Abstract
Bosentan is a mixed endothelin receptor antagonist widely used to treat patients with pulmonary arterial hypertension, and the emerging literature suggests bosentan as a potent anti-inflammatory drug. Superoxide anion is produced in large amounts during inflammation, stimulates cytokine production, and thus contributes to inflammation and pain. However, it remains to be determined whether endothelin contributes to the inflammatory response triggered by the superoxide anion. The present study investigated the effects of bosentan in a mouse model of inflammation and pain induced by potassium superoxide, a superoxide anion donor. Male Swiss mice were treated with bosentan (10–100 mg/kg) by oral gavage, 1 h before potassium superoxide injection, and the inflammatory response was evaluated locally and at spinal cord (L4–L6) levels. Bosentan (100 mg/kg) inhibited superoxide anion-induced mechanical and thermal hyperalgesia, overt pain-like behavior (abdominal writhings, paw flinching, and licking), paw edema, myeloperoxidase activity (neutrophil marker) in the paw skin, and leukocyte recruitment in the peritoneal cavity. Bosentan also inhibited superoxide anion-induced interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) production, while it enhanced IL-10 production in the paw skin and spinal cord. Bosentan inhibited the reduction of antioxidant capacity (reduced glutathione, ferric reducing antioxidant power, and ABTS radical scavenging ability) induced by the superoxide anion. Finally, we demonstrated that intraplantar injection of potassium superoxide induces the mRNA expression of prepro-endothelin-1 in the paw skin and spinal cord. In conclusion, our results demonstrated that superoxide anion-induced inflammation, pain, cytokine production, and oxidative stress depend on endothelin; therefore, these responses are amenable to bosentan treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelin-1 (ET-1) was discovered in 1988 as a peptide secreted by endothelial cells with the highest vasoconstriction capacity known (Yanagisawa et al. 1988). This peptide is first synthesized as inactive prepro-ET-1 (ppET-1) and thus has to be cleaved by endothelin-converting enzymes to generate the ET-1, which activates its receptors (Jandeleit-Dahm and Watson 2012). ET-1 plays relevant roles in cardiovascular diseases, including pulmonary arterial hypertension (Avdeev et al. 2013), systemic hypertension (Morelli et al. 1995), atherosclerosis (Yoon et al. 2013), and cerebral vasospasm (Mascia et al. 2001).
Focusing on inflammation and pain, ET-1 upregulates the expression of adhesion molecules on endothelial cells and fibroblasts, increases neutrophil adhesion and migration, stimulates cytokine production through activation of NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells), increases microvascular permeability, and enhances pain sensitivity (Conte Fde et al. 2008; Verri et al. 2008; Ohanian et al. 2012; Zarpelon et al. 2012). The sources of ET-1 include vascular endothelial cells, macrophages, neutrophils, fibroblasts, and neurons (Barton and Yanagisawa 2008), and these cells also present endothelin receptors that are upregulated during inflammation, emphasizing the role of the endothelin system on maladaptive inflammatory responses and diseases (Pomonis et al. 2001; Motte et al. 2006; Lau et al. 2014). Indeed, increased plasma levels of ET-1 occur in several pathological conditions associated with inflammation and pain, including Crohn’s disease, ulcerative colitis, and rheumatoid arthritis (Miyasaka et al. 1992; Letizia et al. 1998).
ET-1 induces severe pain in rodents and humans (Ferreira et al. 1989; Graido-Gonzalez et al. 1998; Davar et al. 1998; De-Melo et al. 1998; Jarvis et al. 2000; Khodorova et al. 2002; Verri et al. 2004). ET-1 induces pain directly by activating nociceptive neurons (Gokin et al. 2001) and indirectly by inducing the production of other inflammatory mediators that also activate and sensitize nociceptive neurons, including cytokines, prostanoids, and reactive oxygen species (Kress et al. 1995; Scalera et al. 2002; Verri et al. 2006a, b; Jin and Gereau 2006; Verri et al. 2008; Binshtok et al. 2008; Romero et al. 2010; Donate et al. 2012; Keles et al. 2014). At the spinal cord level, ET-1 concentration increases after peripheral tissue injury in rats submitted to chronic post-ischemia pain (Kim et al. 2015). An anti-nociceptive effect of spinal cord ET-1 production has been reported in animal models of neuropathic and inflammatory pain (Hung et al. 2012; Hung et al. 2014), and on the other hand, spinal ET-1 induces mechanical hyperalgesia in a model of ischemia/reperfusion-induced oxidative stress (Kim et al. 2015). Therefore, a pro- or anti-nociceptive role for ET-1 is not totally predictable.
Bosentan is a mixed endothelin receptor antagonist recognized as the first orally bioavailable endothelin receptor antagonist approved by the US Food and Drug Administration for the treatment of patients with pulmonary arterial hypertension (Channick et al. 2001; Rubin and Roux 2002). Pre-clinical studies have suggested the potential of using bosentan to treat a wide range of inflammatory diseases, including arthritis (Donate et al. 2012), cancer (Jewell et al. 2010), uveitis (Keles et al. 2014), and depression (Pinho-Ribeiro et al. 2014). Thus, bosentan has been a crucial tool in the investigation of ET-1 roles in disease and perspective of targeting ET-1 to treat diseases.
It has been extensively described that the maladaptive effects of ET-1 signaling depend on superoxide anion production (Scalera et al. 2002; Fiore et al. 2005; Liu et al. 2005; Matsuo et al. 2009; Hsu et al. 2010; Rancourt et al. 2010; López-Sepúlveda et al. 2011; Piechota et al. 2011; Martínez-Revelles et al. 2012). Furthermore, a superoxide anion increases ET-1 production in pulmonary arteries and hepatic cells (Gabriel et al. 1998; Wang et al. 2006), suggesting that superoxide anion-induced pain and inflammation could be mediated by ET-1. However, to our knowledge, this hypothesis has never been tested. Therefore, the effect of bosentan on potassium superoxide (KO2, a superoxide anion donor)-induced inflammation and pain in mice was evaluated.
Materials and methods
Drugs and reagents
Bosentan (a mixed endothelin receptor antagonist) was a generous gift from Actelion Pharmaceuticals Ltd. (Allschwil, Switzerland). KO2 (potassium superoxide) was purchased from Alfa Aesar (Ward Hill, MA, USA). Arabic gum was obtained from Farmácia Nikkey (Arapongas, PR, Brazil). HTAB (hexadecyl trimethyl ammonium bromide), dihydrochloride o-dianisidine, GSH (reduced glutathione), PBS (phosphate-buffered saline), EDTA sodium salt, ferric chloride hexahydrate, TPTZ (2,4,6-tripyridyl-s-triazine), ABTS [2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)], and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). ELISA kits were obtained from eBioscience Inc. (San Diego, CA, USA).
Animals
Male Swiss mice weighing 25 ± 5 g from Universidade Estadual de Londrina, Paraná, Brazil, were used in this study. Mice were housed in standard clear plastic cages with free access to food and water, with a light/dark cycle of 12:12 h, at 21 ± 1 °C. All behavioral testing was performed between 9 a.m. and 5 p.m. in a temperature-controlled (21 ± 1 °C) room. Animals’ care and handling procedures were in accordance with the International Association for the Study of Pain (IASP) guidelines and with the approval of the Animal Ethics Committee of the Universidade Estadual de Londrina (process number 71.2012.68). All efforts were made to minimize the number of animals used and their suffering.
Experimental protocols
Mice were treated orally (p.o.) with bosentan (10–100 mg/kg, suspended in 100 μL of a 0.5 % arabic gum solution, wt/vol in saline) or vehicle, 1 h before stimulus injection. Mechanical and thermal hyperalgesia and edema were evaluated 0.5–7 h after intraplantar (i.pl.) injection of potassium superoxide (KO2 30 μg/paw, in 25 μL of sterile saline) (Maioli et al. 2015), and paw tissue samples were collected after the last evaluation to measure myeloperoxidase (MPO) activity. Overt pain-like behaviors of paw flinching and licking (i.pl. injection of KO2 30 μg/paw) and abdominal writhings (intraperitoneal injection of KO2 1 mg/cavity, 100 μL of sterile saline) were evaluated in mice treated with bosentan (100 mg/kg, p.o., 1 h) or vehicle. The total number of leukocytes, mononuclear cells, and neutrophils was evaluated 6 h after intraperitoneal (i.p.) injection of KO2 (30 μg/cavity, 200 μL of sterile saline). Paw skin and spinal cord (L4–L6) samples were collected 3 h after i.pl. stimulus with KO2 (30 μg/paw) and used to measure GSH (reduced glutathione) levels, FRAP (ferric reducing antioxidant power), ABTS radical scavenging ability, and the production of tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and IL-10. The mRNA expression of prepro-ET-1 was evaluated in paw skin and spinal cord samples collected 0.5–7 h after KO2 (30 μg/paw) injection.
Mechanical hyperalgesia test
The test consisted of evoking a hindpaw flexion reflex with a hand-held force transducer (electronic anesthesiometer; Insight, Ribeirão Preto, SP, Brazil) adapted with a 0.5-mm2 polypropylene tip. Our group described the detailed methodology previously (Cunha et al. 2004). The results are expressed by delta (∆) withdrawal threshold (in g), calculated by subtracting the zero-time mean measurements from the mean measurements at the indicated time points after stimulus.
Thermal hyperalgesia test
Thermal hyperalgesia was evaluated before and 0.5–7 h after the KO2 stimulus. The test was performed and adapted as reported previously (Ankier 1974; Maioli et al. 2015). In brief, mice were placed in a hot plate apparatus (EFF 361, Insight, Ribeirão Preto, SP, Brazil) maintained at 50 °C (Ankier 1974). The reaction time was registered when the animal jumped or licked the paw. A maximum latency (cutoff) was set at 15 s to avoid tissue damage.
Overt pain-like behavior tests
The number of paw flinches and time spent licking the paw were determined 0–30 min after i.pl. injection of KO2 (Maioli et al. 2015). Results are expressed as the cumulative number of paw flinches and the total time spent licking the paw. For the writhing response test, each mouse was placed in a large glass cylinder, and the number of abdominal writhings occurring between 0 and 20 min after i.p. injection of KO2 was registered (Maioli et al. 2015). Results are expressed as the cumulative number of writhings over 20 min.
Paw edema
Paw edema was measured using a caliper (Digmatic Caliper, Mitutoyo Corporation, Kanagawa, Japan). Values of paw edema are expressed as the difference between the thickness measured before (basal) and after induction of paw inflammation (in mm).
MPO activity
The neutrophil migration to paw skin was evaluated by a MPO activity kinetic-colorimetric assay (Bradley et al. 1982; Casagrande et al. 2006). Briefly, mice were terminally anesthetized, and the paw skin samples were collected in 50 mM K2PO4 buffer (pH 6.0) containing 0.5 % HTAB and stored at −20 °C until assayed. MPO activity was determined at 450 nm (Multiskan GO Microplate Spectrophotometer, Thermo Scientific, Vantaa, Finland). Absorbance of samples was compared with a standard curve of neutrophils and is presented as the number of neutrophils × 104/mg of tissue.
Leukocyte recruitment in the peritoneal cavity
Peritoneal cavities were washed with 200 μL of PBS. Total leukocyte counts were performed in a Neubauer chamber after dilution in Turk’s solution (2 % acetic acid). Differential cell counts were performed using the Fast Panotic Kit for histological analysis (Laborclin, Pinhais, PR, Brazil), and the values are expressed as the number of cells (×106) per cavity. Total and differential cell counts were performed under a light microscope (×400 magnification, Olympus Optical Co., Hamburg, Germany) (Verri et al. 2007).
FRAP and ABTS assays
Samples of paw skin and spinal cord (L4–L6) were homogenized immediately in 500 μL of 1.15 % KCl, and the homogenates were centrifuged (10 min × 200g × 4 °C). The resulting supernatants were used to measure the ability of samples to resist oxidative stress by FRAP and ABTS assays as described previously (Re et al. 1999; Katalinic et al. 2005). For FRAP assay, 50 μL of supernatant was mixed with 150 μL of deionized water plus 1.5 mL of FRAP reagent freshly prepared. The reaction mixture was incubated at 37 °C for 30 min, and the absorbance was measured at 595 nm. For the ABTS assay, 20 μL of supernatant was mixed with 1 mL of diluted ABTS solution and incubated for 6 min at room temperature. The absorbance was measured at 730 nm and equated against a Trolox standard curve (1.5–30 μM). The results are expressed as millimoles of Trolox equivalent per gram of tissue.
GSH measurement
Samples of paw skin and spinal cord (L4–L6) were collected and maintained at −80 °C for at least 48 h before the GSH assay. The samples were homogenized with 200 μL of 0.02 M EDTA, and the homogenates were mixed with 25 μL of 50 % trichloroacetic acid. Samples were centrifuged (15 min × 1500g × 4 °C), and the supernatants were mixed with 200 μL of 0.2 M TRIS buffer (pH 8.2) plus 10 μL of 0.01 M DTNB. After 5 min of incubation at room temperature, the absorbance was measured at 412 nm. A standard curve of GSH was performed (Pinho-Ribeiro et al. 2015). Protein concentration was determined by the Lowry method (Lowry et al. 1951). The results are presented as nanomoles and micromoles of GSH per milligram of protein. The tests were adapted for microplate reading.
Cytokine levels
Samples of paw skin and spinal cord (L4–L6) were homogenized in 500 and 250 μL of buffer containing protease inhibitors, respectively. The homogenates were centrifuged (15 min × 1500g × 4 °C), and the resulting supernatants were stored at −80 °C until further analysis. TNF-α, IL-1β, and IL-10 levels were determined by enzyme-linked immunosorbent assay (ELISA) using eBioscience kits according to the manufacturer’s instructions. The results are expressed as picograms of cytokine per milligram of tissue.
RT-PCR and quantitative PCR
Paw skin and spinal cord samples were homogenized in TRIzol® reagent (Life Technologies), and total RNA was isolated according to the manufacturer’s directions. RNA purity was confirmed by the 260/280 ratio. RT-PCR and quantitative PCR were performed using GoTaq® 2-Step RT-qPCR System (Promega) following the manufacturer’s directions. Complementary DNA was reverse transcribed from 2 μg of total RNA, and quantitative PCR was performed on a LightCycler® Nano Instrument (Roche). The following primer sequences were used: ppEt-1: forward 5′-TGTGTCTACTTCTGCCACCT-3′, reverse 5′-CACCAGCTGCTGATAGATAC-3′; Gapdh: forward 5′-CATACCAGGAAATGAGCTTG-3′, reverse 5′-ATGACATCAAGAAGGTGGTG-3′.
Statistical analysis
Results of three independent repetitions (experiments) are presented as mean ± SD of each repetition as well as the value of each mouse except by Fig. 1a, b, which are presented as the mean ± SD of the experimental groups of the three independent experiments. Tests were performed with 6 mice per group per experiment (total of 18 mice per group) except for qPCR experiments in which we used 6 pools of 4 paws per group per repetition (total of 72 mice per group) or 6 pools of 2 spinal cords per group per repetition (total of 36 mice per group). Independent repetitions are represented as different gray scale; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. Differences between groups were evaluated by analyses of variance (ANOVA) followed by the Bonferroni post hoc using GraphPad Prism 6.01. Statistical differences were considered to be significant when P < 0.05.
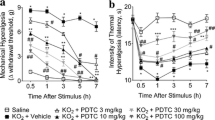
Bosentan, a mixed endothelin receptor antagonist, reduces superoxide anion-induced nociception. Mice were treated with bosentan (10–100 mg/kg, p.o.) or vehicle, 1 h before i.pl. injection of KO2 30 μg/paw (a–d) or i.p. injection of KO2 1 mg/cavity (e). The intensity of mechanical hyperalgesia (a) and thermal hyperalgesia (b) was evaluated 0.5–7 h after KO2 injection using electronic von Frey and hot plate apparatus, respectively. The number of paw flinches (c) and the time spent licking the paw (d) were evaluated during 30 min after KO2 injection. The number of writhings (e) was measured during 20 min after KO2 injection. Results of three independent repetitions (experiments) are presented as mean ± SD (red lines) of each repetition as well as the value of each mouse except for panels a and b, which are presented as mean ± SD of experimental groups of the three independent experiments. Independent repetitions are represented as different gray scales; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. n = 6 mice per group per repetition [*P < 0.05 vs. saline group; #P < 0.05 vs. vehicle group; **P < 0.05 vs. bosentan 10 mg/kg group, (ANOVA followed by Bonferroni’s post hoc)]
Results
Bosentan reduces superoxide anion-induced pain
Treatment with bosentan (10–100 mg/kg, p.o.) reduced the intensity of mechanical hyperalgesia induced by the superoxide anion (30 μg/paw) at all doses and time points evaluated. At all time points, the dose of 100 mg/kg differed from the lower dose (10 mg/kg) (Fig. 1a) [column factor F (16, 340) = 60.74, P < 0.0001]. Thus, the dose of 100 mg/kg was chosen to carry out the following experiments. Treatment with bosentan reduced superoxide anion-induced thermal hyperalgesia at all evaluated time points, abolishing thermal hyperalgesia between 0.5 and 7 h after the stimulus (Fig. 1b) [column factor F (10, 255) = 13.6, P < 0.0001]. Regarding overt pain-like behaviors, treatment with bosentan reduced superoxide anion-induced paw flinches (Fig. 1c) [F (2, 51) = 114.1, P < 0.0001], paw licking (Fig. 1d) [F (2, 51) = 227.2, P < 0.0001], and abdominal writhings (Fig. 1e) [F (2, 51) = 123, P < 0.0001].
Bosentan reduces superoxide anion-induced inflammation
Treatment with bosentan (10–100 mg/kg) reduced superoxide anion-induced edema at 30 min (Fig. 2a) [F (4, 85) = 218.9, P < 0.0001]. Bosentan reduced superoxide anion-induced neutrophil recruitment in the paw skin (MPO activity) at the dose of 100 mg/kg (Fig. 2b) [F (4, 85) = 177.8, P < 0.0001]. As no significant reduction in neutrophil recruitment was observed in mice treated with 10 or 30 mg/kg of bosentan (Fig. 2b), the dose of 100 mg/kg was used in the next experiments. Using another approach, the leukocyte recruitment in the peritoneal cavity was evaluated 6 h after KO2 i.p. injection (30 μg/cavity). Total leukocytes (Fig. 2c) [F (2, 51) = 249.6, P < 0.0001], mononuclear cells (Fig. 2d) [F (2, 51) = 93.64, P < 0.0001], and neutrophil (Fig. 2e) [F (2, 51) = 332.3, P < 0.0001] recruitment induced by the superoxide anion were reduced by bosentan.
Effects of bosentan on superoxide anion-induced inflammation. Mice were treated with bosentan (10–100 mg/kg, p.o.) or vehicle, 1 h before i.pl injection of KO2 (30 μg/paw). Paw edema (a) was evaluated 0.5–7 h after KO2 injection. Neutrophil migration to the paw (b) was evaluated 7 h after KO2 injection by MPO activity assay. Next, bosentan (100 mg/kg, p.o.) was administered 1 h before i.p. injection of KO2 (30 μg/cavity) and recruitment of total leukocytes (c), mononuclear cells (d), and neutrophils (e) to the peritoneal cavity was evaluated under a light microscope, 6 h after KO2 injection. Results of three independent repetitions (experiments) are presented as mean ± SD (red lines) of each repetition as well as the value of each mouse. Independent repetitions are represented as different gray scales; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. n = 6 mice per group per repetition [*P < 0.05 vs. saline group; #P < 0.05 vs. vehicle group; **P < 0.05 vs. bosentan 10 mg/kg group, (ANOVA followed by Bonferroni’s post hoc)]
Bosentan inhibits superoxide anion-induced oxidative stress
Samples of paw skin (Fig. 3a–c) and spinal cord (Fig. 3d–f) were collected 3 h after KO2 i.pl. injection and used in ferric reducing (FRAP, Fig. 3a, d), free radical scavenging ability (ABTS, Fig. 3b, e), and reduced glutathione (GSH, Fig. 3c, f) assays. Bosentan abolished the reduction of ferric reducing ability of paw skin (Fig. 3a) [F (2, 51) = 17.15, P < 0.0001] and spinal cord tissue (Fig. 3d) [F (2, 51) = 31.71, P < 0.0001], and the reduction of the ABTS radical scavenging ability of paw skin (Fig. 3b) [F (2, 51) = 17.66, P = 0.0005] and spinal cord (Fig. 3e) [F (2, 51) = 58.7, P < 0.0001] as well. Bosentan treatment prevented the decrease in GSH levels in paw skin (Fig. 3c) [F (2, 51) = 62.35, P < 0.0001] and in spinal cord (Fig. 3f) [F (2, 51) = 147, P < 0.0001].
Bosentan reduces superoxide anion-induced decrease in antioxidant defenses. Mice were treated with bosentan (100 mg/kg, p.o.) or vehicle, 1 h before i.pl. injection of KO2 (30 μg/paw). Samples from the paw skin (a–c) and spinal cord (d–f) were collected 3 h after stimulus injection, and antioxidant capacity was assessed by determining the ferric reducing antioxidant power (FRAP assay) (a, d), ABTS radical scavenging ability (b, e), and GSH levels (c, f). Results of three independent repetitions (experiments) are presented as mean ± SD (red lines) of each repetition as well as the value of each mouse. Independent repetitions are represented as different gray scales; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. n = 6 mice per group per repetition [*P < 0.05 vs. saline group; #P < 0.05 vs. vehicle group, (ANOVA followed by Bonferroni’s post hoc)]
Bosentan modulates superoxide anion-induced cytokine production
Paw skin (Fig. 4a–c) and spinal cord (Fig. 4d–f) samples were collected 3 h after KO2 injection to measure cytokine levels. The superoxide anion increased the production of TNF-α, IL-1β, and IL-10 in paw skin (Fig. 4a–c, respectively) and in the spinal cord (Fig. 4d–f, respectively). The treatment with bosentan reduced the production of TNF-α in the paw skin (Fig. 4a) [F (2, 51) = 240.9, P < 0.0001] and in the spinal cord (Fig. 4d) [F (2, 51) = 302.3, P < 0.0001]. IL-1β production was also reduced in the paw skin (Fig. 4b) [F (2, 51) = 165.9, P < 0.0001] and in the spinal cord (Fig. 4e) [F (2, 51) = 192.2, P < 0.0001] of mice treated with bosentan. On the other hand, bosentan enhanced superoxide anion-induced IL-10 production in the paw skin (Fig. 4c) [F (2, 51) = 429.8, P < 0.0001] and spinal cord (Fig. 4f) [F (2, 51) = 258.8, P < 0.0001].
Effect of bosentan on superoxide anion-induced cytokine production. Mice were treated with bosentan (100 mg/kg, p.o.) or vehicle, 1 h before KO2 (30 μg/paw) injection. Samples of paw skin (a–c) and spinal cord (d–f) were collected 3 h after KO2 injection and used to measure the cytokine levels by ELISA. Results of the three independent repetitions (experiments) are presented as mean ± SD (red lines) of each repetition as well as the value of each mouse. Independent repetitions are represented as different gray scales; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. n = 6 mice per group per repetition [*P < 0.05 vs. saline group; #P < 0.05 vs. vehicle group, (ANOVA followed by Bonferroni’s post hoc)]
Prepro-endothelin-1 expression is induced by the superoxide anion
Paw skin and spinal cord samples were collected 0.5–7 h after KO2 i.pl. injection to measure ppET-1 mRNA expression. Peripheral stimulus with the superoxide anion increased the expression of ppET-1 mRNA expression in paw skin (Fig. 5a) [F (5, 102) = 99.3, P < 0.0001] in all evaluated time points. The ppET-1 mRNA expression in the paw skin was higher at 5 and 7 h than at 1 h after stimulus injection (Fig. 5a). At the spinal cord level, peripheral stimulus with the superoxide anion increased ppET-1 expression at 0.5, 1, 3, and 5 h after stimulus injection (Fig. 5b) [F (5, 102) = 47.76, P < 0.0001].
Superoxide anion intraplantar stimulus induces local and spinal ppET-1 mRNA expression. Mice were stimulated with KO2 (30 μg/paw). Samples of paw skin (a) and spinal cord (b) were collected 0.5–7 h after KO2 injection and used to measure the ppET-1 mRNA expression by qPCR. Results of three independent repetitions (experiments) are presented as mean ± SD (red lines) of each repetition as well as the value of each mouse. Independent repetitions are represented as different gray scales; the dark gray represents the first repetition, the middle gray the second repetition, and the light gray the last repetition. n = 6 pools of 4 mice paws or n = 6 pools of 2 mice spinal cords (L4–L6) per group per repetition [*P < 0.05 vs. saline group; #P < 0.05 vs. 1 h, (ANOVA followed by Bonferroni’s post hoc)]
Discussion
We demonstrated that intraplantar injection of a superoxide anion donor, potassium superoxide, induces local and spinal cord mRNA expression of prepro-endothelin-1 (ppET-1) and triggers inflammation and pain that are amenable by treatment with bosentan, a mixed endothelin receptor antagonist. Importantly, this is the first study to demonstrate ET-1 mediates superoxide anion-induced inflammation and pain.
Superoxide anion activates pro-inflammatory signaling pathways, leading to the expression of adhesion molecules and chemokines in the vascular endothelium, which contributes to leukocyte recruitment and activation (Dusting et al. 2005). Activated leukocytes produce large amounts of TNF-α, IL-1β, and superoxide anion that, in turn, sensitize and activate nociceptive neurons (Kress et al. 1995; Cunha et al. 2005; Jin and Gereau 2006; Binshtok et al. 2008), leading to inflammatory hyperalgesia. This nociceptor sensitization can occur both in the peripheral tissue as well as in the spinal cord (Verri et al. 2006a, b). Treatment with bosentan inhibited superoxide anion-induced TNF-α and IL-1β production in the paw skin and spinal cord, while it enhanced IL-10 production at these sites. These results indicate that superoxide anion-induced peripheral and spinal cord TNF-α and IL-1β production depends on ET-1 as well as ET-1 attenuates IL-10 production, thus contributing to superoxide anion-induced inflammation and pain. In line with our results, bosentan inhibited TNF-α and IL-1β production in a mouse model of collagen-induced arthritis in mice (Donate et al. 2012), which was correlated with a reduction in the clinical signs of inflammation, including hyperalgesia, leukocyte recruitment, and edema. Bosentan also inhibited TNF-α production during breast cancer bone infiltration (Jewell et al. 2010), in endotoxin-induced uveitis (Keles et al. 2014), and in paracetamol-induced acute liver toxicity (Yayla et al. 2014) in mice.
The pro-oxidant activity of ET-1 was demonstrated in vitro and in vivo (Scalera et al. 2002; López-Sepúlveda et al. 2011; Martínez-Revelles et al. 2012). For instance, the vasoconstriction activity of ET-1 is superoxide anion-dependent in a model of oxidative stress induced by ischemia-reperfusion in rats (Martínez-Revelles et al. 2012). In the present study, we focused on the gap of information on whether ET-1 could mediate oxidative stress-induced inflammation and pain. Potassium superoxide has been used as a generator of superoxide anion in vivo (Wang et al. 2004b; Ndengele et al. 2008; Maioli et al. 2015). It has been previously demonstrated that potassium superoxide induces thermal hyperalgesia in the Hargreaves method, and we extended this observation in the methods used in the present study as standardized previously (Maioli et al. 2015). Superoxide anion induces oxidative stress directly, and it also sustains its own production by triggering inflammation (Morgan and Liu 2011) and inhibiting its metabolism (Wang et al. 2004b). Superoxide anion-induced oxidative stress was amenable to bosentan treatment. Corroborating our results, bosentan also reduced the oxidative stress in endotoxin-induced uveitis (Keles et al. 2014) and paracetamol-induced acute liver toxicity (Yayla et al. 2014).
Peripheral sensitization of nociceptive neurons increases the inputs received by nociceptive neurons in the spinal cord, which reduces the threshold of spinal neurons and leads to sensitization (Ji et al. 2003). We observed a time-dependent increase of ppET-1 mRNA expression in the paw skin and at the spinal cord level following peripheral stimulus with the superoxide anion. The increase of ppET-1 mRNA expression was more pronounced and long lasting as well as initiated earlier in the periphery than in the spinal cord, which is in agreement with the present model in which KO2 was injected in the paw. Therefore, it is reasonable that the peripheral stimulus with KO2 presents an important peripheral component that is responsible for the following spinal cord activation. A drawback of this approach is that mRNA expression does not guarantee the release/processing of active ET-1, but demonstrates that this system is upregulated by the superoxide anion. This increase of ppET-1 mRNA expression correlated with an increased production of TNF-α and IL-1β, and with oxidative stress at the peak of hyperalgesia. Bosentan inhibited these effects in the spinal cord, which also represent an important analgesic mechanism due to the role of TNF-α and IL-1β during the spinal sensitization of nociceptive transmission as occurs in many diseases (Ji et al. 2003). Taking into account the similarities between peripheral and spinal cord responses observed here, we suggest that bosentan inhibits both the peripheral and spinal activation of endothelin receptors induced by superoxide, reducing the activation of nociceptive neurons and, consequently, the nociceptive transmission-induced spinal facilitation. Our results also demonstrated that bosentan enhances peripheral and spinal IL-10 production during inflammatory pain triggered by the superoxide anion. In agreement with our data, bosentan also enhanced IL-10 production in Trypanosoma cruzi-infected rats (Rachid et al. 2006). Enhancing IL-10 production is an important anti-inflammatory and analgesic mechanism as highlighted in other models (Poole et al. 1995; Milligan et al. 2005; Verri et al. 2006a, b) and deserves further investigation.
The inhibition of superoxide anion-induced TNF-α and IL-1β production, enhancement of IL-10 production, and reduction of oxidative stress by bosentan treatment also accounted to diminish the paw edema and leukocyte recruitment. These cytokines and oxidative stress mediate inflammatory edema and leukocyte recruitment (Fuchs et al. 2001; Wang et al. 2004a; Joosten et al. 2006; Bradley 2008; Hattori et al. 2010; Morgan and Liu 2011; Kvietys and Granger 2012; Sadik et al. 2012). Furthermore, recruited leukocytes contribute to the inflammatory hyperalgesia by further production of nociceptive molecules (Guerrero et al. 2008; Ting et al. 2008; Verri et al. 2009).
Conclusion
Bosentan, a mixed endothelin receptor antagonist, inhibited superoxide anion-induced IL-1β and TNF-α production and enhanced IL-10 production. Bosentan also blocked the reduction of antioxidant defenses (reduced glutathione, ferric reducing ability, and ABTS radical scavenging ability) induced by superoxide anion. These effects of bosentan were observed locally (in the site of stimulus injection) and at the spinal cord level, as well as superoxide anion induced ppET-1 mRNA expression in the paw skin and spinal cord, suggesting ET-1 presents an integrative role between peripheral and spinal cord events in superoxide anion-induced inflammation and pain. Our results support the use of bosentan for the treatment of inflammation and pain and reveal a novel role of endothelin in superoxide anion-triggered inflammation and pain.
References
Ankier SI (1974) New hot plate tests to quantify antinociceptive and narcotic antagonist activities. Eur J Pharm 1:1–4. doi:10.1016/0014-2999(74)90195-2
Avdeev SN, Tsareva NA, Nekliudova GV, Chuchalin AG (2013) First clinical experience with endothelin receptor antagonist bosentan used in patients with pulmonary hypertension: results of a one-year study. Ter Arkh 85:38–43
Barton M, Yanagisawa M (2008) Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol 86:485–498. doi:10.1139/Y08-059
Binshtok AM, Wang H, Zimmermann K, et al (2008) Nociceptors are interleukin-1beta sensors. J Neurosci 28:14062–14073. doi:10.1523/JNEUROSCI.3795-08.2008
Bradley JR (2008) TNF-mediated inflammatory disease. J Pathol 214:149–160. doi:10.1002/path.2287
Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60:618–622
Casagrande R, Georgetti SR, Verri Jr WA, et al (2006) Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B 84:21–27. doi:10.1016/j.jphotobiol.2006.01.006
Channick R, Badesch DB, Tapson VF, et al (2001) Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Hear Lung Transpl 20:262–263
Conte Fde P, Barja-Fidalgo C, Verri Jr WA, et al (2008) Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-alpha, and CXCL-1. J Leukoc Biol 84:652–660. doi:10.1189/jlb.1207827
Cunha TM, Verri WA, Silva JS, et al (2005) A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A 102:1755–1760. doi:10.1073/pnas.0409225102
Cunha TM, Verri WA, Vivancos GG, et al (2004) An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res 37:401–407. doi:10.1590/S0100-879X2004000300018
Davar G, Hans G, Fareed MU, et al (1998) Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport 9:2279–2283
De-Melo JD, Tonussi CR, D’Orléans-Juste P, Rae GA (1998) Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain 77:261–269
Donate PB, Cunha TM, Verri Jr WA, et al (2012) Bosentan, an endothelin receptor antagonist, ameliorates collagen-induced arthritis: the role of TNF-alpha in the induction of endothelin system genes. Inflamm Res 61:337–348. doi:10.1007/s00011-011-0415-5
Dusting GJ, Selemidis S, Jiang F (2005) Mechanisms for suppressing NADPH oxidase in the vascular wall. Mem Inst Oswaldo Cruz 97–103
Ferreira SH, Romitelli M, de Nucci G (1989) Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol 13(Suppl 5):S220–S222. doi:10.1097/00005344-198900135-00065
Fiore G, Florio P, Micheli L, et al (2005) Endothelin-1 triggers placental oxidative stress pathways: putative role in preeclampsia. J Clin Endocrinol Metab 90:4205–4210. doi:10.1210/jc.2004-1632
Fuchs J, Zollner TM, Kaufmann R, Podda M (2001) Redox-modulated pathways in inflammatory skin diseases. Free Radic Biol Med 30:337–353
Gabriel A, Kuddus RH, Abdul SR, et al (1998) Superoxide-induced changes in endothelin (ET) receptors in hepatic stellate cells. J Hepatol 29:614–627. doi:10.1016/S0168-8278(98)80157-8
Gokin AP, Fareed MU, Pan HL, et al (2001) Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosc 14:5358–5366
Graido-Gonzalez E, Doherty JC, Bergreen EW, et al (1998) Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood 92:2551–2555
Guerrero AT, Verri Jr WA, Cunha TM, et al (2008) Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol 83:122–130. doi:10.1189/jlb.0207123
Hattori H, Subramanian KK, Sakai J, et al (2010) Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A 107:3546–3551. doi:10.1073/pnas.0914351107
Hsu J-H, Oishi P, Wiseman DA, et al (2010) Nitric oxide alterations following acute ductal constriction in the fetal lamb: a role for superoxide. Am J Physiol Lung Cell Mol Physiol 298:L880–L887. doi:10.1152/ajplung.00384.2009
Hung VKL, Chen SMY, Tai LW, et al (2012) Over-expression of endothelin-1 in astrocytes, but not endothelial cells, ameliorates inflammatory pain response after formalin injection. Life Sci 618–622
Hung VKL, Tai LW, Qiu Q, et al (2014) Over-expression of astrocytic ET-1 attenuates neuropathic pain by inhibition of ERK1/2 and Akt(s) via activation of ETA receptor. Mol Cell Neurosci 60:26–35. doi:10.1016/j.mcn.2014.02.007
Jandeleit-Dahm KA, Watson AM (2012) The endothelin system and endothelin receptor antagonists. Curr Opin Nephrol Hypertens 21:66–71. doi:10.1097/MNH.0b013e32834dde48
Jarvis MF, Wessale JL, Zhu CZ, et al (2000) ABT-627, an endothelin ETA receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur J Pharmacol 388:29–35. doi:10.1016/S0014-2999(99)00865-1
Jewell AN, Swamydas M, Castillo CI, et al (2010) The endothelin axis stimulates the expression of pro-inflammatory cytokines and pro-migratory molecules in breast cancer. Cancer Investig 28:932–943. doi:10.3109/07357907.2010.496757
Ji R-R, Kohno T, Moore KA, Woolf CJ (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26:696–705. doi:10.1016/j.tins.2003.09.017
Jin X, Gereau RW (2006) Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci 26:246–255. doi:10.1523/JNEUROSCI.3858-05.2006
Joosten LA, Netea MG, Kim SH, et al (2006) IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A 103:3298–3303. doi:10.1073/pnas.0511233103
Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2,2′-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol C Toxicol Pharmacol 140:47–52. doi:10.1016/j.cca.2005.01.005
Keles S, Halici Z, Atmaca HT, et al (2014) The ocular endothelin system: a novel target for the treatment of endotoxin-induced uveitis with bosentan. Investig Ophthalmol Vis Sci 55:3517–3524. doi:10.1167/iovs.14-14193
Khodorova A, Fareed MU, Gokin A, et al (2002) Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci 22:7788–7796
Kim YO, Kim IJ, Yoon MH (2015) Antiallodynic effect through spinal endothelin-B receptor antagonism in rat models of complex regional pain syndrome. Neurosci Lett 584:45–49. doi:10.1016/j.neulet.2014.10.005
Kress M, Riedl B, Reeh PW (1995) Effects of oxygen radicals on nociceptive afferents in the rat skin in vitro. Pain 62:87–94
Kvietys PR, Granger DN (2012) Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med 52:556–592. doi:10.1016/j.freeradbiomed.2011.11.002
Lau D, Szöcs K, Klinke A, et al (2014) Myeloperoxidase upregulates endothelin receptor type B expression. J Mol Cell Cardiol 69:76–82. doi:10.1016/j.yjmcc.2013.12.007
Letizia C, Boirivant M, De Toma G, et al (1998) Plasma levels of endothelin-1 in patients with Crohn’s disease and ulcerative colitis. Ital J Gastroenterol Hepatol 30:266–269
Liu JQ, Erbynn EM, Folz RJ (2005) Chronic hypoxia-enhanced murine pulmonary vasoconstriction: role of superoxide and gp91phox. Chest. doi:10.1378/chest.128.6_suppl.594S
López-Sepúlveda R, Gómez-Guzmán M, Zarzuelo MJ, et al (2011) Red wine polyphenols prevent endothelial dysfunction induced by endothelin-1 in rat aorta: role of NADPH oxidase. Clin Sci (Lond) 120:321–333. doi:10.1042/CS20100311
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maioli NA, Zarpelon AC, Mizokami SS, et al (2015) The superoxide anion donor, potassium superoxide, induces pain and inflammation in mice through production of reactive oxygen species and cyclooxygenase-2. Braz J Med Biol Res. doi:10.1590/1414-431X20144187
Martínez-Revelles S, Caracuel L, Márquez-Martín A, et al (2012) Increased endothelin-1 vasoconstriction in mesenteric resistance arteries after superior mesenteric ischaemia-reperfusion. Br J Pharmacol 165:937–950. doi:10.1111/j.1476-5381.2011.01617.x
Mascia L, Fedorko L, Stewart DJ, et al (2001) Temporal relationship between endothelin-1 concentrations and cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Stroke 32:1185–1190
Matsuo J, Oku H, Kanbara Y, et al (2009) Involvement of NADPH oxidase and protein kinase C in endothelin-1-induced superoxide production in retinal microvessels. Exp Eye Res 89:693–699. doi:10.1016/j.exer.2009.06.012
Miyasaka N, Hirata Y, Ando K, et al (1992) Increased production of endothelin-1 in patients with inflammatory arthritides. Arthritis Rheum 35:397–400. doi:10.1002/art.1780350406
Milligan ED, Sloane EM, Langer SJ, et al (2005) Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 1:9. doi:10.1186/1744-8069-1-9
Morelli S, Ferri C, Di Francesco L, et al (1995) Plasma endothelin-1 levels in patients with systemic sclerosis: influence of pulmonary or systemic arterial hypertension. Ann Rheum Dis 54:730–734. doi:10.1136/ard.54.9.730
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21:103–115. doi:10.1038/cr.2010.178
Motte S, McEntee K, Naeije R (2006) Endothelin receptor antagonists. Pharmacol Ther 110:386–414. doi:10.1016/j.pharmthera.2005.08.012
Ndengele MM, Cuzzocrea S, Esposito E, et al (2008) Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J 22:3154–3164. doi:10.1096/fj.08-108159
Ohanian J, Forman SP, Katzenberg G, Ohanian V (2012) Endothelin-1 stimulates small artery VCAM-1 expression through p38MAPK-dependent neutral sphingomyelinase. J Vasc Res 49:353–362. doi:10.1159/000336649
Piechota A, Polanczyk A, Goraca A (2011) Protective effects of endothelin-A receptor antagonist BQ123 against LPS-induced oxidative stress in lungs. Pharmacol Rep 63:494–500
Pinho-Ribeiro FA, Borghi SM, Staurengo-Ferrari L, et al (2014) Bosentan, a mixed endothelin receptor antagonist, induces antidepressant-like activity in mice. Neurosci Lett 560:57–61. doi:10.1016/j.neulet.2013.12.018
Pinho-Ribeiro FA, Hohmann MSN, Borghi SM, et al (2015) Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: role of TRPV1, oxidative stress, cytokines and NF-κB. Chem Biol Interact 228:88–99. doi:10.1016/j.cbi.2015.01.011
Pomonis JD, Rogers SD, Peters CM, et al (2001) Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci 21:999–1006
Poole S, Cunha FQ, Selkirk S, et al (1995) Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol 115:684–688
Rachid MA, Camargos ERS, Barcellos L, et al (2006) Blockade of endothelin ETA/ETB receptors favors a role for endothelin during acute Trypanosoma cruzi infection in rats. Microbes Infect 8:2113–2119. doi:10.1016/j.micinf.2006.03.017
Rancourt M-E, Rodrigue M-E, Agharazii M, et al (2010) Role of oxidative stress in erythropoietin-induced hypertension in uremic rats. Am J Hypertens 23:314–320. doi:10.1038/ajh.2009.242
Re R, Pellegrini N, Proteggente A, et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Romero M, Jimenez R, Sanchez M, et al (2010) Vascular superoxide production by endothelin-1 requires Src non-receptor protein tyrosine kinase and MAPK activation. Atherosclerosis 212:78–85. doi:10.1016/j.atherosclerosis.2010.04.031
Rubin LJ, Roux S (2002) Bosentan: a dual endothelin receptor antagonist. Expert Opin Investig Drugs 11:991–1002. doi:10.1517/13543784.11.7.991
Sadik CD, Noack B, Schacher B, et al (2012) Cytokine production by leukocytes of Papillon-Lefevre syndrome patients in whole blood cultures. Clin Oral Investig 16:591–597. doi:10.1007/s00784-011-0532-0
Scalera F, Dittrich R, Beckmann MW, Beinder E (2002) Effect of endothelin-1 on intracellular glutathione and lipid peroxide availability and on the secretion of vasoactive substances by human umbilical vein endothelial cells. Eur J Clin Investig 32:556–562. doi:10.1046/j.1365-2362.2002.01040.x
Ting E, Guerrero AT, Cunha TM, et al (2008) Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol 153:1043–1053. doi:10.1038/sj.bjp.0707640
Verri Jr WA, Cunha TM, Ferreira SH, et al (2007) IL-15 mediates antigen-induced neutrophil migration by triggering IL-18 production. Eur J Immunol 37:3373–3380. doi:10.1002/eji.200737488
Verri Jr WA, Cunha TM, Magro DA, et al (2009) Targeting endothelin ETA and ETB receptors inhibits antigen-induced neutrophil migration and mechanical hypernociception in mice. Naunyn Schmiedeberg's Arch Pharmacol 379:271–279. doi:10.1007/s00210-008-0360-1
Verri Jr WA, Cunha TM, Parada CA, et al (2006a) IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proc Natl Acad Sci U S A 103:9721–9725. doi:10.1073/pnas.0603286103
Verri Jr WA, Cunha TM, Parada CA, et al (2006b) Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther 112:116–138. doi:10.1016/j.pharmthera.2006.04.001
Verri Jr WA, Guerrero AT, Fukada SY, et al (2008) IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A 105:2723–2728. doi:10.1073/pnas.0712116105
Verri Jr WA, Schivo IR, Cunha TM, et al (2004) Interleukin-18 induces mechanical hypernociception in rats via endothelin acting on ETB receptors in a morphine-sensitive manner. J Pharmacol Exp Ther 310:710–717. doi:10.1124/jpet.103.063990
Wang T, Qin L, Liu B, et al (2004a) Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem 88:939–947
Wang X, Tong M, Chinta S, et al (2006) Hypoxia-induced reactive oxygen species downregulate ETB receptor-mediated contraction of rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 290:L570–L578. doi:10.1152/ajplung.00262.2005
Wang ZQ, Porreca F, Cuzzocrea S, et al (2004b) A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther 309:869–878. doi:10.1124/jpet.103.064154
Yanagisawa M, Kurihara H, Kimura S, et al (1988) A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl 6:S188–S191
Yayla M, Halici Z, Unal B, et al (2014) Protective effect of Et-1 receptor antagonist bosentan on paracetamol induced acute liver toxicity in rats. Eur J Pharmacol 726:87–95. doi:10.1016/j.ejphar.2014.01.022
Yoon MH, Reriani M, Mario G, et al (2013) Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. doi:10.1016/j.ijcard.2012.12.001
Zarpelon AC, Pinto LG, Cunha TM, et al (2012) Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFalpha and CXCL1/CXCR2 in mice. Can J Physiol Pharmacol 90:187–199. doi:10.1139/y11-116
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministério da Ciência, Tecnologia e Inovação (MCTI)/Secretaria da Ciência, Tecnologia e Ensino Superior (SETI)/Fundação Araucária, and Paraná State Government, Brazil.
Compliance with ethical standards
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serafim, K.G.G., Navarro, S.A., Zarpelon, A.C. et al. Bosentan, a mixed endothelin receptor antagonist, inhibits superoxide anion-induced pain and inflammation in mice. Naunyn-Schmiedeberg's Arch Pharmacol 388, 1211–1221 (2015). https://doi.org/10.1007/s00210-015-1160-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-015-1160-z