Abstract
Remote ischemic preconditioning is a well reported therapeutic strategy that induces cardioprotective effects but the underlying intracellular mechanisms have not been widely explored. The current study was designed to investigate the involvement of TRP and especially TRPV channels in remote hind limb preconditioning-induced cardioprotection. Remote hind limb preconditioning stimulus (4 alternate cycles of inflation and deflation of 5 min each) was delivered using a blood pressure cuff tied on the hind limb of the anesthetized rat. Using Langendorff’s system, the heart was perfused and subjected to 30-min ischemia and 120-min reperfusion. The myocardial injury was assessed by measuring infarct size, lactate dehydrogenase (LDH), creatine kinase (CK), LVDP, +dp/dtmax, −dp/dtmin, heart rate, and coronary flow rate. Gadolinium, TRP blocker, and ruthenium red, TRPV channel blocker, were employed as pharmacological tools. Remote hind limb preconditioning significantly reduced the infarct size, LDH release, CK release and improved coronary flow rate, hemodynamic parameters including LVDP, +dp/dtmax, −dp/dtmin, and heart rate. However, gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) significantly attenuated the cardioprotective effects suggesting the involvement of TRP especially TRPV channels in mediating remote hind limb preconditioning-induced cardioprotection. Remote hind limb preconditioning stimulus possibly activates TRPV channels on the heart or sensory nerve fibers innervating the heart to induce cardioprotective effects. Alternatively, remote hind limb preconditioning stimulus may also activate the mechanosensitive TRP and especially TRPV channels on the sensory nerve fibers innervating the skeletal muscles to trigger cardioprotective neurogenic signaling cascade. The cardioprotective effects of remote hind limb preconditioning may be mediated via activation of mechanosensitive TRP and especially TRPV channels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Remote ischemic preconditioning is an intrinsic treatment approach whereby alternate cycles of short ischemic episodes followed by reperfusion to a remote organ (other than heart) protect the target organ (heart) against sustained ischemia-reperfusion injury (Przyklenk and Whittaker 2013; Randhawa et al. 2015). Our own laboratory studies have implicated that delivery of brief episodes of ischemia and reperfusion to the organs including kidney (Kant et al. 2008; Diwan et al. 2008), abdominal aorta, and mesenteric arteries increases the ischemic tolerance of the tissues against prolonged ischemic insult (Rehni et al. 2007). Currently, remote limb preconditioning treatment strategy is increasingly being recognized worldwide due to its non-invasive nature and greater ischemic tolerance of the skeletal tissues in comparison to the other vital organs of the body (Kalogeris et al. 2012). It is clinically applicable and has been employed to reduce post-operative myocardial injury in patients undergoing various surgical interventions including coronary artery bypass graft surgery, abdominal aortic aneurysm repair, and heart valve surgery etc. (Ali et al. 2010). Therefore, in the present study, remote hind limb preconditioning (a blood pressure cuff tied around the hind limb) stimulus has been employed to investigate the possible involvement of mechanosensitive channels in mediating remote hind limb preconditioning-induced cardioprotective effects.
Mechanosensitive channels are membrane proteins capable of mechanotransduction and have the ability to transduce external mechanical stimuli into intracellular signals (Haswell et al. 2011). Opening of these channels allows passage of ions, including Na+ and Ca2+, and regulates a variety of cellular functions including osmoregulation, touch, hearing, etc. (Hamill and Martinac 2001). TRP and TRPV channels are sub-type of mechanosensitive ion channels that are relatively more permeable to Ca2+ ions and also sense broad spectrum of chemical and physical stimuli including heat, low pH, and hypotonicity (Zheng 2013; Randhawa and Jaggi 2015). Mechanosensitive channels are widely distributed in the body regions such as the heart (Kim 1992; Friedrich et al. 2012), brain, colon, sensory nerves, lungs, urinary bladder, prostate glands, and vascular smooth muscles (Davis et al. 1992; Su et al. 2000; Choi et al. 2015). In the cardiovascular system, mechanosensitive channels are localized on vascular endothelial cells, aorta, atria, ventricles, coronary blood vessels (Craelius et al. 1988; Nilius et al. 1997; Lehoux and Tedgui 2003), and sensory nerves innervating the heart (Shenton and Pyner 2014). TRPV channels are also located on the heart, sensory nerve endings, sciatic nerve, and skeletal muscles (Schultz 2003; Fischer et al. 2003).
Apart from the well-documented functional role of mechanosensitive channels in osmoregulation, pain, and hearing regulation, the studies have shown the important role of these channels in regulating cardiovascular function (Bett and Sachs 1997; Friedrich et al. 2012). Previous studies have documented the role of these channels in mediating ischemia-reperfusion injury (Hao et al. 2010), ischemic preconditioning (Gysembergh et al. 1998), and post-conditioning (Babiker et al. 2010) indicating the fundamental role of these channels in regulating cardioprotective signaling. TRPV channels are primarily transducers of pain and have the ability to sense pain sensation induced by various chemical/thermal/mechanical stimuli (Levine and Alessandri-Haber 2007). Besides, activation of these channels during ischemic preconditioning (Zhong and Wang 2007; Lu et al. 2014) and post-conditioning elicits cardioprotective effects indicating their important role in mediating cardioprotection. However, the role of TRP and TRPV channels in mediating remote hind limb preconditioning-induced cardioprotection has not been described yet. Therefore, the present study was designed to investigate the possible involvement of TRP and TRPV channels in mediating remote hind limb preconditioning-induced cardioprotection in rat hearts.
Material and methods
Animals
Wistar albino rats (150–200 g) were employed for the present study. The animals were procured from Lala Lajpat Rai University of Veterinary and Animal Science, Hisar, and were fed on standard laboratory diet (Kisan Feeds Ltd., Chandigarh, India) and had free access to water and food. The experimental protocol was approved by Institutional Animal Ethics Committee, and care of the animals was carried out as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India (Reg. No. 107/99/CPCSEA/2013–37).
Drugs and chemicals
Ruthenium red and gadolinium sulfate were procured from S D Fine-Chem Limited, Maharashtra (India). Creatine phosphokinase (CK) estimation kit was procured from Agappe Diagnostics Limited, Kerala (India). All other chemicals were of analytical grade and were obtained from S D Fine-Chem Limited, Maharashtra (India). Both ruthenium red and gadolinium sulfate were dissolved in distilled water. The doses of ruthenium red and gadolinium sulfate were selected based on the previous literature reports (Du et al. 2010; Kyriakou et al. 2013; Chen et al. 2016) and pilot studies. Previous reports have indicated that gadolinium in salt form (10 mg/kg) protects the liver against acute radiation-induced damage (Du et al. 2010), acute-cadmium induced toxicity (Kyriakou et al. 2013), and ischemia-reperfusion induced injury (Chen et al. 2016). However, if the dose of gadolinium salt increases, i.e., 0.07–0.35 mmol/kg (40–210 mg/kg), it may induce hepatocellular and splenic necrosis in rats (Spencer et al. 1997). The dose of ruthenium red (5 mg/kg) as a TRPV channel blocker has also been selected based on the previous literature reports and pilot studies (Perretti and Manzini 1993). However, if the dose of ruthenium red is increased to ≥20 mg/kg, it may induce hepatotoxicity in rats (Ortiz et al. 1992).
Remote hind limb preconditioning
Rat was anesthetized with thiopental sodium (35 mg kg−1, i.p.). Hind limb preconditioning stimulus was delivered non-invasively by tying the blood pressure cuff at the inguinal level, inflating with air up to 150 mm of Hg to produce ischemia in the limb followed by release of the pressure to reperfuse the tissue. Four brief consecutive cycles of ischemia-reperfusion were delivered by alternatively inflating (up-to supra-systolic pressure levels) and deflating the blood pressure cuff for 5 min each (Wever et al. 2011; Zhang et al. 2012).
Retrograde heart perfusion according to Langendorff’s technique
Rat was heparinized (500 IU, i.p.) 20 min before sacrificing the animal, and the heart was rapidly excised and immediately mounted on the Langendorff apparatus (Langendorff 1885). In the Langendorff perfusion apparatus, the heart is perfused retrogradely by cannulating the aorta. The perfusate flows retrogradely (opposite to normal physiologic flow) down the aorta and closes the aortic valve. The perfusate passes through the vascular bed before being drawn off via the coronary veins to the coronary sinus in the right atria without filling the ventricular chambers with the perfusate. Langendorff perfusion apparatus is simple to operate, exhibits reproducible results, and has exquisite ability to study isolated organs including heart. Isolated heart was retrogradely perfused at constant pressure of 70 mmHg with Kreb’s Henseleit solution, pH 7.4, maintained at 37 °C, bubbled with 95 % O2 and 5 % CO2. Flow rate was between 7 and 8 ml/min. The heart was enclosed in a double wall jacket, the temperature of which was maintained by circulating water maintained at 37 °C. Global ischemia was produced for 30 min by blocking the inflow of Krebs Henseleit solution, and it was followed by reperfusion for 120 min. The left ventricular developed pressure, +dp/dtmax, −dp/dtmin and heart rate was recorded using a fluid filled latex balloon that was inserted into the left ventricle and was connected to a pressure transducer (AD instruments, Australia). These parameters were assessed before subjecting to global ischemia (basal) and during different times of reperfusion, i.e., immediate, 5, 30, 60, and 120 min. Coronary effluent was also collected before global ischemia (basal) and during different times of reperfusion, i.e., immediate, 5 and 30 min after reperfusion for biochemical estimations. Coronary flow rate was also measured at different time intervals to assess the degree of injury to coronary vasculature.
Estimation of infarct size
The heart was removed from the Langendorff apparatus. The heart was kept overnight at 0 °F (−18 °C), and the frozen heart was sliced into uniform sections of 2–3-mm thickness. The slices were incubated in 1 % triphenyltetrazolium chloride (TTC) at 37 °C in 0.2 M Tris buffer (pH 7.4) for 20 min. TTC is converted to red formazone pigment by NADH and dehydrogenase enzyme, and therefore, the viable cells are stained deep red. On the other hand, the infarcted cells remained unstained or dull yellow due to loss of enzymes and cofactors (Vivaldi et al. 1985). The heart slices were placed between two glass plates and a transparent plastic grid with 100 squares in 1 cm2 was placed. The average area of slices was calculated by counting the number of squares on either side. Accordingly, the numbers of squares falling over non-stained dull yellow area and red stained area were counted. The infarct size was expressed as percentage of average area on both slides of the slice. Afterwards, all the infarcted dull yellow parts of heart slices were separated from the red stained slices. Infarct size was expressed as a percentage of total heart weight (Diwan et al. 2008). A blind analysis of infarct size was carried out by both weight method and volume method.
Evaluation of lactate dehydrogenase
Lactate dehydrogenase (LDH) levels were estimated in the samples of coronary effluent collected at different times of reperfusion i.e. immediate, 5 min and 30 min after reperfusion using 2, 4 DNPH method. Limit of detection of LDH was 150 IU/L (King 1959).
Evaluation of creatine kinase
The CK levels were estimated in the samples of coronary effluent collected at different times of reperfusion, i.e., immediate and 5 min after reperfusion using a commercial diagnostic kit (Guzy 1977). Limit of detection of creatine kinase was 20 IU/L.
Experimental protocol
The following groups, each comprising six Wistar albino rats, were employed in the present study:
-
Group I (Control): Rat heart was isolated and perfused on the Langendorff system and was allowed to stabilize for 10 min. Afterwards, the heart was subjected to global ischemia for 30 min followed by reperfusion for 120 min.
-
Group II (Sham): A blood pressure cuff was tied on the hind limb of the anesthetized rat, without inflation or deflation. After 40 min, the rat heart was isolated and perfused on the Langendorff apparatus. The heart was allowed to stabilize for 10 min followed by subjection to global ischemia for 30 min and 120 min reperfusion.
-
Group III (Remote limb preconditioning): A blood pressure cuff was tied on the hind limb of anesthetized rat and was inflated to produce ischemia in the hind limb and deflated to reperfuse the limb. Four episodes of ischemia and reperfusion, each comprising of 5 min of inflation and 5 min of deflation, were used to produce remote limb preconditioning. Immediately after the last episode of remote preconditioning, the heart was isolated and subjected to 30 min global ischemia followed by 120 min reperfusion as described in group I.
-
Groups IV and V (Ruthenium red 4 and 8 mg kg −1 i.p. in remote hind limb preconditioning): Rat was administered 4 or 8 mg kg−1 ruthenium red 30 min before carrying out remote hind limb preconditioning. After the last episode of remote hind limb preconditioning, the heart was subjected to ischemia and reperfusion as described in group I.
-
Group VI and Group VII (Gadolinium 7.5 and 15 mg kg −1 i.p. in remote hind limb preconditioning): Rat was administered gadolinium 7.5 or 15 mg kg−1 30 min before carrying out remote hind limb preconditioning. After remote hind limb preconditioning, the heart was subjected to ischemia and reperfusion as described in group I.
-
Group VIII (Vehicle in remote hind limb preconditioning): Rat was administered saline 30 min before isolating and perfusing the rat heart on Langendorff apparatus. Afterwards, the heart was subjected to ischemia and reperfusion as described in group I.
Statistical analysis
The results were expressed as mean ± standard deviation (S.D.). Statistical analysis for LDH, CK, LVDP, +dp/dtmax, −dp/dtmin, and coronary flow rate and heart rate was done using two way repeated ANOVA, while the results of infarct size were analyzed by one-way ANOVA followed by Bonferroni’s test for post hoc analysis. A value of p < 0.05 was considered to be statistically significant.
Results
Administration of vehicle in remote hind limb preconditioning did not affect infarct size, heart rate, LDH release, CK release, LVDP, +dp/dtmax, −dp/dtmin, and coronary flow rate.
Effects of ischemia-reperfusion injury, remote hind limb preconditioning, gadolinium and ruthenium red on infarct size
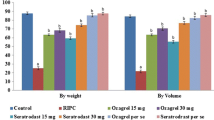
In the present investigation, 30 min of ischemia and 120 min reperfusion produced significant myocardial injury in isolated rat hearts in terms of infarct size, both by weight and volume method. Four consecutive cycles of remote hind limb preconditioning significantly attenuated ischemia-reperfusion-induced increase in myocardial infarct size. However, administration of gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) significantly abolished remote hind limb preconditioning-induced decrease in infarct size (Figs. 1 and 2 ).
Pharmacological effects of gadolinium and ruthenium red on % infarct size by volume and weight method. Statistical analysis of data was done using one-way ANOVA followed by Bonferroni’s post hoc test; values were expressed as mean ± S.D. with n = 6 in each group. For weight method, F (7, 40) = 147; for volume method, F (7, 40) = 145; a = p < 0.05 vs control; b = p < 0.05 vs remote hind limb preconditioning; c = p < 0.05 vs gadolinium
Effect of ischemia-reperfusion injury, remote hind limb preconditioning, gadolinium and ruthenium red on LDH release in the coronary effluent
Sustained ischemia of 30 min and 120 min of reperfusion produced significant increase in LDH release in the coronary effluent at various time periods of reperfusion, i.e., immediately and 30 min after reperfusion, as compared to basal. Remote hind limb preconditioning significantly abolished ischemia-reperfusion-induced increase in LDH release in the coronary effluent. However, administration of gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) significantly attenuated remote hind limb preconditioning-dependent decrease in LDH release (Fig. 3 ).
Pharmacological effects of gadolinium and ruthenium red on remote hind limb preconditioning associated decrease in LDH release in the coronary effluent. Statistical analysis of data was done using two-way ANOVA followed by Bonferroni’s post hoc test; values were expressed as mean ± S.D. with n = 6 in each group. For treatment, F (7, 35) = 6084; for time F (2, 10) = 31,592; a = p < 0.05 vs basal; b = p < 0.05 vs control; c = p < 0.05 vs remote hind limb preconditioning; d = p < 0.05 vs gadolinium
Effect of ischemia-reperfusion injury, remote hind limb preconditioning, gadolinium and ruthenium red on CK release in the coronary effluent
Sustained ischemia of 30 and 120 min of reperfusion produced significant increase in CK release in the coronary effluent at 5 min of reperfusion, as compared to basal. Remote hind limb preconditioning significantly abolished ischemia-reperfusion-induced increase in CK release in the coronary effluent. However, administration of gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) significantly attenuated remote hind limb preconditioning-dependent decrease in CK release (Fig. 4 ).
Pharmacological effects of gadolinium and ruthenium red on remote hind limb preconditioning associated decrease in CK release in the coronary effluent. Statistical analysis of data was done using two-way ANOVA followed by Bonferroni’s post hoc test; values were expressed as mean ± S.D. with n = 6 in each group. For treatment, F (7, 35) = 299.7; for time (1, 5) = 6642; a = p < 0.05 vs basal; b = p < 0.05 vs control; c = p < 0.05 vs remote hind limb preconditioning; d = p < 0.05 vs gadolinium
Effect of ischemia-reperfusion injury, remote hind limb preconditioning, gadolinium and ruthenium red on LVDP, +dp/dtmax, −dp/dtmin
Sustained ischemia of 30 min in the isolated rat hearts produced significant decrease in LVDP, +dp/dtmax, −dp/dtmin during the reperfusion period. Remote hind limb preconditioning markedly improved LVDP, +dp/dtmax, −dp/dtmin during the reperfusion period in the isolated rat hearts. However, remote hind limb preconditioning dependent improvement in LVDP, +dp/dtmax, −dp/dtmin during the reperfusion period was significantly abolished in the presence of gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) (Table 3 ).
Effect of ischemia-reperfusion injury, remote hind limb preconditioning, gadolinium and ruthenium red on heart rate and coronary flow rate
Sustained ischemia of 30 min in the isolated rat hearts produced significant decrease in heart rate and coronary flow rate during the reperfusion period. Remote hind limb preconditioning markedly improved heart rate and coronary flow rate during the reperfusion period in the isolated rat hearts. However, remote hind limb preconditioning dependent improvement in heart rate and coronary flow rate during the reperfusion period was significantly abolished in the presence of and gadolinium (7.5 and 15 mg kg−1) and ruthenium red (4 and 8 mg kg−1) (Tables 1, 2, and 3).
Discussion
In the present study, 30 min of global ischemia and 120 min of reperfusion induced significant ex vivo ischemia-reperfusion injury in isolated rat hearts in terms of increased release of LDH and CK (biochemical markers of myocardial injury) in the coronary effluent. Myocardial cell damage due to ischemia-reperfusion injury leads to release of myocardial enzymes from the cell. LDH is an enzyme that catalyzes the inter-conversion of pyruvate to lactate, with simultaneous inter-conversion of NADH to NAD+. It exists in five different isoforms, i.e., LDH-1-LDH-5, but LDH-1 isoform is predominantly expressed in the heart (Kemp et al. 2004). As various LDH enzyme isoforms are expressed throughout the body, the quantitative estimation of LDH-1 is the true indicator of myocardial injury. CK is an enzyme expressed in a variety of living cells to catalyze the reversible conversion of creatine to phosphocreatine and ADP utilizing an ATP molecule. Although CK enzyme exists in three isoforms, i.e., CK-MM, CK-MB, CK-BB, yet CK-MB isoform is exclusively expressed in the heart muscle and evaluation of CK-MB isozyme would be clear determinant of myocardial injury (Kemp et al. 2004). However, in the present ex-vivo study, the isoform specific forms of LDH and CK were not determined as there was no interference from any other organ and LDH and CK release was determined from the isolated rat heart itself.
In the present study, ischemia-reperfusion injury also led to functional impairment in terms of decrease in contractility parameters including LVDP, +dp/dtmax, −dp/dtmin and heart rate. LVDP is the pressure generated in the left ventricle of the heart; left ventricular dp/dt is the first derivative of left ventricular pressure; its peak value +dp/dtmax represents maximum derivative of change in the systolic pressure over time, and −dp/dtmin represents minimum derivative of change in the diastolic pressure over time (Hamlin and del Rio 2012). Furthermore, in the present study, evaluation of the infarct size by weight and volume method using TTC staining revealed a significant increase in the infarct size after being subjected to sustained ischemia-reperfusion injury. A significant decrease in coronary flow rate during the reperfusion phase was also observed indicating injury to the coronary vasculature.
However, remote hind limb preconditioning remarkably attenuated ischemia-reperfusion-induced LDH, CK release, increase in infarct size and improved the contractility parameters including LVDP, +dp/dtmax, −dp/dtmin, and heart rate. Our previous laboratory studies have shown that the peak release of LDH and CK takes place immediately and 5 min after reperfusion, respectively (Diwan et al. 2008, Kant et al. 2008). Therefore, in the present study, these biochemical parameters were assessed at those particular time periods of reperfusion. Administration of gadolinium (7.5 and 15 mg kg−1) prior to remote hind limb preconditioning significantly attenuated remote hind limb preconditioning-induced cardioprotective effects in the isolated rat hearts. The attenuation of remote hind limb preconditioning-induced cardioprotection in the presence of gadolinium suggests the important role of TRP (a type of mechanosensitive channels) in mediating remote hind limb preconditioning induced cardioprotection. Mechanosensitive channels are localized on different regions of the heart including vascular endothelial cells, aorta, atria, ventricles (Yao et al. 2003), coronary blood vessels (Nilius et al. 1997; Lehoux and Tedgui 2003), and sensory nerves innervating the heart (Xian Tao et al. 2006; Hayabuchi et al. 2011; Shenton and Pyner 2014). These channels are sensitive to mechanical stretch, and there have been studies suggesting the key role of these stretch activated channels in mediating ischemic preconditioning-induced cardioprotection (Ovize et al. 1994; Obadia et al. 1997; Nakagawa et al. 1997; Gysembergh et al. 1998). The opening of these channels leads to inward movement of monovalent and divalent cations including Na+, K+, and Ca2+ ions (Davis et al. 1992; Guibert et al. 2008). Studies have shown that preconditioning increases intracellular Ca2+ to induce cardioprotective effects (Node et al. 1997; Shintani et al. 2004; Bickler and Fahlman 2010) and indeed, intracoronary administration of CaCl2 preconditions the canine heart (Przyklenk et al. 1997). In addition, exercise also preconditions the dog heart via increasing Ca2+ inflow (Parra et al. 2015) indicating that Ca2+ possibly serves as a trigger to induce preconditioning-dependent cardioprotective effects.
The mechanism underlying remote hind limb preconditioning-dependent cardioprotection is possibly an integration of activation of physiological, neural, and humoral pathway. Remote hind limb preconditioning stimulus may lead to opening of mechanosensitive channels (Huang et al. 2004) and increase intracellular Ca2+ to trigger Ca2+-dependent cardioprotective effects against sustained ischemia-reperfusion injury. Meanwhile, remote hind limb preconditioning may also activate the mechanosensitive channels present on the sensory nerve endings innervating the heart and consequently, release chemical mediators including CGRP, substance P to induce cardioprotective effects (Ren et al. 2011; Gao et al. 2015). Furthermore, these channels are also present on the skeletal muscles and the spinal cord regions (Lansman and Franco-Obregón 2006; Kerstein et al. 2013) and hence, these play an important role in modulating mechanosensory information at the spinal cord level. It has been reported that the sensory neurons in the dorsal root ganglia have the ability to transduce various mechanical stimuli due to the presence of mechanosensory nerve endings (Hu and Lewin 2006; Spicarova et al. 2014). Accordingly, it may also be hypothesized that the development mechanical stretch in the sensory nerve endings innervating the skeletal muscles during hind limb occlusion may activate the sensory nerve endings such that they transduce the mechanical stimuli to the spinal cord to induce cardioprotective effects (Luo et al. 2012).
Furthermore, in the present study, administration of ruthenium red, a TRPV antagonist, also abrogated remote hind limb preconditioning-induced cardioprotection. TRPV receptors are the subtype of mechanosensitive channels and are also localized on the sensory nerve endings, heart (Schultz 2003), sciatic nerve (Fischer et al. 2003), and skeletal muscles (Luo et al. 2012; Lotteau et al. 2013). Very recently, Lu et al. reported that hypoxic preconditioning-dependent cardioprotective effects are mediated via activation of TRPV1 receptors (Lu et al. 2014). Furthermore, previous studies have indicated that ischemic preconditioning-induced cardioprotective effects were completely abrogated in TRPV1 −/− mice suggesting key role of these channels in mediating cardioprotection (Zhong and Wang 2007). In addition, Rath and co-workers documented the involvement of TRPV4 channels for preserving hypoxic preconditioning dependent vasorelaxation (Rath et al. 2012). However, it is the first report describing the key role of mechanosensitive channels including TRPV channels in remote limb preconditioning-dependent cardioprotective effects in rat heart. It may be presumed that the mechanosensitive channels including TRPV channels may govern remote hind limb preconditioning-induced cardioprotective effects. It has been reported that the density of the mechanosensitive TRPV channels is higher on the sensory nerve endings innervating the heart than the heart itself (Gao et al. 2015). Therefore, there is a possibility that remote hind limb preconditioning stimulus primarily activates mechanosensitive channels on the sensory nerve endings innervating the heart than the heart itself to induce cardioprotective effects.
Although gadolinium primarily blocks stretch activated channels, yet it also has the tendency to block sodium leaky channels, L- and T-type Ca2+ currents (Biagi and Enyeart 1990; Senatore et al. 2013). Ruthenium red, employed as a TRPV antagonist, also possesses the ability to inhibit mitochondrial Ca2+ uniporter (Liang et al. 2014). Therefore, the contribution of channels/receptors others than mechanosensitive TRPV channels in gadolinium and ruthenium red-mediated attenuation of cardioprotective effects of remote preconditioning may not be completely ruled out. Nevertheless, further studies are warranted to authorize the role of mechanosensitive TRPV channels in mediating remote hind limb preconditioning-dependent cardioprotective effects.
Conclusion
The attenuation of remote hind limb preconditioning-induced cardioprotection in the presence of gadolinium and ruthenium red suggests the possible role of TRP and especially TRPV channels in mediating cardioprotective effects.
References
Ali N, Rizwi F, Iqbal A, Rashid A (2010) Induced remote ischemic pre-conditioning on ischemia-reperfusion injury in patients undergoing coronary artery bypass. J Coll Physicians Surg Pak 20:427–431
Babiker FA, Lorenzen-Schmidt I, Mokelke E, Vanagt WY, Delhaas T, Waltenberger J, Cleutjens JP, Prinzen FW (2010) Long-term protection and mechanism of pacing-induced postconditioning in the heart. Basic Res Cardiol 105:523–533
Bett GC, Sachs F (1997) Cardiac mechanosensitivity and stretch-activated ion channels. Trends Cardiovasc Med 7:4–8
Biagi BA, Enyeart JJ (1990) Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am J Phys 259:C515–C520
Bickler PE, Fahlman CS (2010) Enhanced hypoxic preconditioning by isoflurane: signaling gene expression and requirement of intracellular Ca2+ and inositol triphosphate receptors. Brain Res 1340:86–95
Chen M, Zheng YY, Song YT, Xue JY, Liang ZY, Yan XX, Luo DL (2016) Pretreatment with low-dose gadolinium chloride attenuates myocardial ischemia/reperfusion injury in rats. Acta Pharmacol Sin 37(4):453–462
Choi HJ, Sun D, Jakobs TC (2015) Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol Vis 21:749–766
Craelius W, Chen V, el-Sherif N (1988) Stretch activated ion channels in ventricular myocytes. Biosci Rep 8:407–414
Davis MJ, Donovitz JA, Hood JD (1992) Stretch-activated single-channel and whole cell currents in vascular smooth muscle cells. Am J Phys 262:C1083–C1088
Diwan V, Kant R, Jaggi AS, Singh N, Singh D (2008) Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem 315:195–201
Du SS, Qiang M, Zeng ZC, Ke AW, Ji Y, Zhang ZY, Zeng HY, Liu Z (2010) Inactivation of kupffer cells by gadolinium chloride protects murine liver from radiation-induced apoptosis. Int J Radiat Oncol Biol Phys 76:1225–1234
Fischer MJ, Reeh PW, Sauer SK (2003) Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur J Neurosci 18:803–810
Friedrich O, Wagner S, Battle AR, Schürmann S, Martinac B (2012) Mechano-regulation of the beating heart at the cellular level-mechanosensitive channels in normal and diseased heart. Prog Biophys Mol Biol 110:226–238
Gao Y, Song J, Chen H, Cao C, Lee C (2015) TRPV1 activation is involved in the cardioprotection of remote limb ischemic postconditioning in ischemia-reperfusion injury rats. Biochem Biophys Res Commun 463:1034–1039
Guibert C, Ducret T, Savineau JP (2008) Voltage-independent calcium influx in smooth muscle. Prog Biophys Mol Biol 98:10–23
Guzy PM (1977) Creatine phosphokinase-MB (CPK-MB) and the diagnosis of myocardial infarction. West J Med 127:455–460
Gysembergh A, Margonari H, Loufoua J, Ovize A, André-Fouët X, Minaire Y, Ovize M (1998) Stretch-induced protection shares a common mechanism with ischemic preconditioning in rabbit heart. Am J Phys 274:H955–H964
Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81:685–740
Hamlin RL, del Rio C (2012) dP/dt(max)—a measure of ‘baroinometry’. J Pharmacol Toxicol Methods 66:63–65
Hao J, Kim HS, Choi W, Ha TS, Ahn HY, Kim CH (2010) Mechanical stretch-induced protection against myocardial ischemia-reperfusion injury involves AMP-activated protein kinase. Korean J Physiol Pharmacol 14:1–9
Haswell ES, Phillips R, Rees DC (2011) Mechanosensitive channels: what can they do and how do they do it? Structure 19:1356–1369
Hayabuchi Y, Nakaya Y, Mawatari K, Inoue M, Sakata M, Kagami S (2011) Cell membrane stretch activates intermediate-conductance Ca2+-activated K+ channels in arterial smooth muscle cells. Heart Vessel 26:91–100
Hu J, Lewin GR (2006) Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol 577:815–828
Huang CH, Wang JS, Chiang SC, Wang YY, Lai ST, Weng ZC (2004) Brief pressure overload of the left ventricle preconditions rabbit myocardium against infarction. Ann Thorac Surg 78:628–633
Kalogeris T, Baines CP, Krenz M, Korthuis RJ (2012) Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298:229–317
Kant R, Diwan V, Jaggi AS, Singh N, Singh D (2008) Remote renal preconditioning-induced cardioprotection: a key role of hypoxia inducible factor-prolyl 4-hydroxylases. Mol Cell Biochem 312:25–31
Kemp M, Donovan J, Higham H, Hooper J (2004) Biochemical markers of myocardial injury. Br J Anaesth 93:63–73
Kerstein PC, Jacques-Fricke BT, Rengifo J, Mogen BJ, Williams JC, Gottlieb PA, Sachs F, Gomez TM (2013) Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J Neurosci 33:273–285
Kim D (1992) A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J Gen Physiol 100:1021–1040
King JA (1959) A routine method for estimation of lactate dehydrogenase activity. J Med Lab Tech 16:291–332
Kyriakou LG, Tzirogiannis KN, Demonakou MD, Kourentzi KT, Mykoniatis MG, Panoutsopoulos GI (2013) Gadolinium chloride pretreatment ameliorates acute cadmium-induced hepatotoxicity. Toxicol Ind Health 29:624–632
Langendorff O (1885) Untersuchungen amuber lebenderer saugethierherzen. Pfluger Arch Gesmate Physio 61:291–332
Lansman JB, Franco-Obregón A (2006) Mechanosensitive ion channels in skeletal muscle: a link in the membrane pathology of muscular dystrophy. Clin Exp Pharmacol Physiol 33:649–656
Lehoux S, Tedgui A (2003) Cellular mechanics and gene expression in blood vessels. J Biomech 36:631–643
Levine JD, Alessandri-Haber N (2007) TRP channels: targets for the relief of pain. Biochim Biophys Acta 1772:989–1003
Liang N, Wang P, Wang S, Li S, Li Y, Wang J, Wang M (2014) Role of mitochondrial calcium uniporter in regulating mitochondrial fission in the cerebral cortexes of living rats. J Neural Transm (Vienna) 121:593–600
Lotteau S, Ducreux S, Romestaing C, Legrand C, Van Coppenolle F (2013) Characterization of functional TRPV1 channels in the sarcoplasmic reticulum of mouse skeletal muscle. PLoS ONE 8:e58673
Lu MJ, Chen YS, Huang HS, Ma MC (2014) Hypoxic preconditioning protects rat hearts against ischemia-reperfusion injury via the arachidonate12-lipoxygenase/transient receptor potential vanilloid 1 pathway. Basic Res Cardiol 109:414
Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T, Liu D, Nilius B, Huang Y, Yan Z, Zhu Z (2012) TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res 22:551–564
Nakagawa C, Asayama J, Katamura M, Matoba S, Keira N, Kawahara A, Tsuruyama K, Tanaka T, Kobara M, Akashi K, Ohta B, Tatsumi T, Nakagawa M (1997) Myocardial stretch induced by increased left ventricular diastolic pressure preconditions isolated perfused hearts of normotensive and spontaneously hypertensive rats. Basic Res Cardiol 92:410–416
Nilius B, Viana F, Droogmans G (1997) Ion channels in vascular endothelium. Annu Rev Physiol 59:145–170
Node K, Kitakaze M, Sato H, Minamino T, Komamura K, Shinozaki Y, Mori H, Hori M (1997) Role of intracellular Ca2+ in activation of protein kinase C during ischemic preconditioning. Circulation 96:1257–1265
Obadia JF, Ovize M, Maupoil V, Terrand J, Abadie C, Ovize A, Andre-Fouët X, Minaire Y, Rochette L (1997) Beneficial actions of preconditioning and stretch on postischemic contractile function of isolated working rat heart: effects of staurosporine. J Cardiovasc Pharmacol 30:191–196
Ortiz GG, de la Mora-Rivas G, Cardenas-Ortega A, Orbach-Arbouys S, Bravo-Cuellar A, Feria-Velasco A (1992) Hepatotoxicity induced by a single ip injection of ruthenium red. Biomed Pharmacother 46:115–119
Ovize M, Kloner RA, Przyklenk K (1994) Stretch preconditions canine myocardium. Am J Phys 266:H137–H146
Parra VM, Macho P, Sánchez G, Donoso P, Domenech RJ (2015) Exercise preconditioning of myocardial infarct size in dogs is triggered by calcium. J Cardiovasc Pharmacol 65:276–281
Perretti F, Manzini S (1993) Activation of capsaicin-sensitive sensory fibers modulates PAF-induced bronchial hyperresponsiveness in anesthetized guinea pigs. Am Rev Respir Dis 148(4 Pt 1):927–931
Przyklenk K, Hata K, Kloner RA (1997) Is calcium a mediator of infarct size reduction with preconditioning in canine myocardium? Circulation 96:1305–1312
Przyklenk K, Whittaker P (2013) Genesis of remote conditioning: action at a distance--‘hypotheses non fingo’? J Cardiovasc Med (Hagerstown) 14(3):180–186
Randhawa PK, Jaggi AS (2015) TRPV4 channels: physiological and pathological role in cardiovascular system. Basic Res Cardiol 110:54
Randhawa PK, Bali A, Jaggi AS (2015) RIPC for multiorgan salvage in clinical settings: evolution of concept, evidences and mechanisms. Eur J Pharmacol 746:317–332
Rath G, Saliez J, Behets G, Romero-Perez M, Leon-Gomez E, Bouzin C, Vriens J, Nilius B, Feron O, Dessy C (2012) Vascular hypoxic preconditioning relies on TRPV4-dependent calcium influx and proper intercellular gap junctions communication. Arterioscler Thromb Vasc Biol 32:2241–2249
Rehni AK, Singh N, Jaggi AS (2007) Possible involvement of insulin, endogenous opioids and calcitonin gene-related peptide in remote ischaemic preconditioning of the brain. Yakugaku Zasshi 127:1013–1020
Ren JY, Song JX, Lu MY, Chen H (2011) Cardioprotection by ischemic postconditioning is lost in isolated perfused heart from diabetic rats: involvement of transient receptor potential vanilloid 1, calcitonin gene-related peptide and substance P. Regul Pept 169:49–57
Schultz HD (2003) The spice of life is at the root of cardiac pain. J Physiol 551(Pt 2):400
Senatore A, Monteil A, van Minnen J, Smit AB, Spafford JD (2013) NALCN ion channels have alternative selectivity filters resembling calcium channels or sodium channels. PLoS ONE 8:e55088
Shenton FC, Pyner S (2014) Expression of transient receptor potential channels TRPC1 and TRPV4 in venoatrial endocardium of the rat heart. Neuroscience 267:195–204
Shintani Y, Node K, Asanuma H, Sanada S, Takashima S, Asano Y, Liao Y, Fujita M, Hirata A, Shinozaki Y, Fukushima T, Nagamachi Y, Okuda H, Kim J, Tomoike H, Hori M, Kitakaze M (2004) Opening of Ca2+-activated K+ channels is involved in ischemic preconditioning in canine hearts. J Mol Cell Cardiol 37:1213–1218
Spencer AJ, Wilson SA, Batchelor J, Reid A, Rees J, Harpur E (1997) Gadolinium chloride toxicity in the rat. Toxicol Pathol 25:245–255
Spicarova D, Nerandzic V, Palecek J (2014) Update on the role of spinal cord TRPV1 receptors in pain modulation. Physiol Res 63(Suppl 1):S225–S236
Su X, Wachtel RE, Gebhart GF (2000) Mechanosensitive potassium channels in rat colon sensory neurons. J Neurophysiol 84:836–843
Vivaldi MT, Kloner RA, Schoen FJ (1985) Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol 121:522–530
Wever KE, Warlé MC, Wagener FA, van der Hoorn JW, Masereeuw R, van der Vliet JA, Rongen GA (2011) Remote ischaemic preconditioning by brief hind limb ischaemia protects against renal ischaemiareperfusion injury: the role of adenosine. Nephrol Dial Transplant 26:3108–3117
Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Müller R, Isenberg G, Daut J (2006) The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res 69:86–97
Yao X, Kwan HY, Dora KA, Garland CJ, Huang Y (2003) A mechanosensitive cation channel in endothelial cells and its role in vasoregulation. Biorheology 40:23–30
Zhang Y, Liu X, Yan F, Min L, Ji X, Luo Y (2012) Protective effects of remote ischemic preconditioning in rat hindlimb onischemia- reperfusion injury. Neural Regen Res 7:583–587
Zheng J (2013) Molecular mechanism of TRP channels. Compr Physiol 3:221–242
Zhong B, Wang DH (2007) TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293:H1791–H1798
Acknowledgments
The authors are thankful to Department of Science and Technology F. No. SB/SO/HS/0004/2013, New Delhi, for their gratefulness for providing us financial assistance and Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India for supporting us.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Randhawa, P.K., Jaggi, A.S. Gadolinium and ruthenium red attenuate remote hind limb preconditioning-induced cardioprotection: possible role of TRP and especially TRPV channels. Naunyn-Schmiedeberg's Arch Pharmacol 389, 887–896 (2016). https://doi.org/10.1007/s00210-016-1251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1251-5