Abstract
Rationale
Re-exposing an animal to an environment previously paired with an aversive stimulus evokes large alterations in behavioral and cardiovascular parameters. Dorsal hippocampus (dHC) receives important cholinergic inputs from the basal forebrain, and respective acetylcholine (ACh) levels are described to influence defensive behavior. Activation of muscarinic M1 and M3 receptors facilitates autonomic and behavioral responses along threats. Evidence show activation of cholinergic receptors promoting formation of nitric oxide (NO) and cyclic guanosine monophosphate (cGMP) in dHC. Altogether, the action of ACh and NO on conditioned responses appears to converge within dHC.
Objectives
As answer about how ACh and NO interact to modulate defensive responses has so far been barely addressed, we aimed to shed additional light on this topic.
Methods
Male Wistar rats had guide cannula implanted into the dHC before being submitted to the contextual fear conditioning (3footshocks/085 mA/2 s). A catheter was implanted in the femoral artery the next day for cardiovascular recordings. Drugs were delivered into dHC 10 min before contextual re-exposure, which occurred 48 h after the conditioning procedure.
Results
Neostigmine (Neo) amplified the retrieval of conditioned responses. Neo effects (1 nmol) were prevented by the prior infusion of a M1–M3 antagonist (fumarate), a neuronal nitric oxide synthase inhibitor (NPLA), a NO scavenger (cPTIO), a guanylyl cyclase inhibitor (ODQ), and a NMDA antagonist (AP-7). Pretreatment with a selective M1 antagonist (pirenzepine) only prevented the increase in autonomic responses induced by Neo.
Conclusion
The results show that modulation in the retrieval of contextual fear responses involves coordination of the dHC M1-M3/NO/cGMP/NMDA pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The hippocampus is a complex bilateral and subcortical brain structure that, in its most extension, parallels the lateral ventricle and comprises a reverberant circuit whose neuronal input follows first into the dentate gyrus (DG), then run through the Cornu Ammonis (CA) subfields, to end up in the subiculum, the output pathway (Knierim JJ 2015). Based on its brain connections, pattern of gene expression and particular behavioral functional role, the hippocampus is most commonly divided along its longitudinal axis into a dorsal (dHC) and ventral (vHC) portion, which is respectively analogous to the posterior and anterior portion in primates (Fanselow MS and Dong HW 2010). The dHC function is generally more related to the regulation of spatial memory and navigation, whereas the vHC is supposed to mainly regulate the behavioral and physiological consequences of the stress response (Bannerman DM, et al. 2004). Since contextual fear-conditioning uses of environmental cues, which are previously associated with foot shock, to trigger conditioned responses such as freezing and hyperarousal of the autonomic responses, it is not unexpected that cobalt chloride (a synaptic blocker) infused into the dHC was able to prevent all those expected behavioral and cardiovascular changes (Resstel et al 2008). dHC, in fact, plays a role in contextual fear memory, as optogenetic inhibition of CA1 excitatory neurons was observed to impair both contextual fear acquisition and retrieval (Goshen et al 2011).
Acetylcholine (ACh) has a long history of studies showing its relevance for the modulation of hippocampal-related spatial memory, which includes contextual fear retrieval. For example, animal re-exposure to the same context, where aversive conditioning was stablished a day before, induced a substantial increase of hippocampal ACh levels (Nail-Boucherie et al 2000). Indeed, drugs that interfere with the cholinergic system have been observed to regulate the contextual fear responses when infused into dHC (Wilson and Fadel 2017). For example, dHC ACh has been shown to act through muscarinic receptors to orchestrate the behavioral and autonomic changes triggered by contextual fear retrieval (Diniz et al 2022). Accordingly, a functional role of both nicotinic (nAChR) and muscarinic (mAChR) cholinergic receptors has been determined for hippocampal neuroplasticity and also for a myriad of adaptive behaviors (Picciotto et al 2012). Suitably, the medial septum and vertical limb of the diagonal band of Broca (MSDBB), as part of the basal forebrain, provide the main cholinergic input to the hippocampus (Wainer et al 1985). Such cholinergic connection has consistently been ascertained as important for the formation of spatial memories (Ballinger et al 2016).
Synaptic plasticity has been broadly described as the physiological cellular mechanism underlying learning and memory (Neves et al 2008). Glutamate is another neurotransmitter widely described as being able to modulate hippocampal synaptic plasticity and mutually important for learning and memory (Kim and Linden 2007). Indeed, hippocampal neurons are primarily glutamatergic; and one of its most studied secondary intracellular signaling involves activation of N-methyl-D-aspartate receptors (NMDARs) and the subsequent influx of calcium (Ca2+), which may act then on neuronal nitric oxide synthase (nNOS) to produce nitric oxide — NO (Garthwaite 2008). In turn, NO activates soluble guanylate cyclase (sGC) to finally induce the formation of 3′5-cyclic guanosine monophosphate (cGMP). Likewise, ACh hippocampal NMDA signaling is also involved in regulating the retrieval of contextual fear behavior. For instance, bilateral delivery of a nNOS or sGC inhibitor as well as an NMDAR antagonist or a NO scavenger into dHC, prior to re-exposure the rats to the same context in which they were previously shocked, decreased freezing and fear-related autonomic activity that would otherwise be expected at higher levels (Fabri et al 2014).
Interestingly, a monosynaptic rabies-based retrograde circuit tracing driven by a cell type specific Cre mouse line revealed that a profuse portion of the excitatory dHC CA1 neurons receives inputs from medial septum cholinergic neurons (Sun et al 2014). Additionally, basal forebrain cholinergic hypofunction, achieved by a single intracerebroventricular infusion of the cholinergic immunotoxin 192IgG-saporin, prompted a reduction in the nNOS activity of CA1 and CA3 hippocampal neurons (Hartlage-Rübsamen and Schliebs 2001). Still, endogenously released ACh triggers hippocampal synaptic plasticity when foremost glutamatergic inputs to CA1 are timely controlled (Yi et al 2015; Gu et al 2012). From a behavioral perspective, spatial memory-related deficits induced with an intraperitoneal administration of a NMDAR antagonist or NOS inhibitor were prevented by concurrent intraperitoneal administrations of an acetylcholinesterase (AChE) inhibitor (Csernansky et al 2005 ; Kopf and Baratti 1996). That being so, although some studies have shown an interactivity between cholinergic and glutamatergic hippocampal neurotransmission at both cellular and physiological levels, the outcome of such interaction with regard to learning and memory studies has only been evaluated at the systemic level.
Therefore, this study aimed to better understand how hippocampal cholinergic, nitrergic, and glutamatergic signalings interact with each other to regulate the retrieval of the contextual fear response, including autonomic reactivity. Since dHC has been studied further regarding these interactions, we have focused on this brain area in order to falsify our null hypothesis.
Methods
Animals
Two hundred forty-six male Wistar rats (200–250 g) were initially housed in group of 4, in standard laboratory conditions, which means temperature-controlled room (24 ± 1 °C), 12-h light/12-h dark cycle (light on at 6:30 a.m.) and under ad libitum access to food and water. In vivo experiments were conducted with the approval of the local Animal Ethical Committee (protocol 014/2014) from Ribeirão Preto Medical School of the University of São Paulo, as they are following the Brazilian Council for the Control of Animals Experimentation (CONCEA). All licenses comply with international laws and policies, including the EU Directive 2010/63/EU for animal experiments.
Drug treatments
DL-AP7 (NMDAR antagonist; Tocris), N-propyl-L-arginine (NPLA, selective inhibitor of nNOS; Tocris), 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, potassium salt (cPTIO, NO scavenger; RBI), neostigmine methylsulfate (Neo, AChE inhibitor; Sigma-Aldrich) and pirenzepine dihydrochloride (Pir, selective M1 mAChR; Sigma-Aldrich) were all diluted in sterile saline (NaCl 0.9%). J104129 fumarate (Fum, M3/M1 mAChR antagonist; Tocris) and 1H-[1,2,4]Oxadiazolol [4,3-a]quinoxalin-1-one (ODQ, inhibitor of sGC; Sigma-Aldrich) were both diluted in 10% DMSO (dimethyl sulfoxide) saline solution. All control groups were treated with the respective vehicles according to the excipient used to dilute the drugs, as described above. We chose sub-effective doses of NPLA, cPTIO, ODQ, AP7, Fum, and Pir, which showed no behavioral effect per se in a similar fear-conditioning protocol, based on some of our previous studies (Fabri et al 2014; Diniz et al 2022).
Surgery, intracerebral injections, and histology
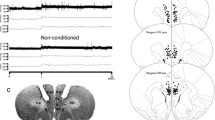
Rats were anesthetized with a 5:1 solution of ketamine hydrochloride (100 mg kg−1, i.p.) and xylazine (20 mg kg−1, i.p.) before being fixed in a stereotaxic frame. Stainless steel guide cannulas (0.7 mm OD) were bilaterally inserted into the dHC (AP = − 4.0 mm from bregma, L = + − 2.8 mm, DV = 2.1 mm), according to the rat brain atlas (Paxinos and Watson 1997), and thus remained attached to the skull bone with stainless steel screws and acrylic cement. A stylet was placed inside the cannulas to avoid any occlusion. Right after the end of the surgery, with the animals still anesthetized, a dose of veterinary pentabiotic (1 mL/kg, i.m.) and the anti-inflammatory Banamine (1 mL/kg, s.c.) was administered in order to prevent postoperative infection and pain. A 10 µL Hamilton microsyringe (Sigma-Aldrich, USA) was connected to a dental needle (0.3 mm OD) through a PE-10 tube where a bubble could be seen moving forward following the drug infusion with a pump (KD Scientific, USA). A total volume of 500 nL/side per min was dHC infused with the dental needle 1.5 mm longer than the cannula length. Infusions were delivered over 1 min and the needles remained in place for another 30 s to prevent reflux. All the animals were deeply anesthetized right after the end of the behavioral procedures with urethane 5% (10 mL/kg, i.p.) for further confirmation of the injection site. Representative of a stained dHC tissue with injection site can be checked in Fig. 1b. Any data corresponding to animals with injections outside the target area were discarded from the statistical analysis. In case the animal’s dHC was collected for the assessment of NO levels (Fig. 1e and f), the injection site was confirmed with the aid of a magnifying glass before the fresh dHC was completely homogenized.
Contextual fear conditioning
The fourth day after the stereotaxic surgery, animals were first pre-exposed (habituation) for 10 min to the conditioning box, a 23 × 20 × 21 cm footshock chamber composed of a grid floor with 18 stainless-steel 2 mm in diameter rods, spaced 1.5 cm apart and wired to a shock generator (automatic reflex conditioner, model 8572; Insight, Brazil). Two hours after habituation, animals returned to the footshock chamber for a low-intensity conditioning protocol in which three inescapable and randomized electric footshocks (20-s to 1-min intervals) of 0.85 mA/2 s were delivered (Uliana et al 2020). In the case of non-conditioned group, the animals were re-exposed to the conditioning chamber without any shock delivery. Previously housed in groups, from now on, the animals were housed individually. Twenty-four hours after the conditioning session, a catheter was implanted into the femoral artery for cardiovascular recordings. The next day, the test session took place 10 min after the last dHC local microinjection and consisted of a 10-min-long re-exposure to the conditioning chamber without shock delivery, where freezing levels and autonomic responses were assessed. Freezing behavior was evaluated by an experienced and treatment-blind observer. Freezing has been defined as the time in which the animals are completely immobilized, except by breathing movements, assuming a characteristic tense posture. Behavioral was expressed as the percentage of freezing time over the 10 min test exposure. Throughout all behavioral procedures, the chamber was thoroughly cleaned with 70% ethanol between each animal performance.
Femoral artery cannulation
Rats were anesthetized again with a 5:1 solution of ketamine hydrochloride (100 mg kg−1, i.p.) and xylazine (20 mg kg−1, i.p) to make a catheter (a 4 cm PE-10 segment heat-bound to a 13 cm PE-50 segment, Clay Adams, USA) reach the abdominal aorta through its insertion into the femoral artery. The catheter was tunneled under the skin and exteriorized on the animal’s dorsum to allow its connection to the transducer system.
Measurement of cardiovascular responses and tail temperature
Animals were brought to the testing room and left undisturbed for at least an hour to quietly adapt to the environment. Then, mean arterial pressure (MAP) and heart rate (HR) were recorded by plugging in the previously heparinized catheter to a signal amplifier (AECADE04P) connected to a computer-processed signal acquisition board (Power Lab 8/30, Australia). The cutaneous temperature (CT) of the tail was recorded with a thermal camera Multi-Purpose Thermal Imager IRI 4010 (Infra-Red Integrated Systems Ltd, USA) at a distance of 50 cm every minute. MAP, HR, and CT values were recorded throughout the 10-min period before and still during the 10 min following re-exposure to the footshock chamber. Such autonomic parameters obtained within the last 5–10 min before context re-exposure were used as baseline values. ΔMAP, ΔHR, and ΔCT were used as changes from the baseline.
Ozone-based reductive chemiluminescence indirect assay NO measure
Right after test session, the animals were decapitated and their dHC were collected. Next, brain samples were homogenized with Rippa buffer (Sigma, EUA) and the total protein levels were measured with Bradford method (BIO-RAD) according to the manufacturer’s instructions. Further, 50 µL of each sample was protein-denatured by incubation with Ethanol 95% at 4 °C for 30 min and then centrifuged for 5 min at 2000 rpm to allow for the supernatant to be collected. Quantitative reduction of nitrate (NO2−) to nitrite (NO3−) and then to NO was done by adding to the supernatant a solution of vanadium (III) 8% in 1 N hydrochloric acid at 96 °C. Briefly, a nitrogen stream was bubbled through the purge vessel (containing vanadium (III) solution) into which plasma samples were injected in-line with a gas-phase chemiluminescence NO analyzer (Sievers Model 280 NO analyzer; Boulder, CO, USA), which detects NO released by its reaction with ozone. Since this is an indirect method to measure NO based on the levels of its metabolites NO2− and NO3− corrected by total protein, the respective outcomes are presented as percentage of NOx (µM/µg protein). Such ozone-based reductive chemiluminescence assay was previously described in Feelisch et al 2002); Pinheiro et al 2012).
Statistical analysis
Data was analyzed with two- or three-way ANOVA, as appropriate. Post hoc has been done by using Tukey’s (two-way) multiple comparisons test. P value < 0.05 was considered as significant. Data are shown as mean ± standard error of the mean (SEM). Graphpad Prism software was used to perform all the analysis.
Results
Due to technical problems involving difficulties with femoral artery cannulation, it was not always possible to assess the cardiovascular parameters of the animals tested behaviorally. Therefore, the number of animals used for the cardiovascular parameters will not always be the same as in behavioral experiments. Of note, behavioral experimental design is depicted in Fig. 1a.
Effect of neostigmine on the retrieval of contextual fear conditioning and dHC NOx levels
First, different doses of Neo were infused into the dHC 10 min before contextual fear retrieval. Indeed, one-way ANOVA showed that the highest two doses (1 nmol and 3 nmol) of Neo increased animals’ freezing levels (F3,39 = 15.09, p < 0.0001; Dunnett’s post hoc with adjusted pVEH x Neo1.0 nmol = 0.0001 and pVEH x Neo3.0 nmol < 0.0001; Fig. 2a). Still, repeated measures two-way ANOVA, followed by Dunnett’s post hoc pairwise column comparison, pointed that the same two doses were still able to increase ΔMAP (Neo factor F3,39 = 10.28, p < 0.0001; Fig. 2c) and ΔHR (Neo factor F3,39 = 6.499, p = 0.0011; Fig. 2d), while decreasing ΔCT (Neo factor F3,39 = 15.48, p < 0.0001; Fig. 2b); Respective Dunnett’s adjusted p values are available in the time curve graphics. Importantly, none of the doses of Neo have altered the basal autonomic levels.
Effect of neostigmine on the retrieval of contextual fear conditioning and dHC NOx levels. Animals were first submitted to the conditioning protocol (day 1), and the day after to the femoral artery cannulation. On the third day, three different doses of Neo (nmol) were infused into the dHC 10 min before the test session. Drug influence on freezing (a), ΔCT (b), ΔMAP (c) and ΔHR (d) was compared to the control group. Differently from the previous experiment, independent groups of animals were either conditioned or not (day 1), and only Neo 1 nmol or VEH were infused into the dHC 10 min before the test session. Interaction between conditioning and Neo was evaluated on the (e) Freezing and (f) dHC NOx levels. All the data are expressed as mean ± SEM. *p < 0.05 compared with VEH (a, b, c, d) or VEH/non-conditioning (e, f). #p < 0.05 compared with VEH/conditioning (e, f). Number of animals are described respectively within the bars or in parentheses according to the color codes
From now on, the minimum effective dose of 1 nmol Neo was used for further experiments in order to minimize likely off targets. By using an independent batch of animals, we found that Neo 1 nmol treatment does interact with conditioning to influence freezing levels (two-way ANOVA shows Neo × conditioning interaction F1,22 = 5.877, p = 0.0240; conditioning factor F1,22 = 104.4, p < 0.0001; Neo factor F1,22 = 12.68, p = 0.0017). Tukey’s post hoc with adjusted p values confirmed, by pairwise comparisons, that the conditioned animals (C) indeed had significantly higher freezing levels and that Neo 1 nmol did not change the freezing levels of those non-conditioned animals (NC), despite its expected effect of amplifying the freezing levels of the conditioned ones (Fig. 2e). Accordingly, 1 nmol treatment also do interact with conditioning to influence NOx levels (two-way ANOVA shows Neo × conditioning interaction F1,22 = 61.75, p < 0.0001; conditioning factor F1,22 = 335.3, p = < 0.0001; Neo factor F1,22 = 49.22, p = 0.0001). Again, pairwise comparisons using Tukey’s post hoc with adjusted p values signaled that the conditioned animals (C) had significantly higher dHC NOx levels and that Neo 1 nmol did not change the dHC NOx levels of those non-conditioned animals (NC), despite its expected effect of amplifying the NOx levels of the conditioned ones (Fig. 2f).
Neostigmine effects on conditioned responses depend on nNOS
To examine whether the Neo effect on conditioned responses depends on intact nitrergic neurotransmission, the nNOS blocker NPLA was infused prior to Neo into the dHC at a sub-effective dose of 0.01 nmol. Two-way ANOVA showed that NPLA completely prevented Neo effect on boosting retrieval of the freezing response (Neo × NPLA interaction F1,30 = 11.27, p = 0.0022; Neo factor F1,30 = 8.474, p = 0.0067; NPLA factor F1,30 = 17.60, p = 0.0002; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo = 0.0017, #pVEH/Neo x NPLA/Neo < 0.0001; Fig. 3a). Repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, has shown that NPLA also abrogated any Neo effect on ΔMAP (Neo factor F1,30 = 12.48, p = 0.0014; NPLA factor F1,30 = 1.7773, p = 0.1930; Neo × NPLA interaction F1,30 = 6.543, p = 0.0158; Fig. 3b), ΔHR (Neo factor F1,30 = 13.76, p = 0.0008; NPLA factor F1,30 = 14.28, p = 0.0007; Neo × NPLA interaction F1,30 = 18.04, p = 0.0002; Fig. 3c) and ΔCT (Neo factor F1,30 = 12.15, p = 0.0015; NPLA factor F1,30 = 9.354, p = 0.0047; Neo × NPLA interaction F1,30 = 6.661, p = 0.0150; Fig. 3d). Respective Tukey’s adjusted p values are available in the time curve graphics. Importantly, none of the drug combinations altered the baseline autonomic levels.
Neostigmine effects on conditioned responses depend on nNOS and were blocked by a NO scavenger. Animals were first submitted to the conditioning protocol (day 1), and the day after to the femoral artery cannulation. On the third day, NPLA 0.01 nmol or cPTIO 0.2 nmol or VEH were infused into the dHC 5 min before Neo 1 nmol or VEH, which in turn were infused 10 min before the test session. The effect of interaction between NPLA and Neo can be seen on freezing (a), ΔMAP (b), ΔHR (c) and ΔCT (d). The effect of interaction between cPTIO and Neo can be seen on freezing (e), ΔMAP (f), ΔHR (g) and ΔCT (h). All the data are expressed as mean ± SEM. *p < 0.05 for comparison with VEH/VEH group and #p < 0.05 for comparison with VEH/Neo group. Number of animals are described respectively within the bars or in parentheses according to the color codes
Neostigmine effects on conditioned responses were blocked by a NO scavenger
NO is an unstable free radical whose easy diffusion over lipid membranes makes it impossible to be stored and, therefore, likely to reach target cells surrounding those neurons that entertained nNOS activity (Garthwaite 2008). Consequently, the NO scavenger cPTIO was infused prior to Neo into the dHC in order to explore whether an autocrine or paracrine action of NO is in fact the mechanism behind the effects of Neo. Two-way ANOVA depicted that a sub-effective dose of cPTIO (0.2 nmol) thoroughly prevented Neo effect on boosting retrieval of the freezing response (Neo × cPTIO interaction F1,27 = 16.28, p = 0.0004; Neo factor F1,27 = 13.39, p = 0.0011; cPTIO factor F1,27 = 8.745, p = 0.0064; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo = 0.0002, #pVEH/Neo x cPTIO/Neo < 0.0006; Fig. 3e). Repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, has shown that cPTIO also abrogated any Neo effect on ΔMAP (Neo factor F1,18 = 5.191, p = 0.0351; cPTIO factor F1,18 = 17.72, p = 0.0351; Neo × cPTIO interaction F1,18 = 27.60, p < 0.0001; Fig. 3f) and ΔHR (Neo factor F1,18 = 6.720, p = 0.0184; cPTIO factor F1,18 = 12.84, p = 0.0021; Neo × cPTIO interaction F1,18 = 15.31, p = 0.001; Fig. 3g), while partially prevented Neo effect on ΔCT (Neo factor F1,27 = 24.17, p < 0.0001; cPTIO factor F1,27 = 4.989, p = 0.0340; Neo × cPTIO interaction F1,27 = 0.1717, p < 0.6819; Fig. 3h). Respective Tukey’s adjusted p values are available in the time curve graphics. None of the drug combinations altered the baseline autonomic levels.
Neostigmine effects on conditioned responses were blocked by an inhibitor of sGC
Once the paracrine action of NO was found to be important for Neo effects on conditioned responses, the obvious next step was to check whether further downstream signaling, which involves the sGC action, would also be relevant. For that reason, the inhibitor of sCG ODQ was infused prior to Neo into the dHC at a sub-effect dose of 0.1 nmol. Two-way ANOVA reported ODQ counteracting Neo’s effect on boosting retrieval of the freezing response (Neo × ODQ interaction F1,22 = 3.405, p = 0.0785; Neo factor F1,22 = 31.47, p < 0.0001; ODQ factor F1,22 = 11.14, p = 0.0030; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo = 0.0002, #pVEH/Neo x ODQ/Neo = 0.0069; Fig. 4a). Repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, showed ODQ also nulling any Neo effect on ΔMAP (Neo factor F1,19 = 4.360, p = 0.0505; ODQ factor F1,19 = 7.400, p = 0.0136; Neo × ODQ interaction F1,19 = 4.345, p = 0.0509; Fig. 4b), ΔHR (Neo factor F1,19 = 9.923, p = 0.0053; ODQ factor F1,19 = 11.54, p = 0.0030; Neo × ODQ interaction F1,19 = 19.50, p = 0.0003; Fig. 4c) and ΔCT (Neo factor F1,22 = 12.28, p = 0.0020; ODQ factor F1,22 = 3.311, p = 0.0825; Neo × ODQ interaction F1,22 = 11.70, p = 0.0024; Fig. 4d). Respective Tukey’s adjusted p values are available in the time curve graphics. None of the drug combinations altered the baseline autonomic levels.
Neostigmine effects on conditioned responses were blocked by a sGC inhibitor and NMDAR antagonist. Animals were first submitted to the conditioning protocol (day 1), and the day after to the femoral artery cannulation. On the third day, ODQ 0.1 nmol or AP7 1 nmol or VEH were infused into the dHC 5 min before Neo 1 nmol or VEH, which in turn were infused 10 min before the test session. The effect of interaction between ODQ and Neo can be seen on freezing (a), ΔMAP (b), ΔHR (c) and ΔCT (d). The effect of interaction between AP7 and Neo can be seen on freezing (e), ΔMAP (f), ΔHR (g) and ΔCT (h). All the data are expressed as mean ± SEM. *p < 0.05 for comparison with VEH/VEH group and #p < 0.05 for comparison with VEH/Neo group. Number of animals are described respectively within the bars or in parentheses according to the color codes
Neostigmine effects on conditioned responses were blocked by a NMDAR antagonist
The NMDAR antagonist AP7 was infused prior to Neo into the dHC at a sub-effect dose of 1 nmol for the purpose of checking whether the cholinergic and glutamatergic systems interact to modulate the conditioned responses. Two-way ANOVA revealed AP7 counteracting Neo’s effect on boosting retrieval of the freezing response (Neo × AP7 interaction F1,30 = 8.419, p = 0.0069; Neo factor F1,30 = 14.28, p = 0.0007; AP7 factor F1,30 = 4.249, p = 0.0480; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo = 0.0006, #pVEH/Neo x AP7/Neo < 0.0056; Fig. 4e). Repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, exhibits AP7 as being able to abolish any Neo effect on ΔMAP (Neo factor F1,26 = 12.79, p = 0.0014; AP7 factor F1,26 = 8.382, p = 0.0076; Neo × AP7 interaction F1,26 = 2.540, p = 0.1231; Fig. 4f), ΔHR (Neo factor F1,26 = 5.447, p = 0.0276; AP7 factor F1,26 = 7.148, p = 0.0128; Neo × AP7 interaction F1,26 = 9.228, p = 0.0054; Fig. 4g) and ΔCT (Neo factor F1,30 = 5.035, p = 0.0324; AP7 factor F1,30 = 7.367, p = 0.0109; Neo × AP7 interaction F1,30 = 2.881, p = 0.100; Fig. 4h). Respective Tukey’s adjusted p values are available in the time curve graphics. As expected, none of the drug combinations altered the baseline autonomic levels.
Neostigmine effects on autonomic responses depend on M1 receptors
As M1R is the most abundant mAChR in the hippocampus (Levey et al 1995), the selective M1R antagonist Pir (0.6 nmol) was infused before Neo into the dHC to check for such a receptor relevance in the actions of Neo. Two-way ANOVA showed that such sub-effective dose of Pir was not able to prevent Neo effect on boosting retrieval of the freezing response (Neo × Pir interaction F1,27 = 0.1120, p = 0.7404, Neo factor F1,27 = 81.90, p < 0.0001; Pir factor F1,27 = 2.302, p = 0.1408; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo < 0.0001, *pVEH/VEH x Pir/Neo = 0.0002, pVEH/Neo x Pir/Neo = 0.5659; Fig. 5a). Strikingly, repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, has shown that Pir anyway prevented Neo effect on ΔMAP (Neo factor F1,27 = 38.46, p < 0.0001; Pir factor F1,27 = 26.38, p < 0.0001; Neo × Pir interaction F1,27 = 8.681, p = 0.0066; Fig. 5b), ΔHR (Neo factor F1,27 = 34.06, p < 0.0001; Pir factor F1,27 = 46.86, p < 0.0001; Neo × Pir interaction F1,27 = 10.13, p = 0.0037; Fig. 5c) and ΔCT (Neo factor F1,27 = 9.093, p = 0.0055; Pir factor F1,27 = 25.93, p < 0.0001; Neo × Pir interaction F1,27 = 0.6251, p = 0.4361; Fig. 5d). Respective Tukey’s adjusted p values are available in the time curve graphics. Again, none of the drug combinations altered the baseline autonomic levels.
Neostigmine effects on conditioned responses depend on M1 or M3 receptors. Animals were first submitted to the conditioning protocol (day 1), and the day after to the femoral artery cannulation. On the third day, Pir 0.6 nmol or Fum 0.6 nmol or VEH were infused into the dHC 5 min before Neo 1 nmol or VEH, which in turn were infused 10 min before the test session. The effect of interaction between Pir and Neo can be seen on freezing (a), ΔMAP (b), ΔHR (c) and ΔCT (d). The effect of interaction between Fum and Neo can be seen on freezing (e), ΔMAP (f), ΔHR (g) and ΔCT (h). All the data are expressed as mean ± SEM. *p < 0.05 for comparison with VEH/VEH group and #p < 0.05 for comparison with VEH/Neo group. Number of animals are described respectively within the bars or in parentheses according to the color codes
Neostigmine effect on freezing levels depends on M3 receptors
Although Fum has been described mainly as a potent and selective M3 receptor antagonist, its respective Ki value for M3 (4.2 nM) and M1 (19 nM) — https://www.tocris.com/products/j-104129-fumarate_2507 — does not allow one to really differentiate how much its action depend on the blockade of one or another receptor. For this reason, Fum was used at a dose equimolar to Pir (0.6 nmol) to ascertain whether these differences around mAChR selectivity make Fum additionally capable of modulating the effect of Neo on freezing levels, in addition to recapitulating the effects of Pir on autonomic responses. Two-way ANOVA showed that a prior dHC infusion of a sub-effective dose (0.6 nmol) of Fum prevented Neo effect on boosting retrieval of the freezing response (Neo × Fum interaction F1,17 = 4.450, p = 0.0500, Neo factor F1,17 = 12.13, p = 0.0028; Fum factor F1,17 = 4.297, p = 0.0537; Tukey’s post hoc with adjusted *pVEH/VEH x VEH/Neo = 0.0061, #pVEH/Neo x Fum/Neo = 0.0349; Fig. 5e). Conversely, repeated measures three-way ANOVA, followed by Tukey’s post hoc pairwise column comparisons, has also shown that Fum still prevented Neo effect on ΔMAP (Neo factor F1,17 = 6.145, p = 0.0240; Fum factor F1,17 = 90.28, p < 0.0001; Neo × Fum interaction F1,17 = 57.95, p < 0.0001; Fig. 5f), ΔHR (Neo factor F1,17 = 3.944, p = 0.0634; Fum factor F1,17 = 30.51, p < 0.0001; Neo × Fum interaction F1,17 = 21.51, p = 0.0002; Fig. 5g) and ΔCT (Neo factor F1,17 = 1.605, p = 0.2223; Fum factor F1,17 = 9.435, p = 0.0069; Neo × cPTIO interaction F1,17 = 12.37, p = 0.0026; Fig. 5h). Respective Tukey’s adjusted p values are available in the time curve graphics. Of note, none of the drug combinations altered the baseline autonomic levels.
Discussion
Provided that ACh, remaining available for longer at the dHC synaptic cleft, overacts on target synaptic receptors to enhance conditioned responses, pharmacological scrutiny suggests the behavioral response being mediated by M3 receptors and autonomic responses presumably by M1 receptors. In addition to the enhancing effects on conditioned responses, dHC infusion of Neo concomitantly increased NO levels. Further pharmacological investigation confirmed dHC nitrergic neurotransmission and respective signaling as key factors for all the effects of Neo on the conditioned responses. At last, NMDARs were also showed to be required for such effects.
Functional role of hippocampal ACh in behavioral and physiological consequences of stress
According to our data, systemic administration of both AChE inhibitors physostigmine and donepezil also increased freezing conditioned response of mice (Csernansky et al 2005). Indeed, acute stress exposure and corticosterone treatment activate the septo-hippocampal cholinergic pathway to induce ACh release (Gilad et al 1990; Gilad 1987; Martinowich et al 2012; Mark et al 1996), and the stimulation of such cholinergic pathway is likely involved with the behavioral consequences of stress exposure as the optogenetic stimulation of septohippocampal terminals or the selective chemogenetic activation of the cholinergic input to hippocampus induced by itself a variety of behavioral changes (Mineur et al 2022). Additionally, shRNAs-based knockdown of AChE within mice hippocampus increased stress-sensitive behaviors as well as decreased resilience to social defeat stress (Mineur et al 2013). According to the function of ACh as a proxy for stress susceptibility, infusion of Neo 1 nmol into the dHC did not increase freezing or NOx levels in non-conditioned animals as it only boosted such phenotypes whose outcomes happened anyway after fear conditioning (Fig. 2e and f). Similarly, systemic pharmacological inhibition or hippocampal knockdown of AChE has been observed not to change the behavioral of nonstressed animals at baseline (Mineur et al 2013; Martinowich et al 2012).
In addition to enhancing the freezing response (Fig. 2a), dHC-infused Neo boosted the autonomic responses normally seen with fear retrieval (Fig. 2b–d). Such increment of ΔMAP and ΔHR is followed by a redistribution of blood flow from the periphery (rat tail) to other inner vascular beds as observed with the decrement of ΔCT, a shift regulated indirectly by cardiac output but also directly by the sympathetic vasoconstrictor tone of the tail skin’s surface arterioles (Vianna and Carrive 2005).
Top-down control of autonomic nervous system responses to stress
In regular occasions, stressors trigger the brainstem activity to signal for body homeostasis and energy mobilization through hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous systems (ANS) responses by directly projecting to the paraventricular nucleus of the hypothalamus — PVN (Ulrich-Lai and Herman 2009). While limbic forebrain regions, including the hippocampus, do not directly connect with HPA axis and ANS, a top-down control happens with such limbic structures using of “middle management” areas as the bed nucleus of the stria terminalis — BNST (Ulrich-Lai and Herman 2009). Indeed, BNST is known to relay vHC connections to modulate hypothalamic and brainstem activity (Herman and Cullinan 1997). Although the dHC does not project to these key areas, vHC and dHC indirectly communicate with each other (Fanselow MS and Dong HW 2010), and thus dHC may still coordinate autonomic responses. In agreement to our reasoning and data, the synaptic blocker cobalt chloride was able to prevent the acute restraint stress effect of increasing such autonomic responses in rats when infused into the dHC, as it did when infused into the vHC (Scopinho et al 2013). Additionally, a genetically encoded fluorescent ACh sensor recorded that fluorescent transients in both dHC and vHC outlasted for at least 10 s after mice had undergone footshock (Mineur et al 2022).
Interaction between ACh and NO in fear memory retrieval
NO has been recognized as a ubiquitous neuromodulator due to its signaling properties and vast distribution throughout the brain. Under ordinary circumstances nNOS-derived NO accounts for 95% of all brain NO (Huang et al 1993); however, keep in mind that other three members of the NOS family does exist: inducible NOS (iNOS), endothelial NOS (eNOS), and mitochondrial NOS — mtNOS — (Biojone et al 2015). Of note, nNOS and eNOS are constitutively expressed in neurons and endothelial cells of blood vessels, respectively, while iNOS is primarily expressed in astrocytes and microglia following immunological challenges (Förstermann et al 1998). Since Neo effect on conditioned responses was casually followed by an increase in dHC NOx levels, as a next step NO signaling was pharmacologically modulated in order to verify whether such a pathway is as expected causally related to the action of Neo. Although ACh is capable of inducing intracellular mAChR-dependent Ca2+ oscillations and additional NO release by brain endothelial cells (Zuccolo et al 2017), prior infusion of NPLA into dHC completely prevented all conditioned responses (Fig. 3a–d), thus suggesting indeed a causal relationship between the effects of Neo and nNOS-dependent NO formation. In agreement with the NPLA data, nNOS KO mice depicted a stringent learning shortage in contextual fear (Kelley et al 2009). Accordingly, nNOS is expressed in several brain areas, including the rat hippocampus (Vincent and Kimura 1992). Although the expression of nNOS in the hippocampus has been mostly described as confined to the cytoplasm of subpopulations of GABAergic interneurons (Tricoire and Vitalis 2012), as well as in long-range inhibitory cells (Christenson Wick et al 2019), in pyramidal CA1 neurons nNOS was found restricted especially to dendritic spines (Burette et al 2002). Additionally, the adaptor protein postsynaptic density-95 was found in a subset of those same spines containing nNOS. Therefore, nNOS is widely expressed throughout the hippocampus and such an arrangement is consistent with the functional role of NO in a broad diversity of physiological conditions such as learning, memory, as well as neuronal disorders (Zhou and Zhu 2009). Properly, cholinergic afferent projections target both glutamatergic and GABAergic hippocampal neurons (Drever et al 2011), and thus ACh might mediate nNOS/NO-induced synaptic neuroplasticity.
NO is an unstable free radical highly diffusible in both aqueous and lipid environments, so it may rapidly diffuse across membranes to act beyond cellular boundaries and on neighboring NO-responsive targets. Indeed, prior dHC infusion of cPTIO prevented Neo effects on all the conditioned responses (Fig. 3e–h), suggesting that Neo-induced NO crosses the cell membrane to act paracrinely on adjacent neurons. Such a scenario for the action of NO makes perfect sense as in rat forebrain, including the hippocampus, both nNOS and sGC were mainly expressed in distinct cell populations (Ding et al 2004). sGC is the enzymatic locus most sensitive to the action of NO, with an EC50 around the low nanomolar range (Roy et al 2008). Once the prior dHC infusion of ODQ prevented all effects of Neo on conditioned responses (Fig. 4a–d), as did NPLA, the action of sGC is strongly suggested to be downstream of NO signaling. Interestingly, sGC may be located either pre- or post-synaptically (Hardingham et al 2013), but mostly in pyramidal cells (Ding et al 2004). Accordingly, presynaptic sGC was found closely juxtaposed to the nNOS-containing dendritic spines of CA1 pyramidal cells (Burette et al 2002). These findings described right above support other additional evidence that suggests NO as a retrograde messenger, which may act through sGC to mediate homosynaptic plasticity.
Indeed, NO is known to regulate glutamate and GABA release as a retrograde messenger that orchestrates several aspects of presynaptic function, including an increase in the neurotransmitter release-probability and in the size of the readily releasable pool (Hardingham et al 2013). In addition, patch-clamp recordings of whole-CA1 pyramidal cells from either ODQ-treated or sGCKO-mice brain slices showed that under both basal and stimulated conditions, glutamate release was sGC-dependent (Neitz et al 2011). A succession of elegant experiments performed with cultured hippocampal pyramidal neurons demonstrated that NO is postsynaptically released, acts retrogradely on the presynaptic sGC to enhance neurotransmitter release, and then finally induces NMDAR-dependent LTP (O'Dell et al 1991; Arancio et al 1995; Arancio et al 1996). Based on the overview of NO functioning as a retrograde messenger, AP7 was infused into dHC before Neo to check for any glutamate-dependent effects. As expected, all effects of Neo on conditioned responses depended on dHC NMDAR (Fig. 4e–h). So far, all data suggest that ACh released within dHC mediates contextual fear retrieval, which includes behavioral and autonomic responses, via the retrograde action of NO on the presynaptic sGC and the subsequent release of glutamate to act on the postsynaptic NMDAR. Accordingly, different forms of hippocampal synaptic plasticity have indeed been precisely triggered by the timing between the reciprocal activation of cholinergic signaling and glutamatergic inputs to CA1 (Gu and Yakel 2011; Gu et al 2012). Additionally, higher doses of NPLA, cPTIO, ODQ, and AP7 infused into dHC were by themselves able to prevent all behavioral and autonomic changes related to contextual fear retrieval (Fabri et al 2014), an outcome that highlights nNOS/NO/sGC and NMDA signaling also as part of the dHC basal fear pathway.
A rationale for the ACh and NO interaction
MSDBB provides about 65% of the hippocampal ACh input (Woolf 1991); however, a small number of cholinergic interneurons are also sparsely distributed throughout the hippocampus (Frotscher et al 2000; Yi et al 2015). ACh acts over a range of nAChRs and mAChRs subtypes. All nAChRs exhibit high conductance to Na+ and K+ ions, but the subtypes diverge in permeability to Ca2+ ions (Drever et al 2011). On the other hand, mAChRs are divided into two further subgroups, in which M1, M3, and M5 receptors (M1R, M3R, and M5R, respectively) are coupled to Gq/11 and activate phospholipase C, resulting in intracellular Ca2+ mobilization; whereas the M2 and M4 receptors are Gi/o-coupled and negatively modulate adenylate cyclase, thus reducing cAMP levels (Lanzafame et al 2003; Drever et al 2011). Since our hypothesis was built to disentangle an eventual cholinergic and nitrergic interaction, we next focused on the M1R and M3R functional role, as they induce Ca2+ mobilization, supposed to be important for nNOS activation, and M5R are expressed at very low levels across the central nervous system (Levey et al 1994). Although the best described stimulus for NO making is the influx of Ca2+ through the aperture of NMDAR, as nNOS and NMDAR are both anchored to PSD95 proteins to make Ca2+ readily available to achieve nNOS (Garthwaite 2008; Zhou and Zhu 2009), carbachol (non-selective mAChR agonist) was also able to activate nNOS and induce NO formation in rat cerebral frontal cortex via Ca2+-calmodulin complexes (Borda et al 1998). Correspondingly, NOS inhibitors inhibited any carbachol-induced cGMP formation in primary cortical cultures (Castoldi et al 1993). From rat retina, carbachol still activated nNOS and induced cGMP accumulation via M1R/M3R-induced phospholipase C and Ca2+-calmodulin (Borda et al 2005). Accordingly, mAChR activation in CA1 pyramidal neurons initially evoked a local rise in cytosolic Ca2+ from the apical dendrites that was later diffused as a wave toward the soma (Power and Sah 2002).
Untangling the functional role of mAChRs for the effects of ACh on behavioral and autonomic conditioned responses
M1R is the most abundant mAChR in the hippocampus and is located in pyramidal cell bodies, along apical or basal dendrites, and on spines (Levey et al 1991; Levey et al 1995; Yamasaki et al 2010). Interestingly, low carbachol concentration or direct stimulation of acetylcholine release enhanced the CA1 long-term potentiation induced by high-frequency stimulation of Schaffer collaterals, such an effect that was absent with M1RKO mice (Shinoe et al 2005). At the electron microscopy level, the M1R was found co-localized with the NR1a NMDA receptor subunit in the pyramidal cell soma and dendrites, which could explain the reason why NMDA-receptor currents in hippocampal CA1 pyramidal neurons was potentiated by M1R activation (Marino et al 1998). Another study, based on whole-cell current clamp recordings, showed that M1R activation-induced potentiation of glutamatergic synaptic transmission onto CA1 pyramidal neurons was NMDAR dependent (Dennis et al 2016). Altogether, these studies demonstrate that M1R and NMDAR cooperate reciprocally to modulate the hippocampal excitatory synaptic neurotransmission. In fact, an interplay that is in agreement with our AP7-based outcomes. According to such a view, a prior dHC Pir infusion was able to prevent those effects of Neo on fear retrieval-induced changes in autonomic responses (Fig. 5b–d), but surprisingly not on the freezing response (Fig. 5a).
Since the behavioral effect of Neo doesn't seem to depend on the M1R, the M3R might be in charge of it. As expected, the prior dHC infusion of Fum recapitulated the Pir effect in addition to preventing the behavioral effect of Neo (Fig. 5e–h), confirming that Neo effect on the fear retrieval-induced freezing response is in fact mediated by M3. Similarly to our latest outcome, ACh effects have previously been mutually attached to M3R activation and NO/sGC signaling. For example, M3R-triggered NO/sGC signaling has been shown to be important for the cardiovascular changes induced by ACh infusion into the prelimbic medial prefrontal cortex (Fassini et al 2015). Although M3R is expressed at a much lower level than M1R, its levels are still significant in pyramidal neurons of the hippocampus and consistent with a postsynaptic distribution in both the somata and the spines of the proximal dendrites (Levey et al 1994; Levey et al 1995).
Interestingly, it is not completely unexpected that the modulation of autonomic responses is detached from the behavior response, since a previous study showed that blocking dHC neurotransmission after conditioning prevented fear retrieval-induced cardiovascular changes, but did not change any freezing response (Resstel et al 2008). Modulation of structures other than the dHC also shows that, in fact, the behavior and the autonomic parameters may be controlled apart from each other (LeDoux et al 1988).
Alternative landscapes
Of note, regarding hippocampal basket cells subtypes, the firing frequency of cholecystokinin-positive interneurons (CCK+) was controlled by M3R, while the excitability of parvalbumin-positive interneurons (PV+) was modulated by M1R (Cea-del Rio et al 2010). Additionally, while PV+ expressed exclusively M1R mRNA, CCK+ robustly expressed both M1R and M3R mRNA (Cea-del Rio et al 2010). Since hippocampal GABAergic interneurons express nNOS, which includes PV+ (Tricoire and Vitalis 2012), an alternative or complementary view to our landscape drawn so far is that M1R-induced NO may also be produced within GABAergic interneurons. Another interesting perspective comes from the fact that about 30–50% of the cholinergic neurons from medial septum and vertical limb of the diagonal band of Broca also express NOS mRNA, at low to moderate levels (Kitchener and Diamond 1993, DOI; Sugaya and McKinney 1994). Thereby, these findings described just above suggest that a portion of the hippocampal cholinergic nerve endings from the basal forebrain may also release NO from an autocrine action of ACh accumulated in the synaptic cleft. Although, to the best of our knowledge, neither M1R nor M3R has been described as an autoreceptor on hippocampal cholinergic nerve endings from medial septum, both M1R and M3R have already been described as lying presynaptically in glutamatergic hippocampal neurons (de Vin F, et al. 2015; Palacios-Filardo et al 2021; Kamsler et al 2010).
Conclusion
In conclusion, wherever mAChR-induced NO is produced, whether in excitatory, inhibitory neurons or from cholinergic nerve endings, our data suggest that NO leaks beyond neuronal boundaries to likely release glutamate and activate NMDAR. Therefore, keeping in mind that ACh release in the hippocampus can affect different neuronal types and subcellular compartments, which provides immeasurable network complexity, according to our data, we suggest that ACh orchestrates the action of M1R and M3R to induce a NO leakage in order to construct a spatially sparse set of NMDA-dependent neuronal codes capable of coordinatingly modulating both behavior and autonomic changes induced by contextual fear retrieval. Please, see Fig. 6 for a brief and simplified suggested overview based on our data.
An overview for how ACh and NO would interact to orchestrate all the behavioral and autonomic changes induced by contextual retrieval. Based on our data, we suggest that the most likely scenario should consider cholinergic terminals being activated by fear retrieval, which in turn would cause the released acetylcholine to activate muscarinic metabotropic receptors 1 (M1) and 3 (M3) along the soma or dendrites of glutamatergic dHC neurons. Since M1 and M3 are coupled to Gq/11 protein, phospholipase C (PLC) would be activated to increase inositol-1,4,5-trisphosphate (IP3) levels by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2). IP3 increases the release of calcium (Ca2+) from the endoplasmic reticulum. Ca2+ complexes to calmodulin (CaM) and activates neuronal nitric oxide synthase (nNOS) to then produce nitric oxide (NO) from the substrate l-arginine (L-Arg). As a free radical, NO can rapidly diffuse across lipid membranes and target for example neighboring glutamatergic presynaptic terminals. Then, NO acts retrogradely on the presynaptic soluble guanylate cyclase (sGC) to produce cyclic guanosine monophosphate (cGMP) from the guanosine-5-triphosphate substrate (GTP). This way, sGC enhances glutamate release. Therefore, we propose that ACh orchestrates M1 and M3 action to induce NO leakage from different sets of neurons and subcellular compartments, thus providing a spatially set of NMDA-dependent neuronal codes to coordinatingly modulate both behavior and autonomic changes induced by contextual fear retrieval
Conflict of interest
The authors declare no competing interests.
References
Arancio O, Kandel ER, Hawkins RD (1995) Activity-dependent long-term enhancement of transmitter release by presynaptic 3’,5’-cyclic GMP in cultured hippocampal neurons. Nature 376(6535):74–80. https://doi.org/10.1038/376074a0
Arancio O, Kiebler M, Lee CJ, Lev-Ram V, Tsien RY, Kandel ER, Hawkins RD (1996) Nitric oxide acts directly in the presynaptic neuron to produce long-term potentiation in cultured hippocampal neurons. Cell 87(6):1025–1035. https://doi.org/10.1016/s0092-8674(00)81797-3
Ballinger EC, Ananth M, Talmage DA, Role LW (2016) Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 91(6):1199–1218. https://doi.org/10.1016/j.neuron.2016.09.006
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J (2004) Regional dissociations within the hippocampus–memory and anxiety. Neurosci Biobehav Rev 28(3):273–283. https://doi.org/10.1016/j.neubiorev.2004.03.004
Biojone C, Casarotto PC, Joca SR, Castrén E (2015) Interplay between nitric oxide and brain-derived neurotrophic factor in neuronal plasticity. CNS Neurol Disord Drug Targets 14(8):979–987. https://doi.org/10.2174/1871527314666150909113727
Borda T, Genaro A, Sterin-Borda L, Cremaschi G (1998) Involvement of endogenous nitric oxide signalling system in brain muscarinic acetylcholine receptor activation. J Neural Transm (vienna) 105(2–3):193–204. https://doi.org/10.1007/s007020050048
Borda E, Berra A, Saravia M, Ganzinelli S, Sterin-Borda L (2005) Correlations between neuronal nitric oxide synthase and muscarinic M3/M1 receptors in the rat retina. Exp Eye Res 80(3):391–399. https://doi.org/10.1016/j.exer.2004.09.016
Burette A, Zabel U, Weinberg RJ, Schmidt HH, Valtschanoff JG (2002) Synaptic localization of nitric oxide synthase and soluble guanylyl cyclase in the hippocampus. J Neurosci 22(20):8961–8970. https://doi.org/10.1523/JNEUROSCI.22-20-08961.2002
Castoldi AF, Manzo L, Costa LG (1993) Cyclic GMP formation induced by muscarinic receptors is mediated by nitric oxide synthesis in rat cortical primary cultures. Brain Res 610(1):57–61. https://doi.org/10.1016/0006-8993(93)91216-f
Cea-del Rio CA, Lawrence JJ, Tricoire L, Erdelyi F, Szabo G, McBain CJ (2010) M3 muscarinic acetylcholine receptor expression confers differential cholinergic modulation to neurochemically distinct hippocampal basket cell subtypes. J Neurosci 30(17):6011–6024. https://doi.org/10.1523/JNEUROSCI.5040-09.2010
Christenson Wick Z, Tetzlaff MR, Krook-Magnuson E (2019) Novel long-range inhibitory nNOS-expressing hippocampal cells. Elife 8:e46816. https://doi.org/10.7554/eLife.46816
Csernansky JG, Martin M, Shah R, Bertchume A, Colvin J, Dong H (2005) Cholinesterase inhibitors ameliorate behavioral deficits induced by MK-801 in mice. Neuropsychopharmacol 30(12):2135–2143. https://doi.org/10.1038/sj.npp.1300761
de Vin F, Choi SM, Bolognesi ML, Lefebvre RA (2015) Presynaptic M3 muscarinic cholinoceptors mediate inhibition of excitatory synaptic transmission in area CA1 of rat hippocampus. Brain Res 1629:260–269. https://doi.org/10.1016/j.brainres.2015.10.031
Dennis SH, Pasqui F, Colvin EM, Sanger H, Mogg AJ, Felder CC, Broad LM, Fitzjohn SM, Isaac JT, Mellor JR (2016) Activation of muscarinic M1 acetylcholine receptors induces long-term potentiation in the hippocampus. Cereb Cortex 26(1):414–426. https://doi.org/10.1093/cercor/bhv227
Ding JD, Burette A, Nedvetsky PI, Schmidt HH, Weinberg RJ (2004) Distribution of soluble guanylyl cyclase in the rat brain. J Comp Neurol 472(4):437–448. https://doi.org/10.1002/cne.20054
Diniz CRAF, da Silva LA, Bertacchini GL, da Silva-Júnior AF, Resstel LBM (2022) Dorsal hippocampal muscarinic cholinergic receptors orchestrate behavioral and autonomic changes induced by contextual fear retrieval. Pharmacol Biochem Behav 218:173425. https://doi.org/10.1016/j.pbb.2022.173425
Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res 221(2):505–514. https://doi.org/10.1016/j.bbr.2010.11.037
Fabri DR, Hott SC, Reis DG, Biojone C, Corrêa FM, Resstel LB (2014) The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur Neuropsychopharmacol 24(10):1676–1686. https://doi.org/10.1016/j.euroneuro.2014.08.002
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65(1):7–19. https://doi.org/10.1016/j.neuron.2009.11.031
Fassini A, Antero LS, Corrêa FM, Joca SR, Resstel LB (2015) The prelimbic cortex muscarinic M3 receptor-nitric oxide-guanylyl cyclase pathway modulates cardiovascular responses in rats. J Neurosci Res 93(5):830–838. https://doi.org/10.1002/jnr.23537
Feelisch M, Rassaf T, Mnaimneh S, Singh N, Bryan NS, Jourd’Heuil D, Kelm M (2002) Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: implications for the fate of NO in vivo. FASEB J 16(13):1775–1785. https://doi.org/10.1096/fj.02-0363com
Förstermann U, Boissel JP, Kleinert H (1998) Expressional control of the “constitutive” isoforms of nitric oxide synthase (NOS I and NOS III). FASEB J 12(10):773–790 (PMID: 9657518)
Frotscher M, Vida I, Bender R (2000) Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience 96(1):27–31. https://doi.org/10.1016/s0306-4522(99)00525-4
Garthwaite J (2008) Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci 27(11):2783–2802. https://doi.org/10.1111/j.1460-9568.2008.06285.x
Gilad GM (1987) The stress-induced response of the septo-hippocampal cholinergic system A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinol 12(3):167–84. https://doi.org/10.1016/0306-4530(87)90002-3
Gilad GM, Gilad VH, Tizabi Y (1990) Aging and stress-induced changes in choline and glutamate uptake in hippocampus and septum of two rat strains differing in longevity and reactivity to stressors. Int J Dev Neurosci 8(6):709–713. https://doi.org/10.1016/0736-5748(90)90064-9
Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Dynamics of retrieval strategies for remote memories. Cell 147(3):678–89. https://doi.org/10.1016/j.cell.2011.09.033
Gu Z, Yakel JL (2011) Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron 71(1):155–165. https://doi.org/10.1016/j.neuron.2011.04.026
Gu Z, Lamb PW, Yakel JL (2012) Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. J Neurosci 32(36):12337–12348. https://doi.org/10.1523/JNEUROSCI.2129-12.2012
Hardingham N, Dachtler J, Fox K (2013) The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci 7:190. https://doi.org/10.3389/fncel.2013.00190
Hartlage-Rübsamen M, Schliebs R (2001) Rat basal forebrain cholinergic lesion affects neuronal nitric oxide synthase activity in hippocampal and neocortical target regions. Brain Res 889(1–2):155–164. https://doi.org/10.1016/s0006-8993(00)03128-0
Herman JP, Cullinan WE (1997) Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20(2):78–84. https://doi.org/10.1016/s0166-2236(96)10069-2
Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75(7):1273–1286. https://doi.org/10.1016/0092-8674(93)90615-w
Kamsler A, McHugh TJ, Gerber D, Huang SY, Tonegawa S (2010) Presynaptic m1 muscarinic receptors are necessary for mGluR long-term depression in the hippocampus. Proc Natl Acad Sci U S A 107(4):1618–1623. https://doi.org/10.1073/pnas.0912540107
Kelley JB, Balda MA, Anderson KL, Itzhak Y (2009) Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem 16(6):371–378. https://doi.org/10.1101/lm.1329209
Kim SJ, Linden DJ (2007) Ubiquitous plasticity and memory storage. Neuron 56(4):582–592. https://doi.org/10.1016/j.neuron.2007.10.030
Kitchener PD, Diamond J (1993) Distribution and colocalization of choline acetyltransferase immunoreactivity and NADPH diaphorase reactivity in neurons within the medial septum and diagonal band of Broca in the rat basal forebrain. J Comp Neurol 335(1):1–15. https://doi.org/10.1002/cne.903350102
Knierim JJ (2015) The hippocampus. Curr Biol 25(23):R1116–R1121. https://doi.org/10.1016/j.cub.2015.10.049
Kopf SR, Baratti CM (1996) Enhancement of the post-training cholinergic tone antagonizes the impairment of retention induced by a nitric oxide synthase inhibitor in mice. Neurobiol Learn Mem 65(3):207–212. https://doi.org/10.1006/nlme.1996.0025
Lanzafame AA, Christopoulos A, Mitchelson F (2003) Cellular signaling mechanisms for muscarinic acetylcholine receptors. Recept Channels 9(4):241–260 (PMID: 12893537)
LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8(7):2517–2529. https://doi.org/10.1523/JNEUROSCI.08-07-02517.1988
Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11(10):3218–3226. https://doi.org/10.1523/JNEUROSCI.11-10-03218.1991
Levey AI, Edmunds SM, Heilman CJ, Desmond TJ, Frey KA (1994) Localization of muscarinic m3 receptor protein and M3 receptor binding in rat brain. Neuroscience 63(1):207–221. https://doi.org/10.1016/0306-4522(94)90017-5
Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995) Expression of m1–m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15(5 Pt 2):4077–4092. https://doi.org/10.1523/JNEUROSCI.15-05-04077.1995
Marino MJ, Rouse ST, Levey AI, Potter LT, Conn PJ (1998) Activation of the genetically defined m1 muscarinic receptor potentiates N-methyl-D-aspartate (NMDA) receptor currents in hippocampal pyramidal cells. Proc Natl Acad Sci U S A 95(19):11465–11470. https://doi.org/10.1073/pnas.95.19.11465
Mark GP, Rada PV, Shors TJ (1996) Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74(3):767–774. https://doi.org/10.1016/0306-4522(96)00211-4
Martinowich K, Schloesser RJ, Lu Y, Jimenez DV, Paredes D, Greene JS, Greig NH, Manji HK, Lu B (2012) Roles of p75(NTR), long-term depression, and cholinergic transmission in anxiety and acute stress coping. Biol Psychiatry 71(1):75–83. https://doi.org/10.1016/j.biopsych.2011.08.014
Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto MR (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110(9):3573–3578. https://doi.org/10.1073/pnas.1219731110
Mineur YS, Mose TN, Vanopdenbosch L, Etherington IM, Ogbejesi C, Islam A, Pineda CM, Crouse RB, Zhou W, Thompson DC, Bentham MP, Picciotto MR (2022) Hippocampal acetylcholine modulates stress-related behaviors independent of specific cholinergic inputs. Mol Psychiatry. https://doi.org/10.1038/s41380-021-01404-7
Nail-Boucherie K, Dourmap N, Jaffard R, Costentin J (2000) Contextual fear conditioning is associated with an increase of acetylcholine release in the hippocampus of rat. Brain Res Cogn Brain Res 9(2):193–197. https://doi.org/10.1016/s0926-6410(99)00058-0
Neitz A, Mergia E, Eysel UT, Koesling D, Mittmann T (2011) Presynaptic nitric oxide/cGMP facilitates glutamate release via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Eur J Neurosci 33(9):1611–1621. https://doi.org/10.1111/j.1460-9568.2011.07654.x
Neves G, Cooke SF, Bliss TV (2008) Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci 9(1):65–75. https://doi.org/10.1038/nrn2303
O’Dell TJ, Hawkins RD, Kandel ER, Arancio O (1991) Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A 88(24):11285–11289. https://doi.org/10.1073/pnas.88.24.11285
Palacios-Filardo J, Udakis M, Brown GA, Tehan BG, Congreve MS, Nathan PJ, Brown AJH, Mellor JR (2021) Acetylcholine prioritises direct synaptic inputs from entorhinal cortex to CA1 by differential modulation of feedforward inhibitory circuits. Nat Commun 12(1):5475. https://doi.org/10.1038/s41467-021-25280-5
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. Compact, 3rd edn. Academic, San Diego
Picciotto MR, Higley MJ, Mineur YS (2012) Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76(1):116–129. https://doi.org/10.1016/j.neuron.2012.08.036
Pinheiro LC, Montenegro MF, Amaral JH, Ferreira GC, Oliveira AM, Tanus-Santos JE (2012) Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. Free Radic Biol Med 53(4):701–709. https://doi.org/10.1016/j.freeradbiomed.2012.06.001
Power JM, Sah P (2002) Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci 22(9):3454–3462. https://doi.org/10.1523/JNEUROSCI.22-09-03454.2002
Resstel LB, Joca SR, Corrêa FM, Guimarães FS (2008) Effects of reversible inactivation of the dorsal hippocampus on the behavioral and cardiovascular responses to an aversive conditioned context. Behav Pharmacol 19(2):137–144. https://doi.org/10.1097/FBP.0b013e3282f62c9e
Roy B, Halvey EJ, Garthwaite J (2008) An enzyme-linked receptor mechanism for nitric oxide-activated guanylyl cyclase. J Biol Chem 283(27):18841–18851. https://doi.org/10.1074/jbc.M801712200
Scopinho AA, Lisboa SF, Guimarães FS, Corrêa FM, Resstel LB, Joca SR (2013) Dorsal and ventral hippocampus modulate autonomic responses but not behavioral consequences associated to acute restraint stress in rats. PLoS ONE 8(10):e77750. https://doi.org/10.1371/journal.pone.0077750
Shinoe T, Matsui M, Taketo MM, Manabe T (2005) Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci 25(48):11194–11200. https://doi.org/10.1523/JNEUROSCI.2338-05.2005
Sugaya K, McKinney M (1994) Nitric oxide synthase gene expression in cholinergic neurons in the rat brain examined by combined immunocytochemistry and in situ hybridization histochemistry. Brain Res Mol Brain Res 23(1–2):111–125. https://doi.org/10.1016/0169-328x(94)90217-8
Sun Y, Nguyen AQ, Nguyen JP, Le L, Saur D, Choi J, Callaway EM, Xu X (2014) Cell-type-specific circuit connectivity of hippocampal CA1 revealed through Cre-dependent rabies tracing. Cell Rep 7(1):269–280. https://doi.org/10.1016/j.celrep.2014.02.030
Tricoire L, Vitalis T (2012) Neuronal nitric oxide synthase expressing neurons: a journey from birth to neuronal circuits. Front Neural Circuits 6:82. https://doi.org/10.3389/fncir.2012.00082
Uliana DL, Antero LS, Borges-Assis AB, Rosa J, Vila-Verde C, Lisboa SF, Resstel LB (2020) Differential modulation of the contextual conditioned emotional response by CB1 and TRPV1 receptors in the ventromedial prefrontal cortex: possible involvement of NMDA/nitric oxide-related mechanisms. J Psychopharmacol 34(9):1043–1055. https://doi.org/10.1177/0269881120928201
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10(6):397–409. https://doi.org/10.1038/nrn2647
Vianna DM, Carrive P (2005) Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 21(9):2505–2512. https://doi.org/10.1111/j.1460-9568.2005.04073.x
Vincent SR, Kimura H (1992) Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46(4):755–784. https://doi.org/10.1016/0306-4522(92)90184-4
Wainer BH, Levey AI, Rye DB, Mesulam MM, Mufson EJ (1985) Cholinergic and non-cholinergic septohippocampal pathways. Neurosci Lett 54(1):45–52. https://doi.org/10.1016/s0304-3940(85)80116-6
Wilson MA, Fadel JR (2017) Cholinergic regulation of fear learning and extinction. J Neurosci Res 95(3):836–852. https://doi.org/10.1002/jnr.23840
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37(6):475–524. https://doi.org/10.1016/0301-0082(91)90006-m
Yamasaki M, Matsui M, Watanabe M (2010) Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci 30(12):4408–4418. https://doi.org/10.1523/JNEUROSCI.5719-09.2010
Yi F, Catudio-Garrett E, Gábriel R, Wilhelm M, Erdelyi F, Szabo G, Deisseroth K, Lawrence J (2015) Hippocampal “cholinergic interneurons” visualized with the choline acetyltransferase promoter: anatomical distribution, intrinsic membrane properties, neurochemical characteristics, and capacity for cholinergic modulation. Front Synaptic Neurosci 7:4. https://doi.org/10.3389/fnsyn.2015.00004
Zhou L, Zhu DY (2009) Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20(4):223–230. https://doi.org/10.1016/j.niox.2009.03.001
Zuccolo E, Lim D, Kheder DA, Perna A, Catarsi P, Botta L, Rosti V, Riboni L, Sancini G, Tanzi F, D’Angelo E, Guerra G, Moccia F (2017) Acetylcholine induces intracellular Ca2+ oscillations and nitric oxide release in mouse brain endothelial cells. Cell Calcium 66:33–47. https://doi.org/10.1016/j.ceca.2017.06.003
Acknowledgements
The authors thank to Laura H. A Camargo for the technical support. This work was supported by research grants from FAPESP (2013/07419–7) and CNPq.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, L.A., Diniz, C.R.A.F., Uliana, D.L. et al. The interaction between hippocampal cholinergic and nitrergic neurotransmission coordinates NMDA-dependent behavior and autonomic changes induced by contextual fear retrieval. Psychopharmacology 239, 3297–3311 (2022). https://doi.org/10.1007/s00213-022-06213-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06213-6