Abstract
Aging is known as a main risk factor in the development of cardiovascular diseases. Naringin (NRG) is a flavonoid compound derived from citrus fruits. It possesses a wide spectrum of pharmacological properties, including antioxidant anti-inflammatory, and cardioprotective. This investigation aimed to assess the cardioprotective effect of NRG against the ischemia/reperfusion (I/R) injury in aged rats. In this study, D-galactose (D-GAL) at the dose of 150 mg/kg/day for 8 weeks was used to induce aging in rats. Rats were orally gavaged with NRG (40 or 100 mg/kg/day), in co-treatment with D-GAL, for 8 weeks. The Langendorff isolated heart was used to evaluate the effect of NRG on I/R injury in aged rats. NRG treatment diminished myocardial hypertrophy and maximum contracture level in aged animals. During the pre-ischemic phase, reduced heart rate was normalized by NRG. The effects of D-GAL on the left ventricular end diastolic pressure (LVDP), the rate pressure product (RPP), and the minimum and maximum rate of left ventricular pressure (±dp/dt) improved by NRG treatment in the perfusion period. NRG also enhanced post-ischemic recovery of cardiac functional parameters (± dp/dt, and RPP) in isolated hearts. An increase in serum levels of the lactate dehydrogenase (LDH), the creatine kinase-MB (CK-MB), and the tumor necrosis factor-alpha (TNF-α) were reversed by NRG in aged rats. It also normalized the D-GAL-decreased the superoxide dismutase (SOD) activity in the heart tissue. NRG treatment alleviated cardiac injury in aged hearts under conditions of I/R. NRG may improve aging-induced cardiac dysfunction through anti-oxidative and anti-inflammatory mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is known as a prominent risk factor in the development of multiple chronic diseases, such as diabetes, cancer, cardiovascular diseases (CVDs), dementia, and chronic obstructive pulmonary disease (Franceschi et al. 2018). Organismal aging is characterized as a progressive decline in physiological functions (Aman et al. 2021). Aging in cardiovascular system refers to an increase in ventricular wall thickness, diastole prolongation, myocardial fibrosis, fibrocalcification, arterial stiffness, loss of compliance in the coronary vasculature, and endothelial dysfunction (Wu et al. 2019; de Almeida et al. 2020). In particular, cardiovascular aging is one of the main determinants of various disorders including hypertension, atherosclerosis, heart failure, myocardial infarction (MI), and stroke (Liberale et al. 2020). Experimental and clinical studies have shown that aging markedly reduces the adaptive response during ischemic pre- and postconditioning (Randhawa et al. 2018; Kleinbongard et al. 2020). Aging induces excessive ROS generation and decreases antioxidant gene expression during preconditioning (Randhawa et al. 2018; Kleinbongard et al. 2020). Oxidative stress and inflammation are the major mechanisms involved in the pathophysiology of aging-related CVDs (de Almeida et al. 2020). Mitochondrial dysfunction-induced excess reactive oxygen species (ROS) generation is recognized as a major mechanism of cardiac oxidative stress related to aging (Papaconstantinou 2019). During aging, the accumulation of damaged proteins and organelles due to impairments in the autophagy pathways (an intracellular cleanup system) causes the activation of inflammatory responses (de Almeida et al. 2020). Low-grade and persistent inflammation (inflammageing) has been reported that participates pivotally in the development of hypertension and arteriosclerosis (Ferrucci and Fabbri 2018). It also has been suggested that elevated levels of proinflammatory cytokines are related to a diversity change in the gut microbiota in older adults (Sanchez-Morate et al. 2020).
Naringin (NRG) is a flavonoid isolated from citrus fruits such as orange, grapefruit, and lemons (Heidary Moghaddam et al. 2020). It is well known in the treatment or prevention of diabetes, metabolic syndrome, cancer, and cardiac diseases (Heidary Moghaddam et al. 2020; Ghanbari-Movahed et al. 2021). NRG plays a protective role in pathophysiology conditions through anti-oxidative, anti-apoptotic, and anti-inflammatory properties (Heidary Moghaddam et al. 2020; Ghanbari-Movahed et al. 2021; AKİN et al. 2022). NRG has poor bioavailability because of its hydrophobic nature (Bhia et al. 2021). Therefore, a wide range of nanocarriers have been used as delivery systems for NRG, including liposomes, micelles, nanosuspensions, and nanoemulsions (Bhia et al. 2021). One of the main strategies for reducing drug dose and toxicity is to increase bioavailability (Alotaibi 2023). NRG has been found to inhibit the cytochrome P450, which is the main catalyst involved in drug metabolism (Fuhr and Kummert 1995). NRG is well-known for improving the bioavailability of various medications such as diltiazem, verapamil, and ranolazine by inhibiting cytochrome P450-mediated metabolism (Alotaibi 2023). The therapeutic potential of NRG on CVDs have been investigated in several studies in vitro and in vivo (Moghaddam et al. 2020). NRG treatment also has provided protection against fructose-induced cardiomyocyte apoptosis (Park et al. 2018). NRG also attenuated cardiac oxidative stress and apoptosis in myocardial ischemia reperfusion injury in rats (Li et al. 2021). In a study, the NRG protected cardiomyocytes from anoxia/reoxygenation injury by the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor (Chen et al. 2015). Various antioxidant enzymes genes, including glutamate cysteine ligase (GCL), superoxide dismutase (SOD), heme oxygenase-1 (HO-1), and glutathione peroxidase (GPx) are upregulated by Nrf2 (He et al. 2020). According to this background information, the aim of the current study was to evaluate the cardioprotective effect of NRG against ischemia/reperfusion injury in aged rats.
Materials and methods
Compounds
NRG (Cat#10236-47-2, > 90% purity), D-GAL (Cat#59-23-4, > 99% purity), and Sodium pentobarbital (Cat#57-33-0) were purchased from Merck Company, Germany. Sodium chloride (NaCl), potassium chloride (KCl), sodium bicarbonate (NaHCO3), monopotassium phosphate (KH2PO4), magnesium sulfate (MgSO4), glucose, calcium chloride (CaCl2) were bought from the Merck Company, Germany.
Animals and experimental design
Forty-two Wistar male rats (300 ± 20 g) were obtained from the School of pharmacy, Kermanshah University of Medical Sciences, Kermanshah, Iran. Animals were housed in a temperature (25 °C ± 2 °C) and relative humidity of 50% controlled room under a 12:12 light/dark cycle with free access to pelleted rat chow (Behparvar®, Tehran, Iran) and water. Aging causes a significant reduction in female estrogen levels (Korzick and Lancaster 2013). Aging has been reported to decrease ischemic tolerance in heart, resulting from a reduction in estrogen levels in females (Korzick and Lancaster 2013). In line with this, estrogen has been suggested as a confounding factor in the effectiveness of cardioprotective agents against I/R injury in rodents (Korzick and Lancaster 2013). Therefore, only male rats were used in this study. This study was approved by the Animal Ethics Committee of Kermanshah University of Medical sciences (Ethics Committee permission No. IR.KUMS.REC.1397.902).
After seven days of acclimatization, rats were randomly divided into following groups (n = 6-7 in each group): control, D-GAL, D-Gal + NRG40, D-GAL + NRG100, NRG40, and NRG100. The control group received 0.9% normal saline (1 mL/kg/day, intraperitoneally). Group 2 rats received D-GAL (150 mg/kg, intraperitoneally) for eight weeks (Maharajan and Cho 2021). Group 3 received D-GAL (150 mg/kg/day, intraperitoneally) and NRG (40 mg/kg/day, via oral gavage) for eight weeks. Group 4 received D-GAL (150 mg/kg/day, intraperitoneally) and NRG (100 mg/kg/day, via oral gavage). Group 5 received NRG (40 mg/kg/day, via oral gavage) for eight weeks. Group 6 received NRG (100 mg/kg/day, via oral gavage) for eight weeks. D-GAL and NRG was prepared by dissolving in 0.9% normal saline.
At the end of experimental period, blood (4–5 mL) was immediately collected from the abdominal aorta in rats anesthetized with sodium pentobarbital (60 mg/kg of body weight, intraperitoneally). Then serum (1.5–2 mL) was separated through centrifugation at 4000 rpm for 10 min and stored at −20 ºC until used for biochemical analysis.
Langendorff isolated heart experiments
The Langendorff isolated heart model was used to evaluate the effect of NRG on myocardial injury in ischemic-aged rats. Animals were anesthetized with sodium pentobarbital and their hearts were rapidly separated. After aortic cannulation, the hearts were mounted on a Langendorff apparatus and were retrograde perfused with Krebs solution (118 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 11 mM glucose, and 1.2 mM CaCl2, pH 7.4) at a constant hydrostatic pressure of 60 mm Hg (95% oxygen and 5% carbon dioxide at 37 ºC). Isolated hearts were equilibrated for 15 min (Shackebaei et al. 2022).
The left ventricle function was measured using an intraventricular water-filled balloon connected to a pressure transducer (MLT 844; AD Instruments, New South Wales, Australia). Left ventricular end diastolic pressure (LVEDP) was adjusted by nearly 5–10 mmHg by the volume of the balloon. Cardiac function parameters, including heart rate (HR, beats/minute), left ventricular systolic pressure (LVSP), left ventricular developed pressure (LVDP = LVSP – LVEDP, mm Hg), rate pressure product (RPP = LVDP × HR), and as well as minimum and maximum rate of left ventricular pressure (± dp/dt) were recorded and documented by the Power Lab system and Lab Chart 5 software (AD Instruments, Australia). Coronary effluent was collected per minute during the experiment for the measurement of coronary flow (CF). Isolated rat hearts were subjected to 40 min no-flow global normothermic ischemia (via effluent clamping) followed by 45 min of reperfusion, according to previous studies (Shackebaei et al. 2022). The level of maximum contracture (MC), as a maximum rise in LVEDP, was detected after the onset of ischemia in heart of aged rats. The recovery percentage of cardiac function (± dp/dt ratio and RPP ratio) was recorded at the 45th minute of reperfusion to the 15th minute of baseline (Fig. 1). Finally, the heart was removed and weighed. Cardiac hypertrophy was regarded as the heart weight (HW, g)/body weight (BW, g) ratio. Heart tissue was stored at −20 °C until used for SOD activity analysis (Zarei et al. 2023).
Colorimetric assay
Cardiac damage was assessed using the determination of lactate dehydrogenase (LDH) and creatine kinase (CK-MB) levels in serum. The serum level of LDH and CK-MB was detected by specific LDH and CK-MB kits (Pars Azmoon, Tehran, Iran). They were determined by mixing the reagents and serum samples, incubating at 37 C for 5 min, and measuring the absorbance at 340 nm with an ELISA reader following the protocol of the manufacturer. Levels were expressed in units per liter of sample (U/L) (Zarei et al. 2023).
Heart samples were homogenized with ice-cold phosphate buffer saline (PBS) (pH 7.4) for the determination of SOD activity by a colorimetric method (Kiazist SOD kit, Kiazist, Iran) at 570 nm according to the instruction of the manufacture. A unit of SOD activity was calculated as the amount of SOD that reduced the resazurin production by 50%. Data were presented as unit per milligram of tissue (U/mg tissue) (Yarmohammadi et al. 2023).
Enzyme-linked immunosorbent assay (ELISA)
The tumor necrosis factor-alpha (TNF-α) was measured to indicate the levels of intracellular inflammation by a commercial ELISA kit (Karmania Pars Gene, Kerman, Iran), following the instruction of the manufacture (Shackebaei et al. 2022).
Statistical analysis
Experimental data were analyzed with the SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard deviation (SD). The statistical differences between groups were compared with one-way analysis of variance (ANOVA) followed with Tukey-Kramer post hoc test. Moreover, p < 0.05 was statistically considered as significant.
Results
Effect of NRG on cardiac hypertrophy
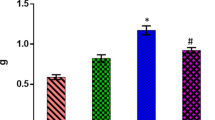
Cardiac hypertrophy was significantly increased in D-GAL group compared to control group (p <0.05). NRG significantly (p < 0.01) decreased myocardial hypertrophy in comparison to the D-GAL group but only at the low-dose of 40 mg/kg (Fig. 2).
Effect of NRG on the level of maximum contracture
The level of MC in heart of aged rats was recorded during the global ischemia period at 37 °C. D-GAL-exposed rats showed a significant increase in MC level as compared to the control group (p < 0.05). NRG 40 and 100 mg/kg significantly decreased the MC level compared to the D-GAL group (p < 0.05 and p < 0.01, respectively) (Fig. 3).
Effect of NRG on hemodynamic parameters
Baseline period
Values of cardiac function variables obtained during the pre-ischemic phase were summarized in Table 1. Exposure to the D-GAL resulted in a significant reduction in HR compared to the control group (p < 0.01), whereas no significant alterations were revealed in the other parameters, including LVDP, CF, ± dp/dt and RPP. Co-treatment of NRG at 40 and 100 mg/kg and D-GAL raised HR, however, the improvement was not significant compared with the D-GAL group. NRG treatment at 100 mg/kg in the D-GAL group significantly increased RPP compared to the D-GAL group.
Reperfusion period
As shown in Table 1, LVDP (p < 0.05), ± dp/dt (p < 0.05), and RPP (p < 0.05) changed following D-GAL exposure at the end of reperfusion. NRG at 40 mg/kg exhibited a significantly decrease in LVDP (p < 0.05) compared to the D-GAL group. Furthermore, NRG treatment at 100 mg/kg significantly modulated the LVDP (p < 0.01), dp/dt Max (p < 0.01), and RPP (p < 0.05) values in comparison to the D-GAL group.
Effect of NRG on cardiac function recovery percentage
In the D-GAL group, the percentage of recovery of dP/dt Max (p < 0.001), dP/dt Min (p < 0.05), and RPP (p < 0.05) were low compared to the control group. NRG treatment at 40 and 100 mg/kg significantly enhanced post-ischemic recovery of cardiac functional parameters in isolated hearts. This enhancement by NRG 100 mg/kg was especially manifested by increased recovery of dP/dt Max (p < 0.05), dP/dt Min (p < 0.05), and RPP (p < 0.05) compared with D-GAL group (Figs. 4, 5, and 6). In comparison, no change was observed at the low dose of NRG (40 mg/kg).
Effect of NRG on dP/dt Max recovery percentage. Data were analyzed with one-way ANOVA followed with Tukey-Kramer post hoc test and expressed as the mean ± SD (n = 6-7). ***p < 0.001 compared with control group. #p < 0.05 compared with D-GAL group. D-GAL, D-galactose; dP/dt Max, the maximum rate of left ventricular pressure; NRG, naringin
Effect of NRG on dP/dt Min recovery percentage. Data were analyzed with one-way ANOVA followed with Tukey-Kramer post hoc test and expressed as the mean ± SD (n = 6-7). *p < 0.05 compared with control group. #p< 0.05 compared with D-GAL group. D-GAL, D-galactose; dp/dt Min, minimum rate of left ventricular pressure; NRG, naringin
Effect of NRG on RPP recovery percentage. Data were analyzed with one-way ANOVA followed with Tukey-Kramer post hoc test and expressed as the mean ± SD (n = 6-7). *p < 0.05 compared with control group. #p < 0.05 compared with D-GAL group. D-GAL, D-galactose; NRG, naringin; RPP, rate pressure product
Effect of NRG on the serum level of LDH
LDH, as a non-specific marker for myocardial injury (Dumea et al. 2022), was evaluated in the current study. As shown in Fig. 7, elevated serum LDH observed in the D-GAL group (p < 0.05) was decreased by NRG at 40 mg/kg (p < 0.01) and NRG at 100 mg/kg (p < 0.01).
Effect of NRG on the serum level of CK-MB
Our findings indicated that CK-MB level enhanced dramatically in the D-GAL rats compared to the control group (p < 0.05). Treatment with NRG at 40 and 100 mg/kg significantly reduced CK-MB level in rats treated by D-GAL (p < 0.01, Fig. 8).
Effect of NRG on the serum level of TNF-α
TNF-α is mainly secreted by macrophages and used for the studies of aging (Zhong et al. 2020). The serum level of TNF-α in animals treated by D-GAL was significantly increased compared with the control rats (p < 0.01). An increase in the level of TNF-α was reversed significantly by NRG 40 mg/kg (p < 0.05) and NRG 100 mg/kg (p < 0.001) (Fig. 9).
Effect of NRG on serum level of TNF-α. Data were analyzed with one-way ANOVA followed with Tukey-Kramer post hoc test and expressed as the mean ± SD (n = 6-7). **p < 0.01 compared with control group. #p < 0.05 and ###p < 0.001 compared with D-GAL group. D-GAL, D-galactose; NRG, naringin; TNF-α, tumor necrosis factor alpha
Effect of NRG on the SOD activity
The SOD activity was assessed to illustrate the antioxidant effect of NRG in heart injury. SOD activity was significantly (p < 0.01) reduced by D-GAL exposure compared to the control group. NRG, however, significantly improved the D-GAL-reduced SOD activity at dose of 40 mg/kg (p < 0.01) (Fig. 10).
Effect of NRG on SOD activity in heart tissue. Data were analyzed with one-way ANOVA followed with Tukey-Kramer post hoc test and expressed as the mean ± SD (n = 6-7). **p < 0.01 compared with control group. ##p < 0.01 compared with D-GAL group. D-GAL, D-galactose; NRG, naringin; SOD, superoxide dismutase
Discussion
Our finding represents that NRG improved cardiac dysfunction in aged rats through anti-oxidative and anti-inflammatory mechanisms. Aging is a critical risk factor that increases susceptibility to developing CVDs (Martín-Fernández and Gredilla 2016). Ischemic heart disease is one of the main causes of premature mortality among the elderly population (Martín-Fernández and Gredilla 2016). NRG treatment may be a promising approach against aging-heart complications. Herein, elevated cardiac SOD activity by NRG suggests a possible involvement of its anti-oxidative effect in preventing oxidative stress mediated by D-GAL. The anti-inflammatory effect of NRG was shown with a reduction in the serum level of TNF-α in aged rats. The serum levels of LDH and CK-MB, cardiac markers for diagnosis of early MI, were decreased by NRG treatment. It also diminished cardiac hypertrophy. The most important cardiac hemodynamic parameters, including HR, LVDP, ±dP/dt, and RPP, were normalized with NRG during baseline and reperfusion period. Moreover, the recovery percentage of cardiac function in NRG-treated hearts was improved in comparison to aged hearts. Generally, our data demonstrated that NRG reduced the severity of cardiac aging by modulating oxidative stress and inflammation.
Oxidative stress and inflammation are strongly associated with the pathogenesis of vascular aging, especially arterial stiffness (Mikael et al. 2017). Aging-related arterial stiffness leads to different adverse hemodynamic consequences such as a rise in systolic blood pressure, which promotes left ventricular hypertrophy and dysfunction (Yucel et al. 2015; Vatner et al. 2021; Castelli et al. 2023). An abnormal accumulation of D-GAL, a monosaccharide sugar, in the body could accelerate the aging process in different organs such as the heart (Bo‐Htay et al. 2018). According to past studies, the current study used D-GAL (150 mg/kg/day for 8 weeks) to generate a useful model to study heart aging (Bo‐Htay et al. 2018; Azman and Zakaria 2019). Oxidative and inflammation damage mediated by excessive D-GAL metabolism is a main factor in accelerating mechanisms that contribute to aging (Cheng et al. 2021; Chen et al. 2022). SOD has an important antioxidant effect against oxidative stress by detoxifying toxic O2 in cells (Montllor-Albalate et al. 2022). It has been documented that SOD depletion can result in oxidative stress (Montllor-Albalate et al. 2022). Here, we showed a significant decrease in cardiac SOD activity during D-GAL exposure, which represented cardiac oxidative stress. Numerous studies revealed that NRG ameliorated CVDs, such as diabetic cardiomyopathy and ischemic heart diseases, by up-regulating antioxidant pathways (Gelen and Şengül 2020; Viswanatha et al. 2022). In this study, NRG modulated SOD activity which was altered by D-GAL. Oxidative stress is important for the development of cardiac hypertrophy in rats exposed to D-GAL (Bo‐Htay et al. 2018). Our findings indicated that pathological cardiac hypertrophy, as a result of increased muscle mass, was elevated in D-GAL-treated rats. Moreover, D-GAL-induced myocardial injury was reflected in this study by the elevation of cardiac enzymes (LDH, CK-MB) in the serum. Park et al. reported the protective effect of NRG against fructose-induced cardiac hypertrophy by suppressing mitochondrial ROS generation and mitochondrial dysfunction (Park et al. 2018). NRG also mitigated cardiac hypertrophy by inhibiting oxidative stress in diabetic rats (Adebiyi et al. 2016). Our study has confirmed the reduction of cardiac hypertrophy and cardiac enzyme serum levels following NRG treatment, probably through increasing cardiac SOD activity.
Moreover, cardiac structural alteration during the aging process is associated with heart rate reduction (Hosseini et al. 2020). The relationship between oxidative stress and reduced HR has been proven in different diseases (Lee et al. 2020). In the present study, NRG normalized the D-GAL-reduced heart rate at the baseline period, which was probably by its antioxidant effect.
It has been demonstrated that long-term administration of D-GAL resulted in activating the inflammatory pathways (Azman and Zakaria 2019). The therapeutic benefits of NRG in various inflammatory related diseases have been reported (Adebiyi et al. 2016; Viswanatha et al. 2022). NRG attenuated the cardiac inflammation in the lipopolysaccharide-induced sepsis (Xianchu et al. 2016). Volkan study has shown an anti-inflammatory effect of NRG on the cisplatin-induced cardiac damage (Gelen and Şengül 2020). Our results revealed that level of inflammatory marker TNF-α was increased in aging group that was effectively reversed following NRG treatment.
It has been reported that aging increases susceptibility to myocardial I/R injury and decreases cardiac function recovery after damage (Dong et al. 2023). Our findings indicated that D-GAL injection resulted in a cardiac I/R injury, which was probably through a reduction in the activity of SOD in the heart tissue in aged rats. Following I/R injury, cardiac function was improved by NRG treatment which may result from the elevation of the cardiac SOD activity.
Limitations
Our findings did not reveal the exact molecular mechanisms involved in the protective effects of NRG. Thus, in future studies, it would be better to determine the pathways through which NRG exerts its antioxidant and anti-inflammatory effects in aging rats. Moreover, to better understand the protective effects of NRG on heart function, we could record the electrocardiogram.
In sub-chronic and chronic oral toxicity studies, different daily doses of NRG were well-tolerated and did not cause toxic clinical symptoms (Li et al. 2020). However, in the current study, NRG at 100 mg/kg showed a statistically significant effect on cardiac hypertrophy compared with the control group. This difference may be due to the low statistical power of our small sample size. In future studies, NRG at multiple doses should be examined further to determine its potential effects on the structure and function of the heart.
Conclusion
Generally, the results from this study evidence the preclinical effectiveness of NRG in both ischemic and reperfusion phases of aged hearts. This effect may be described by the decreasing oxidative stress and inflammatory pathways. Therefore, NRG supplementation could be used as a promising treatment strategy for cardiovascular aging. Further studies, however, are required to confirm the cardioprotective properties of NRG. The no-observed-adverse-effect level (NOAEL) of NRG in rats is reported to be greater than 1250 mg/kg, which is equal to 200 mg/kg in humans (Li et al. 2013). In current study, rats were orally exposed to NRG for 8 weeks at dosages of 40 and 100 mg/kg body weight, both of which were lower than the NOAEL. Clinical trials are needed to elucidate the safety and effectiveness of NRG as a cardioprotective drug in aging.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BW:
-
Body weight
- CaCl2:
-
Calcium chloride
- CF:
-
Coronary flow
- CK-MB:
-
Creatine kinase-MB
- CVDs:
-
Cardiovascular diseases
- D-GAL:
-
D-Galactose
- dp/dt Max :
-
Maximum rate of left ventricular pressure
- dp/dt Min :
-
Minimum rate of left ventricular pressure
- ELISA:
-
Enzyme-linked immunosorbent assay
- GCL:
-
Glutamate cysteine ligase
- GPx:
-
Glutathione peroxidase
- HR:
-
Heart rate
- HO-1:
-
Heme oxygenase-1
- HW:
-
Heart weight
- I/R:
-
Ischemia/reperfusion
- KCl:
-
Potassium chloride
- KH2PO4:
-
Potassium dihydrogenephosphate
- LDH:
-
Lactate dehydrogenase
- LVDP:
-
Left ventricular developed pressure
- LVEDP:
-
Left ventricular end diastolic pressure
- LVSP:
-
Left ventricular systolic pressure
- MC:
-
Maximum contracture
- MgSO4:
-
Magnesium sulfate
- MI:
-
Myocardial infarction
- NaCl:
-
Sodium chloride
- NaHCO3:
-
Sodium bicarbonate
- NOAEL:
-
No-observed-adverse-effect level
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NRG:
-
Naringin
- PBS:
-
Phosphate buffer saline
- ROS:
-
Reactive oxygen species
- RPP:
-
Rate pressure product
- SD:
-
Standard deviation
- SEM:
-
Standard error of measurement
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
- U/L:
-
Units per liter of sample
- U/mg:
-
Unit per milligram
References
Adebiyi AO, Adebiyi OO, Owira PMO (2016) Naringin mitigates cardiac hypertrophy by reducing oxidative stress and inactivating c-Jun nuclear kinase-1 protein in type I diabetes. J Cardiovasc Pharmacol 67:136–144. https://doi.org/10.1097/FJC.0000000000000325
Aki̇n AT, El Bechir ML, Kaymak E, et al (2022) Anti-inflammatory and anti-apoptotic effects of naringin on bacterial endotoxin-induced small intestine damage in rats. Cukurova Med J 47:1137–1146. https://doi.org/10.17826/cumj.1124641
Alotaibi F (2023) Naringenin alters the pharmacokinetics of ranolazine in part through the inhibition of cytochrome P450 (3A4) and P-glycoprotein. Futur J Pharm Sci 9:23. https://doi.org/10.1186/s43094-023-00477-1
Aman Y, Schmauck-Medina T, Hansen M et al (2021) Autophagy in healthy aging and disease. Nat Aging 1:634–650. https://doi.org/10.1038/s43587-021-00098-4
Azman KF, Zakaria R (2019) d-Galactose-induced accelerated aging model: an overview. Biogerontology 20:763–782. https://doi.org/10.1007/s10522-019-09837-y
Bhia M, Motallebi M, Abadi B et al (2021) Naringenin nano-delivery systems and their therapeutic applications. Pharmaceutics 13:291. https://doi.org/10.3390/pharmaceutics13020291
Bo-Htay C, Palee S, Apaijai N et al (2018) Effects of d-galactose-induced ageing on the heart and its potential interventions. J Cell Mol Med 22:1392–1410. https://doi.org/10.1111/jcmm.13472
Castelli R, Gidaro A, Casu G, et al (2023) Aging of the arterial system. Int J Mol Sci 24:. https://doi.org/10.3390/ijms24086910
Chen RC, Sun GB, Wang J et al (2015) Naringin protects against anoxia/reoxygenation-induced apoptosis in H9c2 cells via the Nrf2 signaling pathway. Food Funct 6:1331–1344. https://doi.org/10.1039/c4fo01164c
Chen X, Yu J, Zheng L et al (2022) Quercetin and lycopene co-administration prevents oxidative damage induced by d-galactose in mice. Food Biosci 50:102042. https://doi.org/10.1016/j.fbio.2022.102042
Cheng J, Ren C, Cheng R et al (2021) Mangiferin ameliorates cardiac fibrosis in D-galactose-induced aging rats by inhibiting TGF-β/p38/MK2 signaling pathway. Korean J Physiol Pharmacol 25:131–137. https://doi.org/10.4196/kjpp.2021.25.2.131
de Almeida AJPO, de Almeida Rezende MS, Dantas SH et al (2020) Unveiling the role of inflammation and oxidative stress on age-related cardiovascular diseases. Oxid Med Cell Longev 2020:1954398. https://doi.org/10.1155/2020/1954398
Dong M, Chen D, Zhu Y et al (2023) Impaired regulation of MMP2/16–MLCK3 by miR-146a-5p increased susceptibility to myocardial ischaemic injury in aging mice. Cardiovasc Res 119:786–801. https://doi.org/10.1093/cvr/cvac104
Dumea E, Lazar M, Barbu EC et al (2022) Pulmonary involvement in SARS-CoV-2 Infection Estimates Myocardial Injury Risk. Medicina (B Aires) 58:1436. https://doi.org/10.3390/medicina58101436
Ferrucci L, Fabbri E (2018) Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15:505–522. https://doi.org/10.1038/s41569-018-0064-2
Franceschi C, Garagnani P, Morsiani C et al (2018) The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med 5:61. https://doi.org/10.3389/fmed.2018.00061
Fuhr U, Kummert AL (1995) The fate of naringin in humans: a key to grapefruit juice-drug interactions? Clin Pharmacol Ther 58:365–373. https://doi.org/10.1016/0009-9236(95)90048-9
Gelen V, Şengül E (2020) Antioxidant, anti-inflammatory and antiapoptotic effects of Naringin on cardiac damage induced by cisplatin. Indian J Tradit Knowl 19:459–465. https://doi.org/10.56042/ijtk.v19i2.35371
Ghanbari-Movahed M, Jackson G, Farzaei MH, Bishayee A (2021) A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front Pharmacol 12:639840. https://doi.org/10.3389/fphar.2021.639840
He F, Ru X, Wen T (2020) NRF2, a transcription factor for stress response and beyond. Int J Mol Sci 21:4777. https://doi.org/10.3390/ijms21134777
HeidaryMoghaddam R, Samimi Z, Moradi SZ et al (2020) Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol 887:173535. https://doi.org/10.1016/j.ejphar.2020.173535
Hosseini L, Vafaee MS, Badalzadeh R (2020) Melatonin and nicotinamide mononucleotide attenuate myocardial ischemia/reperfusion injury via modulation of mitochondrial function and hemodynamic parameters in aged rats. J Cardiovasc Pharmacol Ther 25:240–250. https://doi.org/10.1177/1074248419882002
Kleinbongard P, Bøtker HE, Ovize M et al (2020) Co-morbidities and co-medications as confounders of cardioprotection-does it matter in the clinical setting? Br J Pharmacol 177:5252–5269. https://doi.org/10.1111/bph.14839
Korzick DH, Lancaster TS (2013) Age-related differences in cardiac ischemia–reperfusion injury: effects of estrogen deficiency. Pflügers Arch J Physiol 465:669–685. https://doi.org/10.1007/s00424-013-1255-7
Lee C-H, Shin H-W, Shin D-G (2020) Impact of oxidative stress on long-term heart rate variability: linear versus non-linear heart rate dynamics. Hear Lung Circ 29:1164–1173. https://doi.org/10.1016/j.hlc.2019.06.726
Li F, Zhan Z, Qian J et al (2021) Naringin attenuates rat myocardial ischemia/reperfusion injury via PI3K/Akt pathway-mediated inhibition of apoptosis, oxidative stress and autophagy. Exp Ther Med 22:1–8. https://doi.org/10.3892/etm.2021.10243
Li P, Wang S, Guan X et al (2013) Acute and 13weeks subchronic toxicological evaluation of naringin in Sprague-Dawley rats. Food Chem Toxicol 60:1–9. https://doi.org/10.1016/j.fct.2013.07.019
Li P, Wu H, Wang Y et al (2020) Toxicological evaluation of naringin: acute, subchronic, and chronic toxicity in Beagle dogs. Regul Toxicol Pharmacol 111:104580. https://doi.org/10.1016/j.yrtph.2020.104580
Liberale L, Montecucco F, Tardif J-C et al (2020) Inflamm-ageing: the role of inflammation in age-dependent cardiovascular disease. Eur Heart J 41:2974–2982. https://doi.org/10.1093/eurheartj/ehz961
Maharajan N, Cho G-W (2021) Camphorquinone promotes the antisenescence effect via activating AMPK/SIRT1 in stem cells and D-galactose-induced aging mice. Antioxidants 10:1916. https://doi.org/10.3390/antiox10121916
Martín-Fernández B, Gredilla R (2016) Mitochondria and oxidative stress in heart aging. Age (Omaha) 38:225–238. https://doi.org/10.1007/s11357-016-9933-y
de Mikael RL, de Paiva AMG, Gomes MM et al (2017) Vascular aging and arterial stiffness. Arq Bras Cardiol 109:253–258. https://doi.org/10.5935/abc.20170091
Moghaddam RH, Samimi Z, Moradi SZ et al (2020) Naringenin and naringin in cardiovascular disease prevention: a preclinical review. Eur J Pharmacol 887:173535. https://doi.org/10.1016/j.ejphar.2020.173535
Montllor-Albalate C, Kim H, Thompson AE et al (2022) Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc Natl Acad Sci 119:e2023328119. https://doi.org/10.1073/pnas.2023328119
Papaconstantinou J (2019) The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 8:1383. https://doi.org/10.3390/cells8111383
Park JH, Ku HJ, Kim JK et al (2018) Amelioration of high fructose-induced cardiac hypertrophy by naringin. Sci Rep 8:9464. https://doi.org/10.1038/s41598-018-27788-1
Randhawa PK, Bali A, Virdi JK, Jaggi AS (2018) Conditioning-induced cardioprotection: Aging as a confounding factor. Korean J Physiol Pharmacol Off J Korean Physiol Soc Korean Soc Pharmacol 22:467–479. https://doi.org/10.4196/kjpp.2018.22.5.467
Sanchez-Morate E, Gimeno-Mallench L, Stromsnes K et al (2020) Relationship between diet, microbiota, and healthy aging. Biomedicines 8:287. https://doi.org/10.3390/biomedicines8080287
Shackebaei D, Hesari M, Ramezani-Aliakbari S et al (2022) Gallic acid protects against isoproterenol-induced cardiotoxicity in rats. Hum Exp Toxicol 41:09603271211064532. https://doi.org/10.1177/09603271211064532
Vatner SF, Zhang J, Vyzas C et al (2021) Vascular stiffness in aging and disease. Front Physiol 12:762437. https://doi.org/10.3389/fphys.2021.762437
Viswanatha GL, Shylaja H, Keni R et al (2022) A systematic review and meta-analysis on the cardio-protective activity of naringin based on pre-clinical evidences. Phyther Res 36:1064–1092. https://doi.org/10.1002/ptr.7368
Wu NN, Zhang Y, Ren J (2019) Mitophagy, mitochondrial dynamics, and homeostasis in cardiovascular aging. Oxid Med Cell Longev 2019:9825061. https://doi.org/10.1155/2019/9825061
Xianchu L, Lan PZ, Qiufang L et al (2016) Naringin protects against lipopolysaccharide-induced cardiac injury in mice. Environ Toxicol Pharmacol 48:1–6. https://doi.org/10.1016/j.etap.2016.09.005
Yarmohammadi F, Barangi S, Aghaee-Bakhtiari SH et al (2023) Melatonin ameliorates arsenic-induced cardiotoxicity through the regulation of the Sirt1/Nrf2 pathway in rats. BioFactors. https://doi.org/10.1002/biof.1934
Yucel C, Demir S, Demir M et al (2015) Left ventricular hypertrophy and arterial stiffness in essential hypertension. Bratisl Lek Listy 116:714–718. https://doi.org/10.4149/bll_2015_140
Zarei M, Sarihi A, Zamani A, et al (2023) Mitochondrial biogenesis and apoptosis as underlying mechanisms involved in the cardioprotective effects of Gallic acid against D-galactose-induced aging. Mol Biol Rep 1–10. https://doi.org/10.1007/s11033-023-08670-4
Zhong W, Rao Z, Rao J et al (2020) Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell 19:e13186. https://doi.org/10.1111/acel.13186
Acknowledgements
The authors are grateful to the Kermanshah University of Medical Sciences, Health Technology Institute, Medical Biology Research Center, Kermanshah, Iran, for financial support.
Funding
This work was supported by Kermanshah University of Medical Sciences, Kermanshah, Iran (IR.KUMS.REC.1397.902).
Author information
Authors and Affiliations
Contributions
D. Sh. contributed to data collection and interpretation, and wrote the manuscript, M. H. performed the experiments, and contributed reagents, S. R-A. Contributed reagents, materials, and analysis tools or data, M. P. conceived and designed the experiments, F. Y. contributed to data collection and analysis, and wrote the manuscript, F. R-A. Designed the study, contributed to data collection and interpretation, and wrote the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Animal Ethics Committee of Kermanshah University of Medical sciences (Ethics Committee Permission No. IR.KUMS.REC.1397.902).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shackebaei, D., Hesari, M., Ramezani-Aliakbari, S. et al. Cardioprotective effect of naringin against the ischemia/reperfusion injury of aged rats. Naunyn-Schmiedeberg's Arch Pharmacol 397, 1209–1218 (2024). https://doi.org/10.1007/s00210-023-02692-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02692-2