Abstract
Rationale
The midbrain periaqueductal gray (PAG) is part of the brain system involved in active defense reactions to threatening stimuli. Glutamate N-methyl-d-aspartate (NMDA) receptor activation within the dorsal column of the PAG (dPAG) leads to autonomic and behavioral responses characterized as the fear reaction. Nitric oxide (NO) has been proposed to be a mediator of the aversive action of glutamate, since the activation of NMDA receptors in the brain increases NO synthesis.
Objectives
We investigated the effects of intra-dPAG infusions of NMDA on defensive behaviors in mice pretreated with a neuronal nitric oxide synthase (nNOS) inhibitor [Nω-propyl-l-arginine (NPLA)], in the same midbrain site, during a confrontation with a predator in the rat exposure test (RET).
Materials and methods
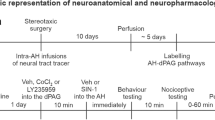
Male Swiss mice received intra-dPAG injections of NPLA (0.1 or 0.4 nmol/0.1 μl), and 10 min later, they were infused with NMDA (0.04 nmol/0.1 μl) into the dPAG. After 10 min, each mouse was placed in the RET.
Results
NMDA treatment enhanced avoidance behavior from the predator and markedly increased freezing behavior. These proaversive effects of NMDA were prevented by prior injection of NPLA. Furthermore, defensive behaviors (e.g., avoidance, risk assessment, freezing) were consistently reduced by the highest dose of NPLA alone, suggesting an intrinsic effect of nitric oxide on defensive behavior in mice exposed to the RET.
Conclusions
These findings suggest a potential role of glutamate NMDA receptors and NO in the dPAG in the regulation of defensive behaviors in mice during a confrontation with a predator in the RET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The periaqueductal gray (PAG) is a midbrain structure proposed to be involved in the modulation of several brain functions, such as nociception, sexual, and defensive behaviors (for review, see Bandler and Depaulis 1991; Behbehani 1995). Anatomical and functional data suggest four longitudinal columns within the PAG, namely, the dorsomedial, dorsolateral, lateral, and ventrolateral (Bandler et al. 1991; Carrive 1993). The dorsal columns (dPAG) have been referred as a site of integration and modulation of the behavioral and autonomic expression of defensive reactions (for reviews, see Bandler and Shipley 1994; Graeff 1981, 1994). Electrical and chemical (by excitatory amino acids) stimulation of this site induces fight/flight reactions, which are similar to unconditioned fear responses to proximal threat (Bandler and Carrive 1988; Schenberg et al. 2001; Carvalho-Netto et al. 2006; Martinez et al. 2007). In addition, many lines of evidence have indicated that aside from the modulation of these fear-like responses (for review, see Graeff 2004), the dPAG mediates more subtle defensive responses related to anxiety such as threat avoidance and risk assessment (Teixeira and Carobrez 1999; McNaughton and Corr 2004; Mendes-Gomes and Nunes-De-Souza 2005; Bertoglio and Zangrossi 2006; Carvalho-Netto et al. 2007).
Recent studies have focused on the relationship between glutamate N-methyl-d-aspartate (NMDA) receptors and anxiety-like responses elicited by the stimulation of the dPAG (for review, see Carobrez et al. 2001; De Oliveira et al. 2001; Bergink et al. 2004). For example, intra-dPAG microinjections of low doses of glutamate, NMDA receptor agonist, or NMDA/glycine receptor agonist facilitate threat avoidance and risk assessment in rats exposed in the elevated plus-maze (EPM) or elevated T-maze (Schmitt et al. 1995; Carobrez et al. 2001; Bertoglio and Zangrossi, 2006; Santos et al. 2006).
It has been proposed that the anxiogenic-like effects following activation of NMDA receptors into the dPAG are mainly mediated by the gas neurotransmitter nitric oxide (NO) (for review, see De Oliveira et al. 2001; Guimarães et al. 2005). Indeed, glutamate NMDA receptor activation leads to cellular calcium influx which triggers a cascade of intracellular events including activation of neuronal nitric oxide synthase (nNOS), an enzyme that produces NO (Garthwaite et al. 1989; Garthwaite 1991; Lohse et al. 1998). In accordance with this, a recent study from our laboratory (Miguel and Nunes-De-Souza 2006) has demonstrated that pretreatment with the selective and potent nNOS inhibitor, Nω-propyl-l-arginine (NPLA), into the mouse dPAG, completely blocked defensive-like behaviors (e.g., jumping, running, and freezing) induced by NMDA receptor agonist injection into the same site.
Preclinical animal models have been widely used to provide behavioral measures related to fear and anxiety states (Markham et al. 2004; Litvin et al. 2008). The rat exposure test (RET) is an animal model of anxiety based on the predator–prey interaction (rat and mouse; Yang et al. 2004). Rats have been shown as actual mice predators both in nature and in the laboratory (O’Boyle 1974, 1975; Calvo-Torrent et al. 1999). When confronted by rats, both wild and laboratory mice show clear innate defensive behaviors (Blanchard et al. 1998). Regarding the RET, recent studies have attempted to identify possible intracerebral neurotransmitter systems involved in the modulation of behavioral defensive responses of mice exposed to this type of prey–predator interaction (Carvalho-Netto et al. 2007; Litvin et al. 2007; Martinez et al. 2008). In line with this view, the present study was designed to investigate the effects of intra-dPAG NMDA infusions on defensive behaviors of mice pretreated with local injection of NPLA and confronted by a predator in the RET.
Materials and methods
Animals
Subjects were male Swiss adults mice weighing 25–35 g (São Paulo State University/UNESP, SP, Brazil), housed in groups of ten per cage (cage size, 41 × 34 × 16 cm). They were maintained under a normal 12-h light cycle (lights on 7:00 a.m.) in a temperature (23 ± 1°C) and humidity (55 ± 5%)-controlled environment. Food and drinking water were freely available except during the brief test periods. All mice were experimentally naïve and used only once. A total of five male Long–Evans rats were used as predator stimuli during the course of the study.
Drugs
The drugs used were NPLA (Tocris Cookson, Ballwin, MO, USA), a highly selective and potent inhibitor of nNOS (Ki = 57 nM), and N-methyl-d-aspartic acid (NMDA; Sigma, USA). The doses used were based on previous studies: NMDA 0.04 nmol/0.1 μl and NPLA 0.1 and 0.4 nmol/0.1 μl (Miguel and Nunes-De-Souza 2006, 2008). The drugs were dissolved in physiological saline (NaCl 0.9%).
d-Amphetamine sulfate (Research Biochemicals, MA, USA) was dissolved in physiological saline and administered i.p. to Long–Evans rats at a single dose of 5.0 mg/kg 15 min prior to placement into the rat exposure chamber. This procedure was used to keep the stimulus rats uniformly active during and across test sessions.
Surgery
Mice were implanted unilaterally with an 8-mm stainless-steel guide cannula (26-gauge) under sodium pentobarbital (90 mg/kg, i.p.) anesthesia. The guide cannula was fixed to the skull using dental cement and jewelers’ screw. Stereotaxic coordinates for the dorsal PAG were 4.16 mm posterior to the bregma, 1.32 mm lateral to the midline, and 2.23 mm ventral to the skull surface, with the guide cannula angled 26° to the vertical axis. A dummy cannula inserted into the guide cannula at the time of surgery, served to reduce the incidence of occlusion. Upon removal from stereotaxic apparatus, mice were administered 1 ml 0.9% saline s.c. in order to prevent dehydration. Postoperative analgesia was provided for 3 days by adding acetaminophen (200 mg/ml) to the drinking water in a ratio of 0.2 ml acetaminophen:250 ml water (i.e., final concentration = 0.16 mg/ml).
Intracerebral drug administration
Six to 7 days after the surgery, mice were transferred from the main holding area to the laboratory and left undisturbed for 1 h prior to drug administration. Each mouse was lightly restrained and a 32-gauge injection cannula (1.0 mm longer than the guide cannula) was inserted into the guide cannula, and the injector was connected via PE-10 polyethylene tubing to a 5-μl Hamilton microsyringe. The administration of each compound was controlled by an infusion pump, (BI 2000, Insight Equipamentos Científicos, Brazil) programmed to deliver a volume of 0.1 μl over a period of 30 s. The injector remained in place for an additional 30 s (before slow removal) to ensure complete diffusion of the drug. Confirmation of successful infusion was obtained by monitoring the movement of a small air bubble in the PE-10 tubing. Ten minutes later, the same procedure was repeated for the second injection. Immediately following drugs infusion, each animal was returned to its home cage and left undisturbed for 10 min prior to behavioral evaluation in the RET.
Apparatus (RET)
The RET was developed and validated by Yang et al. (2004) to facilitate measurement of avoidance and risk assessment behaviors in mice. Testing procedures were conducted in a 46 × 24 × 21 cm clear polycarbonate cage (exposure chamber) covered with a black polycarbonate lid. The exposure chamber was divided into two equal-sized compartments by a wire mesh screen (surface and predator compartment). The home cage was a 7 × 7 × 12 cm box made of black Plexiglas on three sides and clear Plexiglas on the fourth side to facilitate videotaping. The home chamber was connected to the exposure cage by a clear Plexiglas tube tunnel (4.4 cm in diameter, 13 cm in length, 1.5 cm elevated the floor of the two chamber).
Rat exposure test and behavioral analysis
All testing were conducted during the light phase of the light/dark cycle under illumination of a 100-W red light bulb. Each apparatus was cleaned with 20% alcohol and dried with paper towels between trials. One vertically mounted camera linked to a video monitor and DVD was used to record the experiment.
Rat exposure test
Prior to the start of each trial, the individual home cage bedding of each subject was poured into the home chamber and the surface of the RET so as to cover the entire floor of the apparatus.
Phase 1: Habituation
Each subject was allowed one daily habituation sessions during three consecutive days in the apparatus. The mouse was placed in the center of the surface and was allowed to explore freely for 10 min with no rat present.
Phase 2: Exposure test
On test day, each animal received intracerebral pharmacological treatment (see below experiment 1 and 2) and was placed in the center of the surface. An amphetamine-treated male Long–Evans rat was then immediately placed behind the wire mesh. Each trial lasted 10 min, and the following behaviors were scored.
The behavioral parameters comprised both spatiotemporal and ethological measures. The spatiotemporal measures were frequency and time spent in the home chamber, tunnel, and on the surface. Time spent in contact with the wire screen barrier (including climbing) was taken as total (barrier) contact time. The ethological measures included duration of risk assessment behaviors (stretched attend postures, an exploratory posture in which the body is stretched forward but the animal’s hind paws remain in position, and stretched approach, in which the body is stretched while moving forward); freezing (complete cessation of movement except breathing) and self-grooming.
Procedure
Experiment 1. Effects intra-dPAG injections of NPLA on the behavior mice in the RET
On test days, mice were microinjected with saline or NPLA (0.1 and 0.4 nmol/0.1 μl), and 10 min later, they were individually placed in the surface of the RET for recording the behavioral (spatiotemporal and ethological) measures (see above) during a period of 10 min.
Experiment 2. Effects of intra-PAG injection of NPLA on the effect of local infusion of NMDA
Animals received intra-dPAG injections of saline, or NPLA (0.1 or 0.4 nmol) followed 10 min later by saline or NMDA (0.04 nmol/0.1 μl) into the same site. Ten minutes after the second injection, animals were submitted to the RET. A total of four groups were formed: Saline + Saline, Saline + NMDA, NPLA 0.1 + NMDA, NPLA 0.4 + NMDA.
Experiment 3. Effects of NMDA injection on locomotor activity
In order to investigate an intrinsic effect of NMDA on locomotor activity, mice were microinjected with saline or NMDA (0.04 nmol/0.1 μl) into the dorsal PAG and 10 min later were placed in the center of activity monitoring chamber (Columbus Instruments, CA, USA). Photocell counts accumulated in 1-min intervals were recorded during a 10-min testing session.
Histology
Mice were sacrificed with an overdose of sodium pentobarbital and received an infusion of 1% Evans blue intra-dPAG (according to the microinjection procedure described above). The animals were perfused intracardially with 10 cc 0.9% formalin, and their brain were removed from the cranial cavity and stored in 10% formalin/30% sucrose solution for at least 24 h before histological analysis. Mouse brain were coronally sectioned by a cryostat (40 μm) and microscopically verified with reference to the atlas of Paxinos and Franklin (2001). Data from animals with injection sites outside the dPAG were excluded from analysis.
Statistical analyses
The behavioral data from the RET were analyzed by one-way analysis of variance (ANOVA) for parametrically distributed data or Kruskall–Wallis for nonparametrically distributed data. Post hoc tests [Duncan test (parametric) or Mann-Whitney U test (nonparametric) were conducted for significant treatment effects relative to control means]. Locomotor activity data were analyzed by two-way ANOVA with repeated measures. A P value ≤0.05 was considered significant.
Ethics
The experiments carried out in this study comply with the norms of Brazilian Neuroscience and Behavior Society (SBNeC), based on the US National Institutes of Health Guide for Care and Use of Laboratory Animals.
Results
Histology
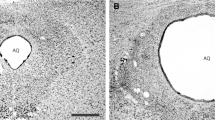
Histology confirmed that a total of 103 mice had cannula placements in the PAG (Fig. 1). Thirty-seven mice were used in experiment 1 [Saline (n = 15), NPLA 0.1 nmol (n = 9), 0.4 nmol (n = 13)], 53 mice were used in experiment 2 [Saline + Saline (n = 16), Saline + NMDA (n = 14), NPLA 0.1 + NMDA (n = 10), and NPLA 0.4 + NMDA (n = 13)], while 13 mice were used in experiment 3 [saline (n = 7) and NMDA (n = 6)].
a Schematic representation of microinfusion sites within (filled circle) and outside (blank circle) the dorsal periaqueductal gray (dPAG). b Photomicrograph of midbrain coronal section from a representative subject showing an injection site into the dPAG. Section correspond to −4.72 mm from bregma in the atlas of Paxinos and Franklin (2001)
Experiment 1. Effects of intra-dPAG injections of NPLA on the behavior mice in the RET
Figure 2 shows that intra-dPAG injections of NPLA (0.4 nmol) changed the spatiotemporal and ethological measures in mice submitted to the RET. NPLA increased surface duration [F(2,34) = 4.26, Duncan test, p < 0.05] and mesh contact [H(2,37) = 7.37, Mann–Whitney test, P < 0.05], while it reduced freezing [H(2,37) = 6.85, P < 0.05] and risk assessment behaviors [F(2,34) = 3. 69, p < 0.05].
Experiment 2. Effects of intra-PAG injection of NPLA on the effect of local infusion of NMDA
Effects of combined microinfusions of NPLA and NMDA into the dPAG on the frequency of entries in the RET compartments are summarized in Table 1. One-way ANOVA followed by Duncan test revealed that intra-dPAG NMDA (saline + NMDA) significantly reduced frequency of entries into the home chamber, tunnel, and surface area. Table 1 also shows that these NMDA effects were blocked by prior infusion of NPLA (0.1 and 0.4 nmol) into the dPAG.
As shown in Fig. 3, intra-dPAG NMDA increased time spent in home chamber [F(3,49) = 2.80, Duncan test, P < 0.05] and decreased mesh contact duration [H(3,53) = 9.47, Mann–Whitney test, P < 0.05] when compared to control group (saline + saline). Prior injection of NPLA (0.4 nmol) into the dPAG prevented the NMDA effects. Regarding the ethological measures, Kruskal–Wallis ANOVA followed by Mann–Whitney U comparisons revealed that NMDA treatment enhanced freezing duration [H(3,53) = 13.3, P < 0.05] when compared with control group. However, prior local infusion of NPLA (0.1 and 0.4 nmol) completely abolished NMDA effects on freezing.
Experiment 3. Effects of intra-dPAG NMDA injection on locomotor activity
As illustrated in Fig. 4, intra-dPAG injection of NMDA did not alter locomotor activity during the 10-min test session in the activity-monitoring chamber. Two-way ANOVA detected a significant effect for time factor (F(9,99) = 3.79, p < 0.05) but no significant effects for drug treatment factor (F(1,11) = 0.15, p > 0.05) or time × treatment interaction (F(9,99) = 0.32, p >0 .05).
Discussion
The present results are in concordance with several pharmacological studies indicating that glutamate NMDA receptors in the dPAG modulate fear and anxiety-related behaviors (Guimarães et al. 1991; Matheus et al. 1994; Schmitt et al. 1995; Teixeira and Carobrez 1999; Carobrez et al. 2001; Molchanov and Guimarães 2002; Bertoglio and Zangrossi 2006; Miguel and Nunes-De-Souza 2006; Santos et al. 2006). Specifically, our results demonstrated that microinfusion of NMDA receptor agonist into the mouse dPAG consistently increased avoidance behavior to the predator in the RET (i.e., increased home chamber time while reducing contact with the wire mesh time). In addition, the glutamate NMDA receptor agonist markedly altered ethological measures as observed by the enhancement of freezing duration when compared with the control group (saline + saline).
Our findings corroborate a consistent literature demonstrating that intra-PAG administration of glutamate, NMDA receptor agonist, or NMDA/glycine receptor agonist enhance avoidance and risk assessment behaviors in the elevated plus-maze (EPM) and elevated T-maze (ETM) (Schmitt et al. 1995; Carobrez et al. 2001; Bertoglio and Zangrossi 2006; Santos et al. 2006). On the other hand, direct injections into this structure of NMDA receptor antagonists (e.g., AP-7) or NMDA/glycine receptor antagonists (e.g., HA-966, 7-Cl-KY) induce opposite effects in these tasks (Guimarães et al. 1991; Matheus et al. 1994; Teixeira and Carobrez 1999; Bertoglio and Zangrossi 2006).
Although many studies have previously emphasized the role of glutamate NMDA receptor subtypes on fear/anxiety-like responses, the present results do not preclude the possibility that the effects of NMDA may have been partially induced by a motoric disruption. Indeed, our results showed that intra-dPAG infusion of NMDA reduced the frequency of crossing between the RET compartments (see Table 1). However, the locomotion test in the activity-monitoring chamber (see Fig. 4) was not significantly different in both control and NMDA groups, suggesting that intra-dPAG NMDA did not change the normal pattern of motoric coordination in mice. Thus, it is likely that animals microinjected with NMDA into the dPAG spent more time expressing defensive behaviors such as freezing in the home chamber instead of crossing the RET compartments.
Evidences from literature have emphasized that the anxiogenic-like effects following activation of NMDA receptors into the dPAG are mainly mediated by the gas neurotransmitter NO (for review, see De Oliveira et al. 2001; Guimarães et al. 2005). In fact, the present results corroborate these findings and indicate that NO displays a fundamental role in the proaversive effects of intra-dPAG NMDA infusion. For example, pretreatment with both doses of the selective nNOS inhibitor, NPLA, into the mouse dPAG, was able to prevent most of the anxiogenic-like behaviors (e.g., avoidance and freezing) induced by local NMDA treatment in the RET. These results are in consonance with a recent study carried out in our laboratory demonstrating that intra-dPAG NPLA blocks the anxiogenic-like effects of NMDA infusion into the same brain site in mice exposed to the EPM (Miguel and Nunes-De-Souza 2008). Together, these findings corroborate the hypothesis mentioned above in that the anxiogenic effects induced by NMDA receptors activation in the brain are dependent on endogenous NO.
In terms of the role of NO on defensive behaviors, many pharmacological studies have shown that systemic injections of NOS inhibitors (Volke et al. 1995; Faria et al. 1997; Volke et al. 2003; for review, see Guimarães et al. 2005) as well as intracerebral (e.g., dPAG, dorsal hippocampus, and medial amygdala) microinfusions of NOS inhibitors, guanylate cyclase inhibitors, or NO scavenger elicit anxiolytic-like effects in several animal models of anxiety such as the elevated plus-maze and light–dark compartment test (Guimarães et al. 1994; De Oliveira and Guimarães 1999; Aguiar et al. 2004; for review, see Guimarães et al. 2005; Forestiero et al. 2006; Spolidório et al. 2007).
In line with these studies, the present results demonstrate that NPLA treatment per se produced a dose–response curve with the highest dose (0.4 nmol) increasing the time in the surface and in contact with the wire screen barrier between the subject and predator. In addition, intra-dPAG NPLA (0.4 nmol) markedly reduced the freezing and risk assessment behavior duration, indicating that this drug significantly reduced the spatiotemporal (avoidance) and ethological (freezing and risk assessment) measures in the RET, reinforcing the suggestion of a potential role for nitric oxide system in the dPAG in the regulation of anxiety.
Based on previous studies (for review, see De Oliveira et al. 2001; Guimarães et al. 2005) as well as the present findings, it could be assumed the effects of NPLA represent an inhibition of the nitric oxide production that is mediated by endogenous glutamatergic activation and acting via NMDA receptors. Indeed, it has been established that NMDA receptor activation is the main stimulus for NO production in the central nervous system (for review, see Esplugues 2002), and reciprocal regulatory mechanisms between these two neuronal pathways (glutamatergic and nitrergic) are likely to occur in the dPAG (Lin et al. 2000). In this context, an elegant study reported by Beijamini and Guimarães (2006) showed that cat exposure increases activation of neurons containing NOS in the rat dPAG, an effect that was attenuated by prior intracerebroventricular microinjection of AP-7, a competitive NMDA receptor antagonist.
However, the hypothesis that other neurotransmitter systems (not associated with the glutamate-NMDA receptor) are involved with the nitrergic modulation should not be rejected. For instance, a number of studies have shown that NO enhancement can also occur following other glutamatergic receptor subtypes activation such as AMPA, kainate, and metabotropic receptors (Southam et al. 1991; Okada 1992). Furthermore, it has been suggested that a set of neurotransmitters including serotonin, bradykinin, endothelin, acetylcholine, noradrenaline and GABA might be involved in the nitric oxide modulation (Reiser 1990a, b; Lovick et al. 2000; Moreira and Guimarães 2004). In this context, there are evidences emphasizing that NO production following NMDA receptor activation plays a role over some neurotransmitters release such as serotonin and dopamine in different brain regions (e.g., hippocampus and striatum; Whitton et al. 1994; Wegener et al. 2000).
In conclusion, the present data indicate that NO located within the dPAG modulates anxiety like-behaviors in mice confronted with a predator in the RET, and that this response is mediated, at least in part, by NMDA glutamate receptor activation. Moreover, our results strongly support previous findings (Carobrez et al. 2001; McNaughton and Corr 2004; Bertoglio and Zangrossi 2006; Carvalho-Netto et al. 2007) that emphasize the importance of the dorsal region of PAG in the modulation of more subtle defensive behaviors related to anxiety, such as risk assessment and threat avoidance.
References
Aguiar DC, De Lucca AC, Guimarães FS (2004) Role of nitric oxide on chemically-induced defensive reactions in the dorsolateral periaqueductal grey. Eur Neuropsychopharmacol 14(suppl. 3):S305
Bandler R, Carrive P (1988) Integrated defense reaction elicited by excitatory amino acid in the midbrain periaqueductal gray region of the unrestrained cat. Brain Res 439:95–106
Bandler R, Depaulis A (1991) Midbrain periaqueductal gray control of defensive behavior in the cat and the rat. In: Bandler R, Depaulis A (eds) The midbrain periaqueductal gray matter: functional, anatomical and neurochemical organization. Plenum, New York, pp 175–197
Bandler R, Shipley MT (1994) Columnar organization in the midbrain periaqueductal gray: modulates for emotional expression? Trends Neurosci 17:379–389
Bandler R, Carrive P, Zhang SP (1991) Integration of somatic autonomic reactions within the midbrain periaqueductal gray: viscerotopic, somatotopic and functional organization. Prog Brain Res 87:269–305
Behbehani MM (1995) Functional characteristics of the midbrain periaqueductal gray. Progress Neurobiol 46:575–605
Beijamini V, Guimarães FS (2006) c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res 170:52–61
Bergink V, Van Megen HJGM, Westenberg HGM (2004) Glutamate and anxiety. Eur Neuropharmacol 14:175–183
Bertoglio LJ, Zangrossi H Jr (2006) Involvement of dorsolateral periaqueductal gray N-methyl-d-aspartic acid glutamate receptors in the regulation of risk assessment and inhibitory avoidance behaviors in the rat elevated T-maze. Behav Pharmacol 17:589–596
Blanchard RJ, Hebert MA, Ferrari PF, Palanza P, Figueira R, Blanchard DC, Parmigiani S (1998) Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol Behav 65(2):201–209
Calvo-Torrent A, Brain PF, Martinez M (1999) Effect of predatory stress on sucrose intake and behavior on the plus-maze in male mice. Physiol Behav 67:189–196
Carobrez AP, Teixeira KV, Graeff FG (2001) Modulation of defensive behavior by periaqueductal gray NMDA/glycine-B receptor. Neurosci Biobehav Rev 25:697–709
Carrive P (1993) The periaqueductal gray and defensive behavior: functional significance and neuronal organization. Behav Brain Res 58:27–47
Carvalho-Netto EF, Markham C, Blanchard DC, Nunes-De-Souza RL, Blanchard RJ (2006) Physical environment modulates the behavioral responses induced by chemical stimulation of dorsal periaqueductal gray in mice. Pharmacol Biochem Behav 85:140–147
Carvalho-Netto EF, Litvin Y, Nunes-De-Souza RL, Blanchard DC, Blanchard RJ (2007) Effects of intra-PAG infusion of ovine CRF on defensive behaviors in Swiss-Webster mice. Behav Brain Res 176:222–229
De Oliveira RW, Guimarães FS (1999) Anxiolytic effect of methylene blue microinjected into the dorsal periaqueductal gray. Braz J Med Biol Res 32:1529–1532
De Oliveira RW, Del Bel EA, Guimarães FS (2001) Effects of excitatory amino acids and nitric oxide on flight behavior elicited from the dorsolateral periaqueductal gray. Neurosci Biobehav Rev 25:679–685
Esplugues J (2002) NO as a signalling molecule in the nervous system. Br J Pharmacol. 135:1079–1095
Faria MS, Muscara MN, Moreno H Jr, Dias SA, Oliveira B, Graeff FG, De Nucci G (1997) Acute inhibition of nitric oxide synthesis induces anxiolysis in the plus maze test. Eur J Pharmacol 323:37–343
Forestiero D, Manfrim CM, Guimarães FS, De Oliveira RM (2006) Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology (Berl) 184:166–172
Garthwaite J (1991) Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci 14:60–67
Garthwaite J, Garthwaite G, Palmer RM, Moncada S (1989) NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol 172:413–416
Graeff FG (1981) Minor tranquilizers and brain defense system. Braz J Med Biol Res 14:239–265
Graeff FG (1994) Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res 27(4):811–829
Graeff FG (2004) Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev 28:239–259
Guimarães FS, Carobrez AP, De Aguiar JC, Graeff FG (1991) Anxiolytic effect in the elevated plus maze of NMDA receptor antagonist AP 7 microinjected into the dorsal periaqueductal gray. Psychopharmacology (Berl) 103:91–94
Guimarães FS, De Aguiar JC, Del-Bel EA, Ballejo G (1994) Anxiolytic effect of NO synthase inhibitors microinjected into the dorsal central grey. Neuro Report 5:123–126
Guimarães FS, Beijamini V, Moreira FA, Aguiar DC, De Luca ACB (2005) Role of nitric oxide on brain regions related to defensive reactions. Neurosci Biobehav Rev 29:1313–1322
Lin HC, Kang BH, Wan FJ, Huang ST, Tseng CJ (2000) Reciprocal regulation of nitric oxide and glutamate in the nucleus tractus solitarii of rats. Eur J Pharmacol 407:83–89
Litvin Y, Pentkowski NS, Blanchard DC, Blanchard RJ (2007) CRF type 1 receptors in the dorsal periaqueductal gray modulate anxiety-induced defensive behaviors. Horm Behav 52:244–251
Litvin Y, Pentkowski NS, Pobbe RL, Blanchard DC, Blanchard RJ (2008) Unconditioned models of fear and anxiety. In: Blanchard DC, Blanchard RJ, Griebel G, Nutt D (eds) Handbook of anxiety and fear. Academic Press, Amsterdam, pp 82–99
Lohse MJ, Forstermann U, Schmidt HHHW (1998) Pharmacology of NO: cGMP signal transduction. Naunyn-Schmiedeberg’s Arch Pharmacol 358:111–112
Lovick TA, Parry DM, Stezhka VV, Lumb BM (2000) Serotonergic transmission in the periaqueductal gray matter in relation to aversive behaviour: morphological evidence for direct modulatory effects on identified output neurons. Neurosci 95:763–772
Markham CM, Blanchard DC, Canteras NS, Cuyno CD, Blanchard RJ (2004) Modulation of predatory odor processing following lesions to the dorsal premammillary nucleus. Neurosci Lett. 372:22–26
Martinez RC, Ribeiro De Oliveira A, Brandão ML (2007) Serotonergic mechanisms in the basolateral amygdala differentially regulate the conditioned and unconditioned fear organized in the periaqueductal gray. Eur Neuropychopharmacol 17:717–724
Martinez RC, Carvalho-Netto EF, Amaral VC, Nunes-de-Souza RL, Canteras NS (2008) Investigation of the hypothalamic defensive system in the mouse. Behav Brain Res 192(2):185–190
Matheus MG, Nogueira RL, Carobrez AP, Graeff FG, Guimarães FS (1994) Anxiolytic effect of glycine antagonists microinjected into the dorsal periaqueductal grey. Psychopharmacology (Berl) 113:565–569
McNaughton N, Corr PJ (2004) A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev 28:285–305
Mendes-Gomes J, Nunes-De-Souza RL (2005) Concurrent nociceptive stimulation impairs the anxiolytic effect of midazolam injected into the periaqueductal gray in mice. Brain Res 1047:97–104
Miguel TT, Nunes-De-Souza RL (2006) Defensive-like behaviors and antinociception induced by NMDA injection into the periaqueductal gray of mice depend on nitric oxide synthesis. Brain Res 1076:42–48
Miguel TT, Nunes-De-Souza RL (2008) Anxiogenic-like effects induced by NMDA receptor activation are prevented by inhibition of neuronal nitric oxide synthase in the periaqueductal gray in mice. Brain Res 1240:39–46
Molchanov ML, Guimarães FS (2002) Anxiolytic-like effects of AP7 injected into the dorsolateral or ventrolateral columns of the periaqueductal gray of rats. Psychopharmacology (Berl) 160:30–38
Moreira FA, Guimarães FS (2004) Benzodiazepine receptor and serotonin 2A receptor modulate the aversive-like effects of nitric oxide in the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 176:362–368
O’Boyle M (1974) Rats and mice together: the predatory nature of the rat’s mouse-killing response. Psychol Bull 81(4):261–269
O’Boyle M (1975) The rat as a predator. Psychol Bull 82:460–462
Okada D (1992) Two pathways of cyclic GMP production through glutamate receptor-mediated nitric oxide synthesis. J Neurochem 59:1203–1210
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, California
Reiser G (1990a) Mechanism of stimulation of cyclic-GMP level in a neuronal cell line mediated by serotonin (5-HT3) receptors. Involvement of nitric oxide, arachidonic-acid metabolism and cytosolic Ca2+. Eur J Biochem 189:547–552
Reiser G (1990b) Endothelin and a Ca2+ ionophore raise cyclic GMP levels in a neuronal cell line via formation of nitric oxide. Br J Pharmacol 101:722–726
Santos P, Bittencourt AS, Schenberg LC, Carobrez AP (2006) Elevated T-maze evaluation of anxiety and memory effects of NMDA/glycine-B site ligands injected into the dorsal periaqueductal gray matter and the superior colliculus of rats. Neuropharmacol 51:203–212
Schenberg LC, Bittencourt AS, Sudré ECM, Vargas LC (2001) Modeling panic attacks. Neurosci Behav Rev 25:647–659
Schmitt ML, Coelho W, Lopes-De-Souza AS, Guimarães FS, Carobrez AP (1995) Anxiogenic-like effect of glycine and D-serine microinjected into dorsal periaqueductal gray matter of rats. Neurosci Lett 189:93–96
Southam E, East SJ, Garthwaite J (1991) Excitatory amino acid receptors coupled to the nitric oxide/cyclic GMP pathway in rat cerebellum during development. J Neurochem 56:2072–2081
Spolidório PC, Echeverry MB, Iyomasa M, Guimarães FS, Del Bel EA (2007) Anxiolytic effects induced by inhibition of the nitric oxide-cGMP pathway in the rat dorsal hippocampus. Psychopharmacoly (Berl) 195:183–192
Teixeira KV, Carobrez AP (1999) Effects of glycine or (+/−)-3-amino-1-hydroxy-2-pyrrolidone microinjections along the rostrocaudal axis of the dorsal periaqueductal gray matter on rats’ performance in the elevated plus-maze task. Behav Neurosci 113:196–203
Volke V, Soosaar A, Koks S, Bourin M, Mannisto PT (1995) Inhibition of nitric oxide synthase causes anxiolytic-like behavior in an elevated plus-maze. NeuroReport 6:1413–1416
Volke V, Wegener G, Bourin M, Vasar E (2003) Antidepressant- and anxiolytic-like effects of selective neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole in mice. Behav Brain Res 140:141–147
Wegener G, Volke V, Rosenberg R (2000) Endogenous nitric oxide decreases hippocampal levels of serotonin and dopamine in vivo. Br J Pharmacol 130:575–580
Whitton PS, Richards DA, Biggs CS, Fowler LJ (1994) N-methyl-d-aspartate receptors modulate extracellular 5-hydroxytryptamine concentration in rat hippocampus and striatum in vivo. Neurosci Lett 169:215–218
Yang M, Augustsson H, Markham C, Hubbard DT, Webster D, Wall PM, Blanchard RJ, Blanchard DC (2004) The rat exposure test: a model of mouse defensive behaviors. Physiol Behav 81:465–473
Acknowledgements
The authors thank Dr. Chris Markham (Center for Behavioral Neuroscience, Georgia State University) for suggestions and English corrections, Dr. João Ricardo Sato (University of ABC) for statistical assistance, as well as Elisabete Lepera and Rosana Silva for technical support. The study was supported by FAPESP, CNPq, and PADC/FCF-Unesp.
E. F. Carvalho-Netto was recipient of CAPES, K.S. Gomes was recipient of FAPESP and V.C.S. Amaral and R.L. Nunes-de-Souza were recipients of CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carvalho-Netto, E.F., Gomes, K.S., Amaral, V.C.S. et al. Role of glutamate NMDA receptors and nitric oxide located within the periaqueductal gray on defensive behaviors in mice confronted by predator. Psychopharmacology 204, 617–625 (2009). https://doi.org/10.1007/s00213-009-1492-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1492-9