repeated stress during adulthood: an electrochemical study in the rat. Neuroscience 77:1067–1076
Brake WG, Sullivan RM, Gratton A (2000) Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci 20:5538–5543
Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A (2004) Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci 19:1863–1874
Brown GW, Moran P (1994) Clinical and psychosocial origins of chronic depressive episodes. I: a community survey. Br J Psychiatry 165:447–456
Burke RE (2004) Ontogenic cell death in the nigrostriatal system. Cell Tissue Res 318:63–72
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95:5335–5340
Cannon TD, van Erp TG, Bearden CE, Loewy R, Thompson P, Toga AW, Huttunen MO, Keshavan MS, Seidman LJ, Tsuang MT (2003) Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull 29:653–669
Carroll BJ, Curtis GC, Mendels J (1976) Cerebrospinal fluid and plasma free cortisol concentrations in depression. Psychol Med 6:235–244
Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R (2002) Role of genotype in the cycle of violence in maltreated children. Science 297:851–854
Catalani A, Marinelli M, Scaccianoce S, Nicolai R, Muscolo LA, Porcu A, Koranyi L, Piazza PV, Angelucci L (1993) Progeny of mothers drinking corticosterone during lactation has lower stress-induced corticosterone secretion and better cognitive performance. Brain Res 624:209–215
Cerqueira JJ, Mailliet F, Almeida OF, Jay TM, Sousa N (2007a) The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci 27:2781–2787
Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N (2007b) Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cereb Cortex 17:1998–2006
Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF (2004) Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 82:217–225
Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI (2009) Subchronic effects of phencyclidine on dopamine and serotonin receptors: implications for schizophrenia. J Mol Neurosci 38:227–235
Cools AR (1991) Differential role of mineralocorticoid and glucocorticoid receptors in the genesis of dexamphetamine-induced sensitization of mesolimbic, alpha 1 adrenergic receptors in the ventral striatum. Neuroscience 43:419–428
Cools R (2006) Dopaminergic modulation of cognitive function-implications for l-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 30:1–23
Crow TJ (1972) A map of the rat mesencephalon for electrical self-stimulation. Brain Res 36:265–273
Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS (1994) Corticosteroids and the control of function in the hypothalamo-pituitary–adrenal (HPA) axis. Ann N Y Acad Sci 746:22–31. discussion 31–22, 64–27
de Kloet ER, Rots NY, Cools AR (1996) Brain–corticosteroid hormone dialogue: slow and persistent. Cell Mol Neurobiol 16:345–356
Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL (1993) Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA 90:7966–7969
Diana M, Melis M, Muntoni AL, Gessa GL (1998) Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA 95:10269–10273
Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N (2009) Chronic stress causes frontostriatal reorganization and affects decision-making. Science 325:621–625
Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA (2009) Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry 66:206–213
Doherty MD, Gratton A (1992) High-speed chronoamperometric measurements of mesolimbic and nigrostriatal dopamine release associated with repeated daily stress. Brain Res 586:295–302
Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ (1999) Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet 354:2132–2133
Drury SS, Theall KP, Smyke AT, Keats BJ, Egger HL, Nelson CA, Fox NA, Marshall PJ, Zeanah CH (2010) Modification of depression by COMT val158met polymorphism in children exposed to early severe psychosocial deprivation. Child Abuse Negl 34:387–395
Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003) Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics 111:564–572
Dunn AJ (1988) Stress-related changes in cerebral catecholamine and indoleamine metabolism: lack of effect of adrenalectomy and corticosterone. J Neurochem 51:406–412
Edwards VJ, Holden GW, Felitti VJ, Anda RF (2003) Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 160:1453–1460
El Mansari M, Guiard BP, Chernoloz O, Ghanbari R, Katz N, Blier P (2010) Relevance of norepinephrine–dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther 16:e1–17
Ellenbroek BA, Derks N, Park HJ (2005) Early maternal deprivation retards neurodevelopment in Wistar rats. Stress 8:247–257
Espejo EP, Hammen CL, Connolly NP, Brennan PA, Najman JM, Bor W (2007) Stress sensitization and adolescent depressive severity as a function of childhood adversity: a link to anxiety disorders. J Abnorm Child Psychol 35:287–299
Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW (2008) Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363:3125–3135
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Fahlke C, Engel JA, Eriksson CJ, Hard E, Soderpalm B (1994) Involvement of corticosterone in the modulation of ethanol consumption in the rat. Alcohol 11:195–202
Faraone SV, Doyle AE, Mick E, Biederman J (2001) Meta-analysis of the association between the 7-repeat allele of the dopamine D(4) receptor gene and attention deficit hyperactivity disorder. Am J Psychiatry 158:1052–1057
Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258
Ferguson SA, Holson RR (1999) Neonatal dexamethasone on day 7 causes mild hyperactivity and cerebellar stunting. Neurotoxicol Teratol 21:71–76
French NP, Hagan R, Evans SF, Godfrey M, Newnham JP (1999) Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol 180:114–121
Fride E, Weinstock M (1988) Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 42:1059–1065
Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S (2006) Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141:907–915
Genro JP, Kieling C, Rohde LA, Hutz MH (2010) Attention-deficit/hyperactivity disorder and the dopaminergic hypotheses. Expert Rev Neurother 10:587–601
German DC, Manaye KF (1993) Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol 331:297–309
Glick SD, Cox RS, Crane AM (1975) Changes in morphine self-administration and morphine dependence after lesions of the caudate nucleus in rats. Psychopharmacologia 41:219–224
Goeders NE, Guerin GF (1994) Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology 114:63–70
Gutman DA, Nemeroff CB (2003) Persistent central nervous system effects of an adverse early environment: clinical and preclinical studies. Physiol Behav 79:471–478
Hall FS, Wilkinson LS, Humby T, Robbins TW (1999) Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse 32:37–43
Heim C, Nemeroff CB (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49:1023–1039
Heim C, Nemeroff CB (2002) Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry 7:147–159
Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB (2000) Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 284:592–597
Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB (2001) Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry 158:575–581
Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33:693–710
Henry C, Guegant G, Cador M, Arnauld E, Arsaut J, Le Moal M, Demotes-Mainard J (1995) Prenatal stress in rats facilitates amphetamine-induced sensitization and induces long-lasting changes in dopamine receptors in the nucleus accumbens. Brain Res 685:179–186
Holsboer F (2001) Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 62:77–91
Hosak L (2007) Role of the COMT gene Val158Met polymorphism in mental disorders: a review. Eur Psychiatry 22:276–281
Howes OD, Kapur S (2009) The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull 35:549–562
Huang W, Ma JZ, Payne TJ, Beuten J, Dupont RT, Li MD (2008) Significant association of DRD1 with nicotine dependence. Hum Genet 123:133–140
Huizink AC, Ferdinand RF, Ormel J, Verhulst FC (2006) Hypothalamic–pituitary–adrenal axis activity and early onset of cannabis use. Addiction 101:1581–1588
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM (2001) Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology 158:366–373
Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ (2000) Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci 20:7489–7495
Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675:325–328
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Kapur S, Mann JJ (1992) Role of the dopaminergic system in depression. Biol Psychiatry 32:1–17
Kawamura T, Chen J, Takahashi T, Ichitani Y, Nakahara D (2006) Prenatal stress suppresses cell proliferation in the early developing brain. NeuroReport 17:1515–1518
Kawano H, Ohyama K, Kawamura K, Nagatsu I (1995) Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res Dev Brain Res 86:101–113
Kendler KS, Kuhn JW, Prescott CA (2004) Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med 34:1475–1482
Kikusui T, Faccidomo S, Miczek KA (2005) Repeated maternal separation: differences in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology 178:202–210
Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE (2006) MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry 11:903–913
Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE (2008) Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology 33:769–782
Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM (2000) Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 122:81–103
Leao P, Sousa JC, Oliveira M, Silva R, Almeida OF, Sousa N (2007) Programming effects of antenatal dexamethasone in the developing mesolimbic pathways. Synapse 61:40–49
Levine S (1957) Infantile experience and resistance to physiological stress. Science 126:405
Levine S (1967) Maternal and environmental influences on the adrenocortical response to stress in weanling rats. Science 156:258–260
Li Y, Robinson TE, Bhatnagar S (2003) Effects of maternal separation on behavioural sensitization produced by repeated cocaine administration in adulthood. Brain Res 960:42–47
Lindley SE, Bengoechea TG, Schatzberg AF, Wong DL (1999) Glucocorticoid effects on mesotelencephalic dopamine neurotransmission. Neuropsychopharmacology 21:399–407
Lindley SE, Bengoechea TG, Wong DL, Schatzberg AF (2002) Mesotelencephalic dopamine neurochemical responses to glucocorticoid administration and adrenalectomy in Fischer 344 and Lewis rats. Brain Res 958:414–422
Linseman MA (1976) Effects of lesions of the caudate nucleus on morphine dependence in the rat. Pharmacol Biochem Behav 5:465–472
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26:7870–7874
Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277:1659–1662
Luscher C, Nicoll RA, Malenka RC, Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci 3:545–550
MacArthur BA, Howie RN, Dezoete JA, Elkins J (1982) School progress and cognitive development of 6-year-old children whose mothers were treated antenatally with betamethasone. Pediatrics 70:99–105
Macri S, Granstrem O, Shumilina M, Gomes A, dos Santos FJ, Berry A, Saso L, Laviola G (2009) Resilience and vulnerability are dose-dependently related to neonatal stressors in mice. Horm Behav 56:391–398
Makino S, Smith MA, Gold PW (2002) Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res 943:216–223
Malaspina D, Corcoran C, Kleinhaus KR, Perrin MC, Fennig S, Nahon D, Friedlander Y, Harlap S (2008) Acute maternal stress in pregnancy and schizophrenia in offspring: a cohort prospective study. BMC Psychiatry 8:71
Maricle RA, Nutt JG, Carter JH (1995) Mood and anxiety fluctuation in Parkinson’s disease associated with levodopa infusion: preliminary findings. Mov Disord 10:329–332
Marinelli M, Piazza PV (2002) Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci 16:387–394
Marinelli M, Piazza PV, Deroche V, Maccari S, Le Moal M, Simon H (1994) Corticosterone circadian secretion differentially facilitates dopamine-mediated psychomotor effect of cocaine and morphine. J Neurosci 14:2724–2731
Marinelli M, Rouge-Pont F, De Jesus-Oliveira C, Le Moal M, Piazza PV (1997a) Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology 16:156–161
Marinelli M, Rouge-Pont F, Deroche V, Barrot M, De Jesus-Oliveira C, Le Moal M, Piazza PV (1997b) Glucocorticoids and behavioral effects of psychostimulants. I: locomotor response to cocaine depends on basal levels of glucocorticoids. J Pharmacol Exp Ther 281:1392–1400
Markey KA, Towle AC, Sze PY (1982) Glucocorticoid influence on tyrosine hydroxylase activity in mouse locus coeruleus during postnatal development. Endocrinology 111:1519–1523
Martinez-Tellez RI, Hernandez-Torres E, Gamboa C, Flores G (2009) Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse 63:794–804
McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE (2005) Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol 17:475–482
McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB (1997) Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 277:1362–1368
McFarlane A, Clark CR, Bryant RA, Williams LM, Niaura R, Paul RH, Hitsman BL, Stroud L, Alexander DM, Gordon E (2005) The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. J Integr Neurosci 4:27–40
McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12:342–348
Meaney MJ, Brake W, Gratton A (2002) Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology 27:127–138
Melis M, Spiga S, Diana M (2005) The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol 63:101–154
Mendels J, Frazer A, Fitzgerald RG, Ramsey TA, Stokes JW (1972) Biogenic amine metabolites in cerebrospinal fluid of depressed and manic patients. Science 175:1380–1382
Mesquita AR, Pego JM, Summavielle T, Maciel P, Almeida OF, Sousa N (2007) Neurodevelopment milestone abnormalities in rats exposed to stress in early life. Neuroscience 147:1022–1033
Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW (2007) Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci 8:107
Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ (2006) Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther 317:1210–1218
Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ (2007) Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol 73:321–330
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D (2009) Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci 12:1559–1566
Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J (2006) Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci 24:1477–1487
Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ (2001) Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics 107:217–221
Murray RM, Fearon P (1999) The developmental ‘risk factor’ model of schizophrenia. J Psychiatr Res 33:497–499
Murthy VN, Schikorski T, Stevens CF, Zhu Y (2001) Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32:673–682
Nieoullon A, Coquerel A (2003) Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol 16(Suppl 2):S3–9
Noble EP (2000) Addiction and its reward process through polymorphisms of the D2 dopamine receptor gene: a review. Eur Psychiatry 15:79–89
Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA (2005) The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev Sci 8:122–131
Oliveira M, Bessa JM, Mesquita A, Tavares H, Carvalho A, Silva R, Pego JM, Cerqueira JJ, Palha JA, Almeida OF et al (2006) Induction of a hyperanxious state by antenatal dexamethasone: a case for less detrimental natural corticosteroids. Biol Psychiatry 59:844–852
Oo TF, Burke RE (1997) The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Brain Res Dev Brain Res 98:191–196
Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, Shoemaker W, Duman RS, Nestler EJ (1995) Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse 4:289–298
Ortiz J, Fitzgerald LW, Lane S, Terwilliger R, Nestler EJ (1996) Biochemical adaptations in the mesolimbic dopamine system in response to repeated stress. Neuropsychopharmacology 14:443–452
Otten U, Thoenen H (1975) Circadian rhythm of tyrosine hydroxylase induction by short-term cold stress: modulatory action of glucocorticoids in newborn and adult rats. Proc Natl Acad Sci USA 72:1415–1419
Pantelis C, Yucel M, Wood SJ, McGorry PD, Velakoulis D (2003) Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust N Z J Psychiatry 37:399–406
Papakostas GI (2006) Dopaminergic-based pharmacotherapies for depression. Eur Neuropsychopharmacol 16:391–402
Parsons LH, Smith AD, Justice JB Jr (1991) Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse 9:60–65
Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L et al (2010) Altered histone acetylation is associated with age-dependent memory impairment in mice. Science 328:753–756
Perroud N, Jaussent I, Guillaume S, Bellivier F, Baud P, Jollant F, Leboyer M, Lewis CM, Malafosse A, Courtet P (2010) COMT but not serotonin-related genes modulates the influence of childhood abuse on anger traits. Genes Brain Behav 9:193–202
Piazza PV, Deroche V, Deminiere JM, Maccari S, Le Moal M, Simon H (1993) Corticosterone in the range of stress-induced levels possesses reinforcing properties: implications for sensation-seeking behaviors. Proc Natl Acad Sci USA 90:11738–11742
Piazza PV, Le Moal ML (1996) Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol 36:359–378
Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H (1991) Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci USA 88:2088–2092
Pienaar IS, Kellaway LA, Russell VA, Smith AD, Stein DJ, Zigmond MJ, Daniels WM (2008) Maternal separation exaggerates the toxic effects of 6-hydroxydopamine in rats: implications for neurodegenerative disorders. Stress 11:448–456
Ploj K, Roman E, Nylander I (2003) Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience 121:787–799
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24:3554–3562
Prado-Alcala R, Wise RA (1984) Brain stimulation reward and dopamine terminal fields. I. Caudate-putamen, nucleus accumbens and amygdala. Brain Res 297:265–273
Prakash N, Wurst W (2006) Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci 63:187–206
Prendergast MA, Little HJ (2007) Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav 86:234–245
Pruessner JC, Champagne F, Meaney MJ, Dagher A (2004) Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24:2825–2831
Quinlan MG, Sharf R, Lee DY, Wise RA, Ranaldi R (2004) Blockade of substantia nigra dopamine D1 receptors reduces intravenous cocaine reward in rats. Psychopharmacology 175:53–59
Raison CL, Miller AH (2003) When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554–1565
Rayburn WF, Christensen HD, Gonzalez CL (1997) A placebo-controlled comparison between betamethasone and dexamethasone for fetal maturation: differences in neurobehavioral development of mice offspring. Am J Obstet Gynecol 176:842–850. discussion 850–841
Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W (2002) Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry 52:1102–1112
Robinson TE, Becker JB (1986) Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res 396:157–198
Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604
Robinson TE, Gorny G, Mitton E, Kolb B (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266
Rouge-Pont F, Deroche V, Le Moal M, Piazza PV (1998) Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci 10:3903–3907
Rowe DC, Stever C, Giedinghagen LN, Gard JM, Cleveland HH, Terris ST, Mohr JH, Sherman S, Abramowitz A, Waldman ID (1998) Dopamine DRD4 receptor polymorphism and attention deficit hyperactivity disorder. Mol Psychiatry 3:419–426
Russell V, de Villiers A, Sagvolden T, Lamm M, Taljaard J (1995) Altered dopaminergic function in the prefrontal cortex, nucleus accumbens and caudate-putamen of an animal model of attention-deficit hyperactivity disorder—the spontaneously hypertensive rat. Brain Res 676:343–351
Saal D, Dong Y, Bonci A, Malenka RC (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37:577–582
Savitz J, van der Merwe L, Newman TK, Stein DJ, Ramesar R (2010) Catechol-O-methyltransferase genotype and childhood trauma may interact to impact schizotypal personality traits. Behav Genet 40:415–423
Schneider ML, Clarke AS, Kraemer GW, Roughton EC, Lubach GR, Rimm-Kaufman S, Schmidt D, Ebert M (1998) Prenatal stress alters brain biogenic amine levels in primates. Dev Psychopathol 10:427–440
Secoli SR, Teixeira NA (1998) Chronic prenatal stress affects development and behavioral depression in rats. Stress 2:273–280
Seidel K, Helmeke C, Poeggel G, Braun K (2008) Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol 68:1137–1152
Shaw KM, Lees AJ, Stern GM (1980) The impact of treatment with levodopa on Parkinson’s disease. Q J Med 49:283–293
Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E (2008) Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialysis study in adolescent and young adult rats. Eur J Neurosci 28:744–758
Sinha R (2001) How does stress increase risk of drug abuse and relapse? Psychopharmacology 158:343–359
Sinha R, Lacadie C, Skudlarski P, Fulbright RK, Rounsaville BJ, Kosten TR, Wexler BE (2005) Neural activity associated with stress-induced cocaine craving: a functional magnetic resonance imaging study. Psychopharmacology 183:171–180
Sorg BA, Kalivas PW (1991) Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res 559:29–36
Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM (2000) Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience 97:253–266
Stewart J, Badiani A (1993) Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol 4:289–312
Sullivan RM, Brake WG (2003) What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res 146:43–55
Sullivan RM, Dufresne MM (2006) Mesocortical dopamine and HPA axis regulation: role of laterality and early environment. Brain Res 1076:49–59
Suto N, Vezina P, Wise RA (2004) Electrolytic lesions of the dorsal, central and ventral striatum differentially affect the maintenance of cocaine and morphine self-administration. Abstr Soc Neurosci 576:7
Swanson JM, Kinsbourne M, Nigg J, Lanphear B, Stefanatos GA, Volkow N, Taylor E, Casey BJ, Castellanos FX, Wadhwa PD (2007) Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol Rev 17:39–59
Takahashi LK, Turner JG, Kalin NH (1992) Prenatal stress alters brain catecholaminergic activity and potentiates stress-induced behavior in adult rats. Brain Res 574:131–137
Takahashi H, Takada Y, Nagai N, Urano T, Takada A (1998) Effects of nicotine and footshock stress on dopamine release in the striatum and nucleus accumbens. Brain Res Bull 45:157–162
Tarullo AR, Gunnar MR (2006) Child maltreatment and the developing HPA axis. Horm Behav 50:632–639
Taylor JR, Robbins TW (1986) 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology 90:390–397
Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM (2003) The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev 27:33–44
Thierry AM, Tassin JP, Blanc G, Glowinski J (1976) Selective activation of mesocortical DA system by stress. Nature 263:242–244
Toda M, Abi-Dargham A (2007) Dopamine hypothesis of schizophrenia: making sense of it all. Curr Psychiatry Rep 9:329–336
Valkama AM, Paakko EL, Vainionpaa LK, Lanning FP, Ilkko EA, Koivisto ME (2000) Magnetic resonance imaging at term and neuromotor outcome in preterm infants. Acta Paediatr 89:348–355
Vanderschuren LJ, Di Ciano P, Everitt BJ (2005) Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci 25:8665–8670
Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9:557–569
Voorn P, Kalsbeek A, Jorritsma-Byham B, Groenewegen HJ (1988) The pre- and postnatal development of the dopaminergic cell groups in the ventral mesencephalon and the dopaminergic innervation of the striatum of the rat. Neuroscience 25:857–887
Waldman ID, Rowe DC, Abramowitz A, Kozel ST, Mohr JH, Sherman SL, Cleveland HH, Sanders ML, Gard JM, Stever C (1998) Association and linkage of the dopamine transporter gene and attention-deficit hyperactivity disorder in children: heterogeneity owing to diagnostic subtype and severity. Am J Hum Genet 63:1767–1776
Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ (2004) Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854
Weber K, Rockstroh B, Borgelt J, Awiszus B, Popov T, Hoffmann K, Schonauer K, Watzl H, Propster K (2008) Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry 8:63
Weiner SAFWJ (2002) Parkinson’s disease: diagnosis and clinical management. Demos, New York
Weiss EL, Longhurst JG, Mazure CM (1999) Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry 156:816–828
Wise RA (1981) Intracranial self-stimulation: mapping against the lateral boundaries of the dopaminergic cells of the substantia nigra. Brain Res 213:190–194
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Wise RA (2008) Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14:169–183
Wise RA (2009) Roles for nigrostriatal—not just mesocorticolimbic—dopamine in reward and addiction. Trends Neurosci 32:517–524
Wood PB (2008) Role of central dopamine in pain and analgesia. Expert Rev Neurother 8:781–797
Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW (1991) Hypothalamic–pituitary–adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry 30:1031–1048
Yin HH, Knowlton BJ (2006) The role of the basal ganglia in habit formation. Nat Rev Neurosci 7:464–476
Young EA, Abelson JL, Curtis GC, Nesse RM (1997) Childhood adversity and vulnerability to mood and anxiety disorders. Depress Anxiety 5:66–72
Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF (2010) Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol 67:21–30
Acknowledgments
The authors would like to thank the Institute for Social and Affective Neuroscience, Fundação para a Ciência e Tecnologia, and CRESCENDO (EU Integrated Project FP6-018652) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodrigues, AJ., Leão, P., Carvalho, M. et al. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology 214, 107–120 (2011). https://doi.org/10.1007/s00213-010-2085-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-010-2085-3