Abstract
The transcription factor nuclear factor kappa B (NF-κB) has attracted increasing attention in the field of cancer research from last few decades. Aberrant activation of this transcription factor is frequently encountered in a variety of solid tumors and hematological malignancies. NF-κB family members and their regulated genes have been linked to malignant transformation, tumor cell proliferation, survival, angiogenesis, invasion/metastasis, and therapeutic resistance. In this review, we highlight the diverse molecular mechanism(s) by which the NF-κB pathway is constitutively activated in different types of human cancers, and the potential role of various oncogenic genes regulated by this transcription factor in cancer development and progression. Additionally, various pharmacological approaches employed to target the deregulated NF-κB signaling pathway, and their possible therapeutic potential in cancer therapy is also discussed briefly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
NF-κB signaling pathway
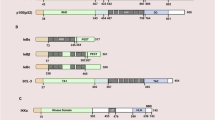
Nuclear factor kappa B (NF-κB) was first identified as a DNA-binding protein that specifically bound to the immunoglobulin κ light-chain enhancer, which is restricted in B cells, by David Baltimore in 1986 (Sen and Baltimore 1986). NF-κB is a Rel family transcription factor that consists of five members in mammalian cells, namely RelA (p65), RelB, Rel (c-Rel), NF-κB1 (p50/p105), and NF-κB2 (p52/p100) (Baldwin 1996). Both classical and alternate pathways can activate NF-κB signaling through an IκB kinase (IKK)-dependent manner (Tergaonkar 2006). In the canonical pathway, the activated β-subunit of IKK (IKKβ) phosphorylates the negative regulator of NF-κB [inhibitor of kappa B-α (IκBα) protein] upon the activation of the IKK complex, and thereafter leads to the ubiquitination and proteasome-mediated degradation of IκBα. This releases the p65/p50 heterodimer and allows the translocation of the NF-κB complex into the nucleus (Hayden and Ghosh 2004).
Activation of the non-canonical pathway involves the NF-κB-inducing kinase (NIK)-mediated activation of the IKKα homodimer, which then activates p100/RelB by proteasomal degradation of its inhibitory C-terminal half for processing into the p52/RelB heterodimer (Senftleben et al. 2001). Various pro-inflammatory cytokines; tumor necrosis factor (TNF); lipopolysaccharide (LPS); and other stimuli such as DNA-damaging agents and viral proteins, working through the TNF receptor (TNFR), Toll-like receptor/interleukin-1 (TLR/IL-1R), and T-cell receptor (TCR), activate the classical NF-κB pathway (Hayden and Ghosh 2004). Following the ligand receptor binding, signaling proceeds through TNFR-associated factor/receptor-interacting protein (TRAF/RIP) complexes, usually with the engagement of TGF-β-activated kinase 1 (TAK1), leading to canonical signaling (Hayden and Ghosh 2008). On the other hand, the alternative pathway is stimulated by a more restricted set of cytokines that belong to the TNF superfamily, such as B-cell-activating factor (BAFF), LTβ, and CD40 (Chen and Greene 2004). It is well established that the canonical NF-κB pathway is essential for inflammation and innate immunity, while the non-canonical pathway plays a central role in the lymphoid organ development and adaptive immunity (Bonizzi and Karin 2004). In general, NF-κB family proteins are evolutionarily conserved mediators that integrate multiple stress stimuli to regulate innate and adaptive immune responses; they act broadly to influence gene expression events that impact cell survival, differentiation, proliferation, adhesion, immunity, and inflammation (Ghosh et al. 1998; Perkins 2007; Shen and Tergaonkar 2009).
Role of NF-κB in cancer initiation and progression
Aberrant NF-κB activation has been implicated in the pathogenesis of various human diseases such as inflammatory diseases; metabolic disorders; cancers that are related to inflammation, oxidative stress, and enhanced cell proliferation; viral infection; and genetic disorders (Kumar et al. 2004; Sarkar et al. 2008; Wong and Tergaonkar 2009). The first evidence implicating the oncogenic potential of NF-κB was the identification of retroviral oncoprotein v-rel, which shares a Rel transactivation domain with the mammalian homologs (Carrasco et al. 1996; Gilmore 1999). Numerous evidences have shown that constitutive activation of NF-κB is prevalent in most major human cancers mainly due to the aberrant activation of upstream signaling molecules, or through the autocrine or paracrine activation by cytokines and growth factors, and sometimes by the genetic alteration of genes encoding NF-κB and IκB proteins (Karin et al. 2002; Van Waes 2007; Table 1). The constitutive activation of NF-κB in specific human malignancies is discussed below.
Molecular mechanism(s) of constitutive NF-κB activation in major solid tumors
Hepatocellular carcinoma (HCC)
Several studies have reported the persistent activation of NF-κB in hepatocellular carcinoma cell lines and tissue samples derived from liver cancer patients (Arsura and Cavin 2005; Qiao et al. 2006; Tai et al. 2000). Experimental mouse models have suggested that the growth factor-mediated NF-κB activation in hepatocytes involving the PI3-kinase (PI3K)/Akt axis (Cavin et al. 2005), hepatocyte growth factor (HGF)/Met signaling (Muller et al. 2002), and TAK1/IKK pathway (Arsura et al. 2003) promotes liver tumor development and progression. Viral proteins of oncovirus have been identified to activate the NF-κB signaling pathway (Block et al. 2003), including hepatitis B virus (HBV) X protein (Diao et al. 2001), as well as hepatitis C virus (HCV) non-structural 5A (NS5A) (Waris et al. 2003), and core proteins (Sato et al. 2006; Shrivastava et al. 1998), which are implicated in hepatocellular transformation (Bouchard and Schneider 2004; McGivern and Lemon 2011). Tight connections between inflammation and cancer have long been established, and tumor-promoting inflammation has been described as one of the hallmarks of cancer by Hanahan and Weinberg (Hanahan and Weinberg 2011; Sethi et al. 2012). As a key player in inflammation and cancer, it is no surprise that NF-κB has been identified to be linked to inflammation-associated liver cancer in an Mdr2-knockout mouse model, from which the animal spontaneously develops cholestatic hepatitis-induced HCC (Pikarsky et al. 2004). The study demonstrated that IκBα super-repressor (IκBα-SR) or anti-TNFα antibodies inhibited the TNFα-induced NF-κB activation in Mdr2 −/− mice, resulted in apoptosis of transformed hepatocytes, and retarded progression to malignancy (Pikarsky et al. 2004). It is correlated with the observation of enhanced carcinogen-mediated hepatocyte death in mice with hepatocyte-specific deletion of IKKβ (Maeda et al. 2005); surprisingly, IKKβ-knockout mice presented a significant increase in diethylnitrosamine (DEN)-induced hepatocarcinogenesis owing to the increased compensatory proliferation of surviving hepatocytes. Additional ablation of IKKβ in adjacent myeloid cells (Kupffer cells) markedly reduced hepatocarcinogenesis, which was attributed to the suppression of NF-κB-dependent production of potent hepatomitogens (TNFα, IL-6, and HGF) that stimulate proliferation of hepatocytes (Maeda et al. 2005).
Colorectal cancer
Further evidence linking NF-κB with inflammation-associated tumor development comes from a mouse model of colitis-associated colon cancer (CAC) (Greten et al. 2004). Specific deletion of IKKβ in intestinal epithelial cells (enterocytes) or myeloid cells led to reduction in tumor formation, or a decrease in both tumor incidence and tumor size, respectively, indicating that enterocytes’ IKKβ contributes to early tumor promotion by inhibiting apoptosis, while it promotes tumor growth by inducing the expression of pro-inflammatory cytokines in myeloid cells (Greten et al. 2004). The study not only revealed the importance of the IKK/NF-κB pathway in the tumor microenvironment for tumorigenesis, but also highlighted its cell type-dependent functions. In fact, in addition to colon cancer cell lines and colorectal carcinoma tissue samples (Kojima et al. 2004; Lind et al. 2001; Yu et al. 2003), NF-κB has been found to be highly expressed and active in the stroma of human colonic adenomatous polyps (Hardwick et al. 2001). Various factors such as pattern recognition receptors (PRRs) (Karin 2006), tumor-promoting cytokines (Terzic et al. 2010), or casein kinase 2 (CK2) (Farah et al. 2003) have been implicated in the constitutive activation of NF-κB in colorectal cancer; however, the molecular mechanisms underlying this response require further investigation. It has been demonstrated, using a mouse colon cancer metastasis model, that inhibition of NF-κB by IκBα-SR could convert LPS-induced tumor growth to tumor regression (Luo et al. 2004). In addition, knockdown of the NF-κB p65 subunit by small interfering RNA (siRNA) has been shown to enhance in vitro and in vivo sensitivity of colon carcinoma to the chemotherapeutic agent CPT-11 (irinotecan) (Guo et al. 2004).
Breast cancer
Several studies have documented the aberrant nuclear expression of different NF-κB family members (c-Rel, p50, and RelA) and constitutive NF-κB DNA-binding activity in many human breast tumor cell lines, human breast cancer specimens, and the majority of carcinogen-induced primary rat mammary tumors (Cogswell et al. 2000; Nakshatri et al. 1997; Sovak et al. 1997). In the transgenic mouse model, 31.6 % of mice that overexpressed c-Rel in the mammary gland developed mammary tumors, in which significant increases in the expression of the cancer-related NF-κB target genes were observed (Romieu-Mourez et al. 2003). These findings reveal the important involvement of constitutive NF-κB activation in the early development of breast cancer. It is suggested that the activation of RelA is associated more with distinct subtypes, as the classic form of NF-κB (RelA/p50 heterodimer) has been detected predominantly in HER2-overexpressing estrogen receptor (ER)-negative breast tumors, which were responsive to treatment with specific NF-κB inhibitors (Biswas et al. 2004; Singh et al. 2007). Another study has shown that an accumulation of nuclear c-Rel, p50, and p52, rather than p65, is differentially activated in breast tumors regardless of ER status (Cogswell et al. 2000). One mechanism, which underlies the elevated activation of NF-κB in breast cancer cells, has been proposed to be regulated by oncogenic signaling. For example, HER2/neu overexpression induces NF-κB through calpain-mediated IκBα degradation by activating the PI3K/Akt pathway (Pianetti et al. 2001; Zhou et al. 2000). Aberrant activation of protein kinase CK2 in breast cancer has also been found to promote the degradation of IκBα by phosphorylating this NF-κB inhibitor, resulting in increased nuclear translocation of NF-κB (Landesman-Bollag et al. 2001; Romieu-Mourez et al. 2001). The essential requirement of IKKα and NF-κB in mammary gland development provides the hint for the importance of increased NF-κB activation in breast cancers (Cao et al. 2001). Evidence has shown that the deletion of IKKα delayed the onset of progesterone-driven mammary cancer with decreased NF-κB activation (Schramek et al. 2010). Besides the substantial role of IKKα in breast carcinogenesis, it has also been found to be important for the self-renewal of HER2-transformed mammary tumor-initiating cells, as well as the metastatic spread of breast cancer (Cao et al. 2007; Tan et al. 2011).
Prostate cancer
NF-κB levels have been found to be constitutively activated in hormone-independent human prostate cell lines that do no response to anti-androgen therapy (Palayoor et al. 1999), while androgen could produce sustained elevation of NF-κB activity in androgen-responsive prostate cancer cells (Ripple et al. 1999). Many studies have also provided evidence of overexpression and activation of NF-κB in human prostate tumors, which correlates with disease progression (Ross et al. 2004; Shukla et al. 2004). Deregulated NF-κB signaling has been implicated in mediating cellular transformation, prostate cancer growth, lymph node metastases, and disease outcome (Fradet et al. 2004; Ismail et al. 2004; Kim et al. 2002; Zhang et al. 2009). The most well-characterized mechanism underlying the constitutive NF-κB activation in prostate cancer has been attributed to Akt activation, which interacts with and stimulates IKK (Dan et al. 2008; Shukla et al. 2005), but other tyrosine kinases such as epidermal growth factor receptor (EGFR), HER2, and NIK are also suggested to be involved in the activation of the pathway (Le Page et al. 2005; Suh et al. 2002). Silencing of IKKα has been found to delay the progression of castration-resistant prostate cancer, whereas IKKβ ablation presents no effect on the development of the tumor (Ammirante et al. 2010). The involvement of IKK in cancer metastasis has been established in the model of prostate cancer, in which IKKα activation mediated through the induction of receptor activator of NF-κB (RANK) promotes metastatic progression by inhibiting the metastasis suppressor Maspin (Luo et al. 2007). Inhibition of NF-κB by the signal-unresponsive IκBα mutant (IκBαM) or peptide antagonist of NF-κB nuclear translocation led to the suppression of prostate cancer cell proliferation, induction of apoptosis, or sensitization to TNFα treatment (Asano et al. 2004; Gasparian et al. 2002; Herrmann et al. 1997). Most importantly, blockade of NF-κB activity decreased angiogenesis, invasion, and metastasis of xeno-transplanted human prostate tumors in nude mice (Huang et al. 2001).
Head and neck squamous cell carcinoma (HNSCC)
Many studies have previously documented the prevalence of constitutively activated NF-κB in diverse HNSCC cell lines and tumor tissue specimens (Bancroft et al. 2001; Mishra et al. 2006; Nakayama et al. 2001; Ondrey et al. 1999). The deregulated NF-κB signaling modulates the expression of programs of functional genes that contributes to different stages of HNSCC development and progression (Allen et al. 2007; Bancroft et al. 2001; Loercher et al. 2004; Molinolo et al. 2009; Ondrey et al. 1999). Global gene profiling analysis has also clearly indicated that NF-κB signaling is a major contributor to metastatic progression of HNSCC (Dong et al. 2001) and a prognostic biomarker of a high-risk disease (Chung et al. 2006). An elevated phosphorylation level of NF-κB in patient samples is associated with poor prognosis in terms of high recurrence and poor survival (Zhang et al. 2005). Autocrine or paracrine secretion of various cytokines or growth factors has been linked to NF-κB activation in HNSCC (Van Waes 2007; Wang et al. 2009). For example, interleukin-1 alpha (IL-1α) has been shown to be overexpressed autonomously by HNSCC and contributes to the activation of NF-κB, thereby promoting cell survival and growth (Wolf et al. 2001).
Major risk factors for head and neck cancer, such as cigarette smoking or human papillomavirus (HPV) infection, have been implicated in NF-κB activation. Cigarette smoke condensate is capable of activating NF-κB via phosphorylation and degradation of IκBα that is mediated through induction of IKK in HNSCC cell lines (Anto et al. 2002). Also, a positive correlation between the nuclear localization of NF-κB and HPV16-encoded E7 protein level has been reported in laryngeal cancer (Du et al. 2003). In HNSCC, several upstream signaling pathways have been indicated to mediate the constitutive activation of NF-κB, namely PI3K/Akt cascade (Bancroft et al. 2002), CK2 (Yu et al. 2006), and the TNF–TNFR1–TRADD–TRAF2–RIP–TAK1–IKK pathway (Jackson-Bernitsas et al. 2007). Different experimental approaches have been designed to block the deregulated NF-κB activation, such as dominant negative IκBαM (Duffey et al. 1999), IKKα, and IKKβ kinase dead mutants (Yu et al. 2006), or pharmacological inhibitors (Bancroft et al. 2002). The inhibition of NF-κB activation in HNSCC significantly suppresses the expression of NF-κB-modulated genes, such as pro-inflammatory cytokines (IL-6, IL-8, and YAP1), and results in the induction of cell death and tumor growth inhibition (Allen et al. 2007; Wang et al. 2009).
Constitutive NF-κB activation in hematological malignancies
Leukemias and lymphomas
Various genetic abnormalities of the NF-κB pathway have been found in human leukemia and lymphoid malignancies. Amplifications of c-Rel are frequently seen in Hodgkin’s lymphomas and diffuse large B-cell lymphomas (DLBCLs), while fewer cases of c-Rel gene rearrangements are found in certain types of B-cell lymphomas (Courtois and Gilmore 2006; Rayet and Gelinas 1999). In contrast to c-Rel, amplifications or chromosomal rearrangements of RelA and RelB have not been consistently reported in human leukemias and lymphomas (Gilmore et al. 2004). In addition, constitutive activation of NF-κB by NFκB2 gene alterations, but not the NFKB1 gene, and inactivating mutations of the IκB gene have been associated with several B- and T-cell lymphomas and chronic lymphocytic leukemia (CLL), as well as Hodgkin’s lymphoma (HL) (Courtois and Gilmore 2006; Karin et al. 2002). Aberrant activation of receptors and key upstream mediators also leads to the persistent NF-κB signaling observed in leukemias and lymphomas (Jost and Ruland 2007). NF-κB activation has been found to be associated with virus-induced leukemias and lymphomas. A study of adult T-cell leukemia (ATL) led to the discovery of the first human retrovirus, human T-cell leukemia virus type 1 (HTLV-1), which was then identified as causal agent of ATL (Matsuoka and Jeang 2007). The HTLV-1 Tax oncoprotein stably associates with IKKγ to form Tax/IKK complexes that persistently activate NF-κB through both canonical and non-canonical pathways (Matsuoka and Jeang 2007). Constitutive activation of NF-κB has been found to be essential for Tax-mediated transformation (Yamaoka et al. 1996).
Infection with Epstein–Barr virus (EBV) is associated with a high risk of Hodgkin’s lymphoma, Burkitt’s lymphoma, and B-cell lymphomas (Young and Rickinson 2004). The EBV-encoded membrane protein LMP1 acts as the member of the TNFR superfamily and constitutively activates the NF-κB pathway in a ligand-independent manner (Young and Rickinson 2004). Another oncogenic protein, Bcr-Abl, which is strongly associated with chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL), is also capable of activating NF-κB (Reuther et al. 1998). The resulting nuclear translocation of NF-κB and enhanced transactivation function has been found to be dependent on the tyrosine kinase activity of Abl (Kirchner et al. 2003; Reuther et al. 1998). Moreover, Bcr-Abl-induced transformation of primary bone marrow cells and tumor growth can be blocked by IκBα-SR-mediated NF-κB inhibition (Reuther et al. 1998). Aberrant NF-κB activation has been demonstrated to be required for the proliferation and survival of Hodgkin’s disease tumor cells and activated B-cell-like (ABC) DLBCL, and contributes to the poor clinical outcome (Bargou et al. 1997; Davis et al. 2001; Jost and Ruland 2007). Introducing IκBα-SR by retroviral transduction caused reduction in cell proliferation rate, blocked cell-cycle progression, and induced apoptosis in HL and ABC DLBCL cells (Bargou et al. 1997; Davis et al. 2001). It was shown in the same study that HL cells depleted of constitutive nuclear NF-κB revealed strongly impaired tumor growth after being xenografted into SCID mice (Bargou et al. 1997).
Multiple myeloma
Diverse genetic or epigenetic alterations that trigger both the canonical and non-canonical NF-κB pathways have been described in multiple myeloma cell lines and patient samples (Annunziata et al. 2007; , Keats et al. (2007) et al. 2007; Demchenko et al. 2010). The alterations include overexpression or activating mutations of positive regulators of NF-κB signaling such as TACI, CD40, LTβR, NIK, NFKB1, and NFKB2 and inactivating abnormalities of negative regulators such as BIRC2/BIRC3, cIAP1/cIAP2, CYLD, TRAF2, and TRAF3 (Annunziata et al. 2007; Keats et al. 2007; Demchenko et al. 2010). Constitutive activation of the NF-κB signaling pathway stimulates cell growth, promotes cell survival, inhibits programmed cell death, and induces drug resistance in myeloma cells (Hideshima et al. 2002, 2004; Richardson et al. 2003). Moreover, production of various cytokines and growth factors such as interleukin-6 (IL-6), TNFα, and vascular endothelial growth factor (VEGF) by the NF-κB pathway facilitates the development of autocrine or paracrine signaling loops involving the interaction of myeloma cells and bone marrow stromal cells, resulting in further progression of the disease (Anderson and Carrasco 2011; Hideshima et al. 2004).
Role of major NF-κB-regulated genes in cancer development
Activated NF-κB binds to specific DNA sequences in target genes, designated as κB elements, and regulates transcription of over five hundred genes (Li and Sethi 2010). A number of important NF-κB-regulated genes involved in tumor cell proliferation, survival, angiogenesis, invasion/metastasis, and therapeutic resistance are listed below, and their roles are discussed.
Cyclooxygenase-2 (COX-2)
Aberrant arachidonic acid metabolism is involved in inflammation and carcinogenesis, owing to the tumor-promoting activities of the metabolic products prostaglandins (PGs) and leukotrienes (Wang and DuBois 2006). Pharmacological intervention in the biosynthesis of these metabolites by inhibiting the relevant enzymes has been considered an effective approach for cancer chemoprevention and treatment (Zha et al. 2004). The PG endoperoxide H synthases (PGHS), also known as cyclooxygenases (COX), catalyze the rate-limiting step in PG synthesis (Smith et al. 2000). There are two isoforms of the enzyme, namely COX-1 and COX-2. COX-1 universally exists in most mammalian tissues, while COX-2 is expressed rapidly in response to pro-inflammatory mediators and mitogenic stimuli (Surh et al. 2001). The expression of COX-2 but not COX-1 has been always observed to be upregulated in a wide variety of human cancers (Fosslien 2000; Surh et al. 2001; Zha et al. 2004). The promoter region of the COX-2 gene contains putative binding sites for NF-κB, which acts as a transcription factor to regulate the induction of COX-2 (Yamamoto et al. 1995).
Activation of NF-κB is required for COX-2 production, as evidenced by the observations of impaired COX-2 expression after specific inhibition of NF-κB (Smith et al. 2000). Moreover, evidence indicates that COX-2 expression is able to promote the activity NF-κB, indicating a positive feedback control mechanism (Poligone and Baldwin 2001). COX-2 can be activated by various chemotherapeutic agents and radiation, and thus contributes to therapeutic resistance (Li and Sethi 2010). Widely used therapeutic microtubule-interfering agents such as taxol, colchicines, and vinblastine have been found to induce activator protein-1 (AP-1) to mediate COX-2 expression via the cyclic AMP response element site in the COX-2 promoter (Subbaramaiah et al. 2000). Taxanes including paclitaxel and docetaxel are able to stabilize COX-2 mRNA through protein kinase C (PKC) and p38 MAPK signaling (Subbaramaiah et al. 2003). Also, levels of COX-2 expression have been reported to be correlated inversely with increased tumor radiation sensitivity in oral squamous cell carcinoma (Terakado et al. 2004). It has been reported that specific COX-2 inhibition by celecoxib synergistically enhances the anti-tumor activity of chemotherapeutic drugs such as CPT-11 (Trifan et al. 2002), COL-3, and docetaxel (Dandekar et al. 2005). In addition to the chemosentization effect, celecoxib augmented the response of A431 human tumor xenografts in nude mice to radiation (Nakata et al. 2004). Another COX-2 inhibitor, SC-236, has been shown to potentiate the effects of radiation therapy in different cancer models including sarcoma (Kishi et al. 2000) and glioma (Petersen et al. 2000). COX-2 has been also found to promote colon carcinoma-induced angiogenesis by producing important angiogenic factors (Tsujii et al. 1998). Jung and his colleagues confirmed, using human lung cancer cells, that the induction of VEGF through COX-2 upregulation is NF-κB-dependent (Jung et al. 2003). Furthermore, COX-2-mediated endothelial cell migration and tube formation could be inhibited by the selective COX-2 inhibitor NS-398 and the non-selective inhibitor aspirin (Tsujii et al. 1998).
Cyclin D1
Cyclin Dl, which is encoded by the CCNDl gene, controls the transition from G1 to S phase in the cell cycle (Sherr 1996). Overexpression of cyclin D1 and amplification or translocation of 11q13 (where CCND1 locates) are found frequently in many human cancers (Donnellan and Chetty 1998; Hunter and Pines 1994; Sherr 1996). NF-κB promotes cell proliferation through transcriptional activation of cyclin D1 by binding to the cyclin D1 promoter (Guttridge et al. 1999; Hinz et al. 1999). The inhibition of NF-κB reduces the cyclin D1 activity, which is associated with delayed phosphorylation of the retinoblastoma (Rb) protein, resulting in impaired cell-cycle progression that can be rescued by ectopic expression of cyclin D1 (Guttridge et al. 1999; Hinz et al. 1999). IKKα has been found to be required for NF-κB-induced cyclin D1 expression, which is proposed as a key element in mammary gland development and mammary carcinogenesis (Cao et al. 2001, 2007). Consistently, transcriptional activation of cyclin D1 by IKKα has been reported in other cell types, but mediated by distinct factors such as β-catenin (Albanese et al. 2003) and ER α (Park et al. 2005). In human breast epithelial cells, IκB homology Bcl-3 in association with p52 homodimers has been demonstrated to stimulate the transcription of the cyclin D1 gene via directly interacting with the NF-κB binding site in the cyclin D1 promoter (Westerheide et al. 2001). This finding is in accordance with the observations of increased nuclear accumulation of p50, p52, and Bcl-3 with elevated expression of cyclin D1 in tumorigenic breast tissues (Cogswell et al. 2000). Inhibition of cyclin D1 expression by using a cyclin D1 antisense construct in a variety of cancers not only resulted in attenuated cancer cell proliferation and loss of tumorigenicity, but also led to an increased growth-inhibitory effect of chemotherapeutic agents. These findings suggest that cyclin D1 may exert a protective effect against drug-induced cytotoxicity and further implies a requirement for cyclin D1 in the maintenance of chemoresistance in these cells (Arber et al. 1997; Kornmann et al. 1998, 1999; Nakashima and Clayman 2000; Schrump et al. 1996; Zhou et al. 1995).
Anti-apoptotic genes
The Bcl-2 family of proto-oncogenes is a critical negative regulator of apoptosis and is frequently dysregulated in wide variety of cancers. The promoter of human Bfl-1/A1, Bcl-xL, and Bcl-2 has been identified to present an NF-κB binding site, which is responsible for its c-Rel/RelA-, c-Rel-, or p50/p65-dependent induction, respectively (Catz and Johnson 2001; Chen et al. 2000; Zong et al. 1999). In IκBα-SR-expressing cells, overexpression of either Bfl-1/A1 or Bcl-xL has been found to confer resistance to apoptosis induced by TNFα (Chen et al. 2000; Karin and Lin 2002; Zong et al. 1999). Furthermore, NF-κB-induced expression of Bfl-1/A1 potently suppressed chemotherapy-induced apoptosis by inhibiting the release of cytochrome c and by blocking caspase-3 activation (Wang et al. 1999). Indeed, most chemotherapeutic drugs and ionizing radiation that activate NF-κB can also activate Bcl-2 family proteins in various cancer cell lines (Li and Sethi 2010). We have previously summarized several studies demonstrating the downregulation of different Bcl-2 family members by antisense oligonucleotides, small-molecule inhibitors, or siRNAs leading to enhanced chemo- or radiosensitization of multiple human cancers (Li and Sethi 2010).
Another important group of survival proteins regulated by NF-κB is the inhibitors of apoptosis (IAPs), which directly bind and inhibit caspase activity (Deveraux and Reed 1999; Tamm et al. 1998). Expression of IAPs is induced in response to several NF-κB-activating stimuli, including TNFα, phorbol myristol acetate (PMA), LPS, and IL-1β, and the induction was blocked by IκBα-SR, suggesting the involvement of IAPs in the anti-apoptotic activity of NF-κB (Chu et al. 1997; Stehlik et al. 1998; Wang et al. 1998). Moreover, cIAP1, cIAP2, and XIAP can further enhance cell survival by their ability to promote NF-κB activation in a positive feedback loop (Chu et al. 1997; Gyrd-Hansen and Meier 2010; Hofer-Warbinek et al. 2000). Survivin, a key regulator of mitosis and programmed cell death, has provided strong evidence for IAP involvement in cancer. RelB and p50 are bound to the NF-κB binding site in the survivin promoter between −354 and −345 (Kawakami et al. 2005). Survivin has been found to be selectively expressed in transformed cells and in most human cancers (LaCasse et al. 1998; Mita et al. 2008). The level of survivin correlates with a decreased tumor cell apoptotic index in gastric cancer (Lu et al. 1998) as well as colorectal cancer (Kawasaki et al. 1998); more importantly, patients with a low apoptotic index or with survivin-negative tumors had shortened 5-year disease survival than the group with high apoptosis or those with survivin-positive tumors (Kawasaki et al. 1998; Sarela et al. 2000). Cancer patients with lymph node invasion, metastases, and recurrent disease displayed significantly higher expression of survivin (Mita et al. 2008). Finally, overexpression of survivin can be useful as a predictive factor to determine response to chemotherapy in patients with bladder cancer, breast cancer multiple myeloma, lymphoma, and ovarian carcinoma (Li and Sethi 2010). Consistent with the findings for survivin, alterations in other IAP members found in many types of human cancer are also associated with chemoresistance, disease progression, and poor prognosis (Gyrd-Hansen and Meier 2010; Hunter et al. 2007; LaCasse et al. 2008). Strategies such as antisense oligonucleotides, siRNA, and small-molecule antagonists have been developed to block IAPs, and have resulted in induction of apoptosis, inhibition of tumor growth, and increased sensitivity to chemo- or radiotherapy in a broad spectrum of human cancer models (Gyrd-Hansen and Meier 2010; Hunter et al. 2007; LaCasse et al. 2008).
Angiogenic factors
Tumor-associated neovasculature, generated by angiogenesis, develops during tumorigenesis to facilitate the tumor acquiring nutrients and oxygen and disposing of metabolic wastes (Hanahan and Weinberg 2011). This process is initiated by an “angiogenic switch” in which the balance between pro-angiogenic and anti-angiogenic factors is tipped toward angiogenesis (Carmeliet and Jain 2000). The deregulated NF-κB signaling also contributes to angiogenesis through modulation of major pro-angiogenic factors such as VEGF and pro-inflammatory cytokines (e.g., IL-8) (Aggarwal 2004; Karin 2006). In highly malignant human prostate cancer cells PC-3M, expression of VEGF and IL-8 is upregulated and correlates with the constitutive NF-κB/relA activity (Huang et al. 2001). Furthermore, bombesin-stimulated expression and secretion of VEGF and IL-8 has been found to be dependent on NF-κB activation (Levine et al. 2003). Administration of a NF-κB antisense oligonucleotide abrogated the TNFα-induced IL-8 and VEGF production, along with inhibition of tubular morphogenesis in vascular endothelial cells (Yoshida et al. 1997). In human melanoma, ovarian, and prostate cancer models, blockade of NF-κB signaling by IκBαM suppressed the in vitro and in vivo expression of VEGF and IL-8, which directly correlated with the reduced neovascularization as well as decreased tumorigenicity (Huang et al. 2000a, b; 2001).
Regulators of invasion/metastasis
The transcription targets of NF-κB also include variety of molecules involved in tumor invasion, migration, and metastasis. NF-κB binding sites were identified in the promoters of genes that encode several matrix metalloproteinases (MMPs) that degrade the extracellular matrix (ECM) to facilitate tumor cell invasion in tissues (Karin et al. 2002). NF-κB activation is also required for the transcription of a group of adhesion molecules [endothelial-leukocyte adhesion molecule-1 (ELAM-1), vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1)] (Collins et al. 1995), which facilitate the extravasation of cancer cells (Kobayashi et al. 2007). Interestingly, VEGF is capable of stimulating the expression of ICAM-1, VCAM-1, and E-selectin, which is mediated through NF-κB activation (Kim et al. 2001). Together with other regulators, MMPs and adhesion molecules cooperate in the induction of epithelial–mesenchymal transition (EMT) for the progression of cancer metastasis (Lopez-Novoa and Nieto 2009). A breast cancer model has exemplified the essential role of NF-κB signaling in the induction and maintenance of EMT, and showed that the reversal of EMT could be achieved by inhibition of NF-κB activity (Huber et al. 2004). Moreover, NF-κB has been implicated in the migration and organ-specific homing of metastatic cancer cells, as demonstrated by Helbig and his co-workers, who showed that NF-κB regulated the motility of breast cancer cells, and promoted tumor migration and metastasis by directly upregulating the expression of CXC-chemokine receptor 4 (CXCR4) (Helbig et al. 2003). Overall, the above studies indicate that it is important to suppress NF-κB activation in order to reduce cancer metastasis.
Potential cross talk between NF-κB and other signal transduction cascades
The full transcriptional activation of NF-κB may involve cross talk with other signal pathways such as EGFR, PI3K/Akt and signal transducer, and activator of transcription 3 (STAT3), and blocking the activity of these signaling pathways can also provide an alternative strategy to regulate NF-κB activity in cancer therapy (Li and Sethi 2010; Fig. 1). The EGFR family acts as a central signal transducer of multiple important signaling pathways that contribute to tumor growth, survival, angiogenesis, invasion, and metastasis (Normanno et al. 2006; Yarden 2001). In many tumors, overexpression of EGFR family members (EGFR or ErbB-2) or their ligand overexpression activates NF-κB, stimulating various intracellular signaling cascades. For example, our group has reported that overexpression of EGFR led to constitutive activation of NF-κB through EGF receptor-kinase-dependent tyrosine 42 phosphorylation of IκBα (Sethi et al. 2007). While Pianetti et al. (2001) found that HER2/neu overexpression also activates NF-κB in an IKK-independent manner, but via the PI3K/Akt pathway in breast cancer.
Schematic diagram of cross talk between NF-κB and other signaling pathways. NF-κB interacts directly or indirectly with multiple signaling pathways or transcription factors in cancer. Abbreviations: IL-6 interleukin-6, STAT3 signal transducer and activator of transcription 3, EGFR epidermal growth factor receptor, PI3K PI3-kinase, CK2 casein kinase 2, TBK1 TANK-binding kinase 1, MEKK1 mitogen-activated protein kinase/ERK kinase kinase, IκBα inhibitor of kappa B-α, IKK IκB kinase, NF-κB nuclear factor kappa B, C/EBPβ CCAAT/enhancer-binding protein beta, HIF-1α hypoxia-inducible transcription factor-1 alpha, MnSOD manganese superoxide dismutase, FHC ferritin heavy chain, XIAP X-linked inhibitor of apoptosis protein, ROS reactive oxygen species, JNK c-Jun NH2-terminal kinase, AP-1 activator protein-1
The kinetics and spectrum of NF-κB activation differs widely in response to various stimuli that require different intermediates to transmit the signal. Several kinases, such as Akt, mitogen-activated protein kinase/ERK kinase kinase (MEKK1), PKC, glycogen synthase kinase-3 beta (GSK-3β), phosphoinositide-dependent protein kinase-1 (PDK1), and TAK1, have been reported to function upstream of NF-κB signaling (Vallabhapurapu and Karin 2009; Viatour et al. 2005). Apart from the canonical and non-canonical NF-κB signaling, PI3K/Akt has been identified as an important mediator to activate NF-κB. Different modes of functional interaction between PI3K/Akt and NF-κB have been discovered in tumors. The most common mechanism has been attributed to direct phosphorylation of IKKα by Akt, thereby leading to the activation of the kinase upstream of NF-κB (Ozes et al. 1999; Romashkova and Makarov 1999). Akt can also indirectly stimulate IKK activity through its downstream effector mammalian target of rapamycin (mTOR) (Dan et al. 2008) or mitogen-activated protein kinase kinase kinase (MAP3K) Cot (Kane et al. 2002). Finally, Akt targets and phosphorylates RelA through a p38- or IKK-dependent mechanism to stimulate NF-κB-dependent transcription by stimulating the transactivation domain of the p65 subunit, rather than inducing NF-κB nuclear translocation via IκBα degradation (Madrid et al. 2000, 2001).
It is possible that oncoproteins that are known to be activated in cancer cells, such as H-Ras, trigger signaling cascades that lead to constitutive NF-κB activation, which is required for efficient Ras-induced cellular transformation (Arsura et al. 2000; Finco et al. 1997; Hanson et al. 2004; Mayo et al. 1997) as well as EMT in Ras-transformed epithelial cells (Huber et al. 2004). The NF-κB activation induced by Ras engages several downstream effector pathways, such as PI3K/Akt signaling or Raf coupling with MEKK1, all of which lead to the activation of the IKK complex (Arsura et al. 2000; Chang et al. 2003; Madrid et al. 2000, 2001). In addition, the IKK-related kinase TANK-binding kinase 1 (TBK1), functioning downstream of Ras, has been shown to trigger the classical NF-κB pathway activation, as judged by the accumulation of nuclear NF-κB (Baldwin 2012; Staudt 2010). Furthermore, Ras is able to stimulate the transcriptional activation function of NF-κB via the targeting of the RelA/p65 subunit, instead of inducing the nuclear translocation of NF-κB (Finco et al. 1997).
In various tumors, the pro-apoptotic c-Jun NH2-terminal kinase (JNK) signaling and NF-κB appear to have opposing biological effects. The activation of JNK results in diverse outcomes of cellular response ranging from the induction of apoptosis to increased survival and altered proliferation, which has been implicated in different stages of cancer development (Herr and Debatin 2001; Wagner and Nebreda 2009). NF-κB activation in response to TNFR1 engagement promotes termination of JNK activation through a mechanism that depends on the induction of anti-oxidant enzymes such as manganese superoxide dismutase (MnSOD) and ferritin heavy chain (FHC), as reactive oxygen species (ROS) help to sustain JNK activity (Kamata et al. 2005; Pham et al. 2004). In addition, interference with JNK activity can be achieved through NF-κB-dependent upregulation of genes encoding inhibitors of JNK signaling such as GADD45β, XIAP, and A20 (De Smaele et al. 2001; Papa et al. 2004; Tang et al. 2001). In many cell types, suppression of JNK-induced apoptosis can contribute to the tumor-promoting activities of NF-κB (Nakano et al. 2006; Papa et al. 2006). Moreover, NF-κB inhibition has been reported to sensitize cells to TNFα-induced or tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through the sustained activation of JNK (Liu et al. 2002; Nakshatri et al. 2004).
As STAT3 supports tumor cell survival and proliferation, and plays important roles in tumor inflammation and immunity, it is not surprising that the activities of STAT3 and NF-κB are closely intertwined. Several NF-κB family members, in particular RelA/p65 and p50, have been found to physically interact with STAT3, resulting in either specific transcriptional synergy or repression of target genes, depending on the cellular context (Grivennikov and Karin 2010). One interesting study showed that STAT3 could prolong NF-κB nuclear retention through acetyltransferase p300-mediated RelA acetylation, thereby interfering with NF-κB nuclear export (Lee et al. 2009). Furthermore, the constitutive activation of NF-κB leads to the production and secretion of cytokines such as IL-6 in an autocrine/paracrine manner, which causes the consequent activation of STAT3 signaling in various human cancers (Grivennikov and Karin 2010; Hodge et al. 2005; Yu et al. 2009). Importantly, STAT3 and NF-κB control both distinct and overlapping groups of genes involved in cell proliferation, survival, angiogenesis, and invasion (Bollrath and Greten 2009; Grivennikov and Karin 2010).
NF-κB is also able to function in concert with other transcription factors, such as CCAAT/enhancer-binding protein beta (C/EBPβ), AP-1, and specificity protein 1 (Sp1) (Perkins 1997, 2007). NF-κB and C/EBPβ either form a complex by direct interaction or cooperatively bind to the same promoter site to transactivate several genes including serum amyloid A2, IL-6, and IL-8 (Stein and Baldwin 1993; Xia et al. 1997). Similarly, the Rel homology domain of p65 is capable of physically interacting with bZIP regions of the AP-1 subunits c-Fos and c-Jun, to mutually stimulate DNA binding and transactivation via both κB and AP-1 response elements in a synergistic manner (Stein et al. 1993). Cellular stress or cytokine stimulation leads to the activation of parallel kinase cascades regulating NF-κB and AP-1, and coordinate the induction of many genes encoding inflammatory mediators, pro-apoptotic and anti-apoptotic proteins, cell-cycle regulators, and enzymes that regulate matrix remodeling (Guha and Mackman 2001; Herr and Debatin 2001; Karin et al. 2002; Tak and Firestein 2001). Moreover, NF-κB regulates the induction of AP-1 activity by promoting the expression of several AP-1 family members, which in turn augments a second wave of NF-κB-dependent gene expression (Fujioka et al. 2004; Krappmann et al. 2004). NF-κB is also a critical transcriptional activator of hypoxia-inducible transcription factor-1 alpha (HIF-1α), and IKKβ-mediated NF-κB activity is required for HIF-1α protein accumulation under hypoxia and induction of HIF-1α target genes (Rius et al. 2008). Figure 1 depicts the potential cross talk of NF-κB signaling pathway with other important oncogenic signal transduction cascades.
Pharmacological strategies to block NF-κB activation
Given the pivotal role of activated NF-κB in the development and progression of human cancer, intensive efforts have been made to explore strategies that block NF-κB signaling in aid of cancer prevention and treatment. A number of compounds with NF-κB inhibitory effects are being developed and tested in translational/clinical studies (Sethi and Tergaonkar 2009). Below, we describe few important classes of major NF-κB blockers and briefly discuss the evidence of their therapeutic promise in cancer therapy.
IKK inhibitors
The idea of specifically blocking IκBα phosphorylation has so far attracted much interest and substantial effort to develop selective inhibitors of IKK kinases via high-throughput screening of candidate compound libraries, or design and synthesis of small-molecule antagonists to IKK (Karin et al. 2004). PS-1145, a small-molecule IKK inhibitor, which was developed from natural β-carboline, has been shown to block TNFα-induced NF-κB activation by blocking the IKK complex and subsequently inhibiting IκBα degradation (Castro et al. 2003; Hideshima et al. 2002). PS-1145 suppresses the proliferation of multiple myeloma cells and exhibits selective toxicity against subtypes of DLBCLs (Hideshima et al. 2002; Lam et al. 2005). BMS-345541 binds to both IKKα and IKKβ at similar allosteric sites, and thus presents as a highly selective inhibitor of IKK (Burke et al. 2003). This compound inhibits the expression of NF-κB-regulated cytokines including TNFα, IL-1β, IL-8, and IL-6 in monocytic cells and the production of TNFα in mice (Burke et al. 2003). In a later study, it was reported that BMS-345541 induced apoptosis of melanoma cell lines and attenuated the growth of melanoma tumors in vivo (Yang et al. 2006). A pyridyl cyanoguanidine, CHS-828, was identified as a potent IKK inhibitor (Olsen et al. 2004) and showed remarkable anticancer effects in several tumor cell lines and different mouse xenograft models (Hjarnaa et al. 1999). However, when the compound was tested in phase I clinical trials, no objective tumor responses were observed on patients with solid tumors (Hovstadius et al. 2002; Ravaud et al. 2005). On the contrary, no specific IKKα inhibitors have been developed, probably because the role of IKKα in NF-κB signaling is not yet fully understood; however, many of the IKKβ inhibitors show considerable high inhibition effects on IKKα as well, with an IC50 value in the low micromolar range (Karin et al. 2004).
Nonsteroidal anti-inflammatory drugs
The NF-κB proteins are evolutionarily conserved mediators that integrate multiple stress stimuli to regulate inflammatory processes by controlling the gene expression of multiple pro-inflammatory molecules (Ghosh et al. 1998; Li and Verma 2002). Thus, conventional anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs (NSAIDs) have been re-evaluated to explore their effects on the NF-κB pathway in the context of cancer treatment. Experimental data, clinical trials, and epidemiologic studies have well documented the preventive role of NSAIDs, including the beneficial effect of aspirin against colorectal adenoma as well as many other cancers (Baron et al. 2003; Schreinemachers and Everson 1994; Smith et al. 2000). While the major targets of NSAIDs are recognized as COX that lead to the inhibition of synthesis of PGs, it has been also found that several NSAIDs inhibit NF-κB activation (Smith et al. 2000).
Aspirin and salicylate drugs exert their anti-inflammatory properties at least partly by their specific inhibition of IKKβ and competing with ATP binding to the molecule, thereby abrogating the subsequent activation of NF-κB (Yin et al. 1998). Similarly, the same group have identified IKKβ kinase as the target of sulindac and its metabolites, and demonstrated that the growth-inhibitory ability of sulindac on a colon cancer cell line is regulated in part by modulating the NF-κB pathway (Yamamoto et al. 1999). A number of commonly used NSAIDs, namely aspirin, ibuprofen, sulindac, phenylbutazone, naproxen, indomethacin, diclofenac, and celecoxib, have been investigated with regard to NF-κB activation (Takada et al. 2004). All these compounds have been shown to inhibit NF-κB activation through suppression of IκBα degradation, but they exhibited a variable inhibitory capacity (Takada et al. 2004). Although the detailed molecular mechanisms of how NSAIDs inhibit the NF-κB pathway still remain unknown, the above-mentioned studies provide evidence that NSAIDs might prevent cancer development through the inhibition of NF-κB signaling.
Immunomodulatory agents
Thalidomide and its analogs are a group of immunomodulatory drugs that have shown therapeutic significance in treating multiple myeloma (Singhal et al. 1999). When thalidomide is used together with dexamethasone (another immunosuppressant), response rates in multiple myeloma patients increased significantly (Kyle and Rajkumar 2004). Currently, thalidomide alone or in combination with other chemotherapeutic agents is used as a standard therapy for treating relapsed and refractory multiple myeloma; however, the mechanisms that underlie the anti-angiogenic and anti-tumor properties of thalidomide are still unclear. Modulation of NF-κB has been proposed as one of the important mechanisms of action by thalidomide. Evidence has shown that thalidomide and its analogs induce apoptosis of multiple myeloma cells, which correlates with the downregulation of constitutive NF-κB activity (Mitsiades et al. 2002b). In addition, thalidomide has also been demonstrated to suppress cytokine-induced NF-κB activation in other cell types such as leukemia, lymphoma, and cervical cell lines (Majumdar et al. 2002). The NF-κB inhibitory effect of thalidomide has been identified as it blocks IKK activity (Keifer et al. 2001). Corticosteroids such as glucocorticoids have been found to inhibit NF-κB activity in studies of their anti-inflammatory and immunosuppressive properties. Two major mechanisms have been described: glucocorticoids increase the transcription of the IκBα gene, which in turn elevates the protein level of this NF-κB inhibitor that retains NF-κB in the cytoplasm (Auphan et al. 1995; Scheinman et al. 1995); also, the ligand-activated glucocorticoid receptor can directly interact with the p65 subunit, resulting in the suppression of NF-κB activation (Ray and Prefontaine 1994).
Proteasome inhibitors
The activation of NF-κB is tightly regulated by the turnover of IκBα protein through ubiquitination and proteasome-mediated degradation. Thus, several proteasome inhibitors have been developed and studied as possible cancer therapy. Bortezomib (former name was PS-341, marketed as Velcade by Millennium Pharmaceuticals) is the first proteasome inhibitor approved by the FDA for the treatment for multiple myeloma and mantle cell lymphoma (Kane et al. 2003). Bortezomib, a boronic acid dipeptide, selectively binds to and inhibits the 26S proteasome, which in turn prevents IκBα degradation, leading to the blockade of NF-κB activation (Richardson et al. 2003). The anti-tumor activities of bortezomib have been well documented as it exhibits cytotoxicity in a variety of cancer cell lines and reduces tumor growth in different in vivo models (Richardson et al. 2003). Accumulating evidence(s) indicate that most chemotherapeutic agents and radiation therapy induce the activation of NF-κB and its mediator genes in different type of cancers, which leads to the chemo- or radioresistance observed in tumors (Li and Sethi 2010). The idea of using bortezomib to inhibit NF-κB activation, in combination with either chemotherapy or radiotherapy, has been tested in variety of cancers. It has been demonstrated that bortezomib could block the chemotherapy- or radiation-induced NF-κB activation, which led to dramatic augmentation of chemo- or radiosensitivity in colorectal cancer cells and a human colon cancer xenograft model (Cusack et al. 2001; Russo et al. 2001).
In the clinical setting, the addition of bortezomib to doxorubicin-based chemotherapy resulted in a significantly higher response and improved the survival of patients with DLBCL (Dunleavy et al. 2009). These effects were associated with the inhibition of NF-κB activation and consequent suppression of NF-κB-regulated genes. Proteasomal ubiquitination regulates the degradation of multiple important proteins involved in cancers including p53, JUN, and β-Catenin (Hershko and Ciechanover 1998), and it is reasonable to believe that the inhibition of NF-κB is not the only mechanism behind the anti-tumor effects of proteasome inhibitors. In fact, it has been reported that bortezomib mediates anti-myeloma activity by inducing p53 phosphorylation and expression, and activating the JNK and caspase-8-dependent apoptotic pathway (Hideshima et al. 2003; Mitsiades et al. 2002a). Besides the synthetic peptide aldehydes, to which bortezomib belongs, the natural compound lactacystin was previously identified as having the ability to irreversibly block the activity of the proteasome via a covalent modification (Voorhees and Orlowski 2006). Indeed, the lactacystin derivative PS-519 (MLN519) is in clinical development for its anti-inflammatory properties; however, other proteasome inhibitors have only been studied in preclinical settings (Adams 2004).
Natural products
Compounds derived from natural products, which have diverse molecular mechanisms of action, have also exhibited inhibitory effects on NF-κB signaling (Aggarwal and Shishodia 2006; Nakanishi and Toi 2005; Surh 2003). A flavonoid-based compound, flavopiridol, blocks the translocation of p65 into the nucleus through inhibition of IKK (Takada and Aggarwal 2004), whereas another extensively investigated phytochemical, curcumin, suppresses NF-κB activation by inhibiting the NIK/IKK signaling complex (Glaser et al. 1973; Plummer et al. 1999). In addition, some studies suggested that curcumin downregulates NF-κB activation by inhibiting Notch-1 signaling (Wang et al. 2006) or disrupting the function of the ubiquitin proteasome system (Dikshit et al. 2006). Similarly, the green tea polyphenol epigallocatechin-3-gallate (EGCG) causes the blockade of the catalytic activities of the proteasome complex, resulting in intracellular accumulation of IκBα (Aktas et al. 2004; Nam et al. 2001). Some natural compounds such as andrographolide (from plant Andrographis paniculata) and sesquiterpene lactone helenalin (from plant Arnica montana and Arnica chamissonis foliosa) can directly interact or modify the p50 or p65 subunit of the NF-κB complex, respectively, and thus prevent the binding of NF-κB to DNA (Lyss et al. 1998; Xia et al. 2004). Resveratrol (from various natural sources such as grapes, berries, or Polygonum Capsidatum) acts as a potent pharmacological agonist of the NAD-dependent deacetylase SIRT1, which in turn inhibits NF-κB transcription by directly deacetylating the RelA/p65 protein (Yeung et al. 2004). Many of these compounds demonstrated promising anticancer properties in preclinical studies, and a few of which have been evaluated in clinical studies. There are several ongoing phase II and phase III clinical trials utilizing curcumin for chemoprevention or as a cancer therapy (Jurenka 2009), and preliminary results from a phase II trial reported beneficial effects of curcumin in patients with advanced pancreatic cancer (Dhillon et al. 2008). It should be noted that some of the therapeutic effects of natural agents may be mediated through pathways other than NF-κB, due to their pleiotropic nature of interfering with numerous molecular targets (Basseres and Baldwin 2006).
Concluding remarks
Several agents such as thalidomide and arsenic trioxide, which are able to inhibit NF-κB function, are currently in clinical use for cancer treatment; however, their NF-κB inhibitory effects have only been indentified after their approval for clinical use. The most well-known case of exploring chemotherapeutics with the intention, at least in part, to target NF-κB is the development of the proteasome inhibitor bortezomib. Since it gained FDA approval in 2003, bortezomib has been used clinically for relapsed multiple myeloma and later for mantle cell lymphoma. In fact, the available NF-κB inhibitors usually present only limited therapeutic efficacy when used as a single agent therapy. There are multiple preclinical studies and clinical trials that successfully demonstrated that NF-κB inhibitors markedly potentiate the effects of chemotherapeutic drugs or radiation, highlighting the promising potential of NF-κB inhibitors as adjuvant therapy. It is very important to take note that prolonged NF-κB inhibition might bring undesirable side effects that comprise the activation or efficacy of the immune system of the patients. Thus, NF-κB inhibition should be transient and reversible to avoid long-term immunosuppression, which makes it more practical to use NF-κB inhibitors in cancer therapy, rather than chemoprevention. In addition, NF-κB inhibitors should be tested and used with caution because NF-κB may promote tumorigenesis under certain circumstances (Perkins 2004; Shishodia and Aggarwal 2004). More detailed understanding of NF-κB signaling and its different roles in diverse tumor types needs to be further addressed in future studies in order to provide new insights into the rational drug design for specific NF-κB inhibition.
Abbreviations
- NF-κB:
-
Nuclear factor kappa B
- IκBα:
-
Inhibitor of kappa B-α
- IKK:
-
IκB kinase
- NIK:
-
NF-κB-inducing kinase
- TNF:
-
Tumor necrosis factor
- LPS:
-
Lipopolysaccharide
- TNFR:
-
TNF receptor
- TLR:
-
Toll-like receptor
- IL-1:
-
Interleukin-1
- TCR:
-
T-cell receptor
- TRAF:
-
TNFR-associated factor
- RIP:
-
Receptor-interacting protein
- TAK1:
-
TGF-β-activated kinase 1
- BAFF:
-
B-cell-activating factor
- HCC:
-
Hepatocellular carcinoma
- PI3K:
-
PI3-kinase
- HGF:
-
Hepatocyte growth factor
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- NS5A:
-
Non-structural 5A
- IκBα-SR:
-
IκBα super-repressor
- DEN:
-
Diethylnitrosamine
- CAC:
-
Colitis-associated colon cancer
- PRR:
-
Pattern recognition receptors
- CK2:
-
Casein kinase 2
- siRNA:
-
Small interfering RNA
- ER:
-
Estrogen receptor
- EGFR:
-
Epidermal growth factor receptor
- RANK:
-
Receptor activator of NF-κB
- IκBαM:
-
IκBα mutant
- HNSCC:
-
Head and neck squamous cell carcinoma
- HPV:
-
Human papillomavirus
- DLBCL:
-
Diffuse large B-cell lymphoma
- CLL:
-
Chronic lymphocytic leukemia
- HL:
-
Hodgkin’s lymphoma
- ATL:
-
Adult T-cell leukemia
- HTLV-1:
-
Human T-cell leukemia virus type 1
- EBV:
-
Epstein–Barr virus
- CML:
-
Chronic myelogenous leukemia
- ALL:
-
Acute lymphoblastic leukemia
- ABC:
-
Activated B-cell-like
- VEGF:
-
Vascular endothelial growth factor
- PGHS:
-
Prostaglandin endoperoxide H synthases
- COX:
-
Cyclooxygenase
- AP-1:
-
Activator protein-1
- PKC:
-
Protein kinase C
- Rb:
-
Retinoblastoma
- IAP:
-
Inhibitors of apoptosis
- PMA:
-
Phorbol myristol acetate
- MMP:
-
Matrix metalloproteinase
- ECM:
-
Extracellular matrix
- ELAM-1:
-
Endothelial-leukocyte adhesion molecule-1
- VCAM-1:
-
Vascular cell adhesion molecule-1
- ICAM-1:
-
Intercellular adhesion molecule-1
- EMT:
-
Epithelial–mesenchymal transition
- CXCR4:
-
CXC-chemokine receptor 4
- MEKK1:
-
Mitogen-activated protein kinase/ERK kinase kinase
- GSK-3β:
-
Glycogen synthase kinase-3 beta
- PDK1:
-
Phosphoinositide-dependent protein kinase-1
- MAP3K:
-
Mitogen-activated protein kinase kinase kinase
- TBK1:
-
TANK-binding kinase 1
- JNK:
-
c-Jun NH2-terminal kinase
- MnSOD:
-
Manganese superoxide dismutase
- FHC:
-
Ferritin heavy chain
- ROS:
-
Reactive oxygen species
- TRAIL:
-
Tumor necrosis factor-related apoptosis-inducing ligand
- STAT3:
-
Signal transducer and activator of transcription 3
- C/EBPβ:
-
CCAAT/enhancer-binding protein beta
- HIF-1α:
-
Hypoxia-inducible transcription factor-1 alpha
- NSAID:
-
Nonsteroidal anti-inflammatory drug
- UPS:
-
Ubiquitin proteasome system
- EGCG:
-
Epigallocatechin-3-gallate
- ATO:
-
Arsenic trioxide
References
Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4(5):349–360. doi:10.1038/Nrc1361
Aggarwal BB (2004) Nuclear factor-kappa-B: the enemy within. Cancer Cell 6(3):203–208. doi:10.1016/j.ccr.2004.09.003
Aggarwal BB, Shishodia S (2006) Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol 71(10):1397–1421. doi:10.1016/j.bcp.2006.02.009
Aktas O, Prozorovski T, Smorodchenko A et al (2004) Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol 173(9):5794–5800
Albanese C, Wu KM, D’Amico M et al (2003) IKK alpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell 14(2):585–599. doi:10.1091/mbc.02-06-0101
Allen CT, Ricker JL, Chen Z, Van Waes C (2007) Role of activated nuclear factor-kappa B in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck J Sci Spec 29(10):959–971. doi:10.1002/Hed.20615
Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M (2010) B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464(7286):302–305. doi:10.1038/Nature08782
Anderson KC, Carrasco RD (2011) Pathogenesis of myeloma. Annu Rev Pathol Mech 6:249–274. doi:10.1146/annurev-pathol-011110-130249
Annunziata CM, Davis RE, Demchenko Y et al (2007) Frequent engagement of the classical and alternative NF-kappa B pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12(2):115–130. doi:10.1016/j.ccr.2007.07.004
Anto RJ, Mukhopadhyay A, Shishodia S, Gairola CG, Aggarwal BB (2002) Cigarette smoke condensate activates nuclear transcription factor-kappa B through phosphorylation and degradation of I kappa B alpha: correlation with induction of cyclooxygenase-2. Carcinogenesis 23(9):1511–1518. doi:10.1093/carcin/23.9.1511
Arber N, Doki Y, Han EKH et al (1997) Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res 57(8):1569–1574
Arsura M, Cavin LG (2005) Nuclear factor-kappaB and liver carcinogenesis. Cancer Lett 229(2):157–169. doi:10.1016/j.canlet.2005.07.008
Arsura M, Mercurio F, Oliver AL, Thorgeirsson SS, Sonenshein GE (2000) Role of the I kappa B kinase complex in oncogenic Ras- and Raf-mediated transformation of rat liver epithelial cells. Mol Cell Biol 20(15):5381–5391. doi:10.1128/Mcb.20.15.5381-5391.2000
Arsura M, Panta GR, Bilyeu JD et al (2003) Transient activation of NF-kappa B through a TAK1/IKK kinase pathway by TGF-beta 1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene 22(3):412–425. doi:10.1038/sj.onc.1206132
Asano T, Yao YX, Zhu JJ, Li DH, Abbruzzese JL, Reddy SAG (2004) The PI 3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-kappa B and c-Myc in pancreatic cancer cells. Oncogene 23(53):8571–8580. doi:10.1038/sj.onc.1207902
Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M (1995) Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270(5234):286–290
Baldwin AS (1996) The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol 14:649–683. doi:10.1146/annurev.immunol.14.1.649
Baldwin AS (2012) Regulation of cell death and autophagy by IKK and NF-kappa B: critical mechanisms in immune function and cancer. Immunol Rev 246:327–345. doi:10.1111/j.1600-065X.2012.01095.x
Bancroft CC, Chen Z, Dong G et al (2001) Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin Cancer Res 7(2):435–442
Bancroft CC, Chen Z, Yeh J et al (2002) Effects of pharmacologic antagonists of epidermal growth factor receptor, PI3K and MEK signal kinases on NF-kappaB and AP-1 activation and IL-8 and VEGF expression in human head and neck squamous cell carcinoma lines. Int J Cancer 99(4):538–548. doi:10.1002/ijc.10398
Bargou RC, Emmerich F, Krappmann D et al (1997) Constitutive nuclear factor-kappa B-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest 100(12):2961–2969. doi:10.1172/Jci119849
Baron JA, Cole BF, Sandler RS et al (2003) A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med 348(10):891–899. doi:10.1056/NEJMoa021735
Basseres DS, Baldwin AS (2006) Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene 25(51):6817–6830. doi:10.1038/sj.onc.1209942
Biswas DK, Shi Q, Baily S et al (2004) NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 101(27):10137–10142. doi:10.1073/pnas.0403621101
Block TM, Mehta AS, Fimmel CJ, Jordan R (2003) Molecular viral oncology of hepatocellular carcinoma. Oncogene 22(33):5093–5107. doi:10.1038/sj.onc.1206557
Bollrath J, Greten FR (2009) IKK/NF-kappa B and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep 10(12):1314–1319. doi:10.1038/embor.2009.243
Bonizzi G, Karin M (2004) The two NF-kappa B activation pathways and their role in innate and adaptive immunity. Trends Immunol 25(6):280–288. doi:10.1016/J.It.2004.03.008
Bouchard MJ, Schneider RJ (2004) The enigmatic X gene of hepatitis B virus. J Virol 78(23):12725–12734. doi:10.1128/Jvi.78.23.12725-12734.2004
Burke JR, Pattoli MA, Gregor KR et al (2003) BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 278(3):1450–1456. doi:10.1074/jbc.M209677200
Cao YX, Bonizzi G, Seagroves TN et al (2001) IKK alpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell 107(6):763–775. doi:10.1016/S0092-8674(01)00599-2
Cao YX, Luo JL, Karin M (2007) I kappa B kinase a kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc Natl Acad Sci USA 104(40):15852–15857. doi:10.1073/pnas.0706728104
Carmeliet P, Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407(6801):249–257. doi:10.1038/35025220
Carrasco D, Rizzo CA, Dorfman K, Bravo R (1996) The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. EMBO J 15(14):3640–3650
Castro AC, Dang LC, Soucy F et al (2003) Novel IKK inhibitors: beta-carbolines. Bioorg Med Chem Lett 13(14):2419–2422. doi:10.1016/S0960-894x(03)00408-6
Catz SD, Johnson JL (2001) Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 20(50):7342–7351. doi:10.1038/sj.onc.1204926
Cavin LG, Wang F, Factor VM et al (2005) Transforming growth factor-alpha inhibits the intrinsic pathway of c-myc-induced apoptosis through activation of nuclear factor-kappa B in murine hepatocellular carcinomas. Mol Cancer Res 3(7):403–412. doi:10.1158/1541-7786.Mcr-04-0186
Chang F, Steelman LS, Lee JT et al (2003) Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17(7):1263–1293. doi:10.1038/sj.leu.2402945
Chen LF, Greene WC (2004) Shaping the nuclear action of NF-kappa B. Nat Rev Mol Cell Bio 5(5):392–401. doi:10.1038/Nrm1368
Chen CL, Edelstein LC, Gelinas C (2000) The Rel/NF-kappa B family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol 20(8):2687–2695. doi:10.1128/Mcb.20.8.2687-2695.2000
Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW (1997) Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappa B control. Proc Natl Acad Sci USA 94(19):10057–10062. doi:10.1073/pnas.94.19.10057
Chung CH, Parker JS, Ely K et al (2006) Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappa B signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res 66(16):8210–8218. doi:10.1158/0008-5472.Can-06-1213
Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS (2000) Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 19(9):1123–1131. doi:10.1038/sj.onc.1203412
Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T (1995) Transcriptional regulation of endothelial-cell adhesion molecules—Nf-Kappa-B and cytokine-inducible enhancers. Faseb J 9(10):899–909
Courtois G, Gilmore TD (2006) Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene 25(51):6831–6843. doi:10.1038/sj.onc.1209939
Cusack JC, Liu R, Houston M et al (2001) Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappa B inhibition. Cancer Res 61(9):3535–3540
Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JPY, Baldwin AS (2008) Akt-dependent regulation of NF-kappa B is controlled by mTOR and raptor in association with IKK. Genes Dev 22(11):1490–1500. doi:10.1101/Gad.1662308
Dandekar DS, Lopez M, Carey RI, Lokeshwar BL (2005) Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and-9 in prostate cancer cells. Int J Cancer 115(3):484–492. doi:10.1002/Ijc.20878
Davis RE, Brown KD, Siebenlist U, Staudt LM (2001) Constitutive nuclear factor kappa B activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 194(12):1861–1874. doi:10.1084/jem.194.12.1861
De Smaele E, Zazzeroni F, Papa S et al (2001) Induction of gadd45 beta by NF-kappa B downregulates pro-apoptotic JNK signalling. Nature 414(6861):308–313. doi:10.1038/35104560
Demchenko YN, Glebov OK, Zingone A, Keats JJ, Bergsagel PL, Kuehl WM (2010) Classical and/or alternative NF-kappa B pathway activation in multiple myeloma. Blood 115(17):3541–3552. doi:10.1182/blood-2009-09-243535
Deveraux QL, Reed TC (1999) IAP family proteins—suppressors of apoptosis. Genes Dev 13(3):239–252. doi:10.1101/Gad.13.3.239
Dhillon N, Aggarwal BB, Newman RA et al (2008) Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res 14(14):4491–4499. doi:10.1158/1078-0432.Ccr-08-0024
Diao JY, Garces R, Richardson CD (2001) X protein of hepatitis B virus modulates cytokine and growth factor related signal transduction pathways during the course of viral infections and hepatocarcinogenesis. Cytokine Growth Fact Rev 12(2–3):189–205. doi:10.1016/S1359-6101(00)00034-4
Dikshit P, Goswami A, Mishra A, Chatterjee M, Jana NR (2006) Curcumin induces stress response, neurite outgrowth and prevent NF-kappa B activation by inhibiting the proteasome function. Neurotox Res 9(1):29–37
Dong G, Loukinova E, Chen Z et al (2001) Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-kappaB signal pathway. Cancer Res 61(12):4797–4808
Donnellan R, Chetty R (1998) Cyclin D1 and human neoplasia. J Clin Pathol Mol Pathol 51(1):1–7
Du J, Chen GG, Vlantis AC, Xu H, Tsang RKY, van Hasselt AC (2003) The nuclear localization of NF kappa B and p53 is positively correlated with HPV16 E7 level in laryngeal squamous cell carcinoma. J Histochem Cytochem 51(4):533–539
Duffey DC, Chen Z, Dong G et al (1999) Expression of a dominant-negative mutant inhibitor-kappaBalpha of nuclear factor-kappaB in human head and neck squamous cell carcinoma inhibits survival, proinflammatory cytokine expression, and tumor growth in vivo. Cancer Res 59(14):3468–3474
Dunleavy K, Pittaluga S, Czuczman MS et al (2009) Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood 113(24):6069–6076. doi:10.1182/blood-2009-01-199679
Farah M, Parhar K, Moussavi M, Eivemark S, Salh B (2003) 5,6-Dichloro-ribifuranosylbenzimidazole- and apigenin-induced sensitization of colon cancer cells to TNF-alpha-mediated apoptosis. Am J Physiol Gastrointest Liver Physiol 285(5):G919–G928. doi:10.1152/ajpgi.00205.2003
Finco TS, Westwick JK, Norris JL, Beg AA, Der CJ, Baldwin AS (1997) Oncogenic Ha-Ras-induced signaling activates NF-kappa B transcriptional activity, which is required for cellular transformation. J Biol Chem 272(39):24113–24116. doi:10.1074/jbc.272.39.24113
Fosslien E (2000) Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit Rev Cl Lab Sci 37(5):431–502. doi:10.1080/10408360091174286
Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AMM, Saad F (2004) Nuclear factor-kappa B nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res 10(24):8460–8464. doi:10.1158/1078-0432.Ccr-04-0764
Fujioka S, Niu J, Schmidt C et al (2004) NF-kappaB and AP-1 connection: mechanism of NF-kappaB-dependent regulation of AP-1 activity. Mol Cell Biol 24(17):7806–7819. doi:10.1128/MCB.24.17.7806-7819.2004
Gasparian AV, Yao YJ, Kowalczyk D et al (2002) The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci 115(Pt 1):141–151
Ghosh S, May MJ, Kopp EB (1998) NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260. doi:10.1146/annurev.immunol.16.1.225
Gilmore TD (1999) Multiple mutations contribute to the oncogenicity of the retroviral oncoprotein v-Rel. Oncogene 18(49):6925–6937. doi:10.1038/sj.onc.1203222
Gilmore TD, Kalaitzidis D, Liang MC, Starczynowski DT (2004) The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene 23(13):2275–2286. doi:10.1038/sj.onc.1207410
Glaser R, Ogino T, Zimmerman J Jr, Rapp F (1973) Thymidine kinase activity in Burkitt lymphoblastoid somatic cell hybrids after induction of the EB virus. Proc Soc Exp Biol Med 142(4):1059–1062
Greten FR, Eckmann L, Greten TF et al (2004) IKK beta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118(3):285–296. doi:10.1016/j.cell.2004.07.013
Grivennikov SI, Karin M (2010) Dangerous liaisons: STAT3 and NF-kappa B collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21(1):11–19. doi:10.1016/j.cytogfr.2009.11.005
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13(2):85–94. doi:10.1016/S0898-6568(00)00149-2
Guo J, Verma UN, Gaynor RB, Frenkel EP, Becerra CR (2004) Enhanced chemosensitivity to irinotecan by RNA interference mediated down-regulation of the nuclear factor-kappa B p65 subunit. Clin Cancer Res 10(10):3333–3341. doi:10.1158/1078-0432.Ccr-03-0366
Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS (1999) NF-kappa B controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19(8):5785–5799
Gyrd-Hansen M, Meier P (2010) IAPs: from caspase inhibitors to modulators of NF-kappa B, inflammation and cancer. Nat Rev Cancer 10(8):561–574. doi:10.1038/Nrc2889
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674. doi:10.1016/j.cell.2011.02.013
Hanson JL, Hawke NA, Kashatus D, Baldwin AS (2004) The nuclear factor kappa B subunits RelA/p65 and c-Rel potentiate but are not required for Ras-induced cellular transformation. Cancer Res 64(20):7248–7255. doi:10.1158/0008-5472.Can-03-3898
Hardwick JCH, van den Brink GR, Offerhaus GJ, van Deventer SJH, Peppelenbosch MP (2001) NF-kappaB, p38 MAPK and JNK are highly expressed and active in the stroma of human colonic adenomatous polyps. Oncogene 20(7):819–827. doi:10.1038/sj.onc.1204162
Hayden MS, Ghosh S (2004) Signaling to NF-kappa B. Genes Dev 18(18):2195–2224. doi:10.1101/Gad.1228704
Hayden MS, Ghosh S (2008) Shared principles in NF-kappa B signaling. Cell 132(3):344–362. doi:10.1016/j.cell.2008.01.020
Helbig G, Christopherson KW, Bhat-Nakshatri P et al (2003) NF-kappa B promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278(24):21631–21638. doi:10.1074/jbc.M300609200
Herr I, Debatin KM (2001) Cellular stress response and apoptosis in cancer therapy. Blood 98(9):2603–2614. doi:10.1182/blood.V98.9.2603
Herrmann JL, Beham AW, Sarkiss M et al (1997) Bcl-2 suppresses apoptosis resulting from disruption of the NF-kappa B survival pathway. Exp Cell Res 237(1):101–109. doi:10.1006/excr.1997.3737
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479. doi:10.1146/annurev.biochem.67.1.425
Hideshima T, Chauhan D, Richardson P et al (2002) NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 277(19):16639–16647. doi:10.1074/jbc.M200360200
Hideshima T, Mitsiades C, Akiyama M et al (2003) Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood 101(4):1530–1534. doi:10.1182/blood-2002-08-2543
Hideshima T, Bergsagel PL, Kuehl WM, Anderson KC (2004) Advances in biology of multiple myeloma: clinical applications. Blood 104(3):607–618. doi:10.1182/blood-2004-01-0037
Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M (1999) NF-kappa B function in growth control: regulation of cyclin D1 expression and G(0)G(1)-to-S-Phase transition. Mol Cell Biol 19(4):2690–2698
Hjarnaa PJV, Jonsson E, Latini S et al (1999) CHS 828, a novel pyridyl cyanoguanidine with potent antitumor activity in vitro and in vivo. Cancer Res 59(22):5751–5757
Hodge DR, Hurt EM, Farrar WL (2005) The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 41(16):2502–2512. doi:10.1016/j.ejca.2005.08.016
Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R (2000) Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem 275(29):22064–22068. doi:10.1074/jbc.M910346199
Hovstadius P, Larsson R, Jonsson E et al (2002) A phase I study of CHS 828 in patients with solid tumor malignancy. Clin Cancer Res 8(9):2843–2850
Huang S, DeGuzman A, Bucana CD, Fidler IJ (2000a) Nuclear factor-kappa B activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res 6(6):2573–2581
Huang S, Robinson JB, DeGuzman A, Bucana CD, Fidler IJ (2000b) Blockade of nuclear factor-kappa B signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res 60(19):5334–5339
Huang SY, Pettaway CA, Uehara H, Bucana CD, Fidler IJ (2001) Blockade of NF-kappa B activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20(31):4188–4197. doi:10.1038/sj.onc.1204535
Huber MA, Azoitei N, Baumann B et al (2004) NF-kappa B is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 114(4):569–581. doi:10.1172/Jci200421358
Hunter T, Pines J (1994) Cyclins and cancer. 2. Cyclin-D and Cdk inhibitors come of age. Cell 79(4):573–582. doi:10.1016/0092-8674(94)90543-6
Hunter AM, LaCasse EC, Korneluk RG (2007) The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 12(9):1543–1568. doi:10.1007/s10495-007-0087-3
Ismail H, Lessard L, Mes-Masson AM, Saad F (2004) Expression of NF-kappa B in prostate cancer lymph node metastases. Prostate 58(3):308–313. doi:10.1002/Pros.10335
Jackson-Bernitsas DG, Ichikawa H, Takada Y et al (2007) Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappa B activation and proliferation in human head and neck squamous cell carcinoma. Oncogene 26(10):1385–1397. doi:10.1038/sj.onc.1209945
Jost PJ, Ruland J (2007) Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood 109(7):2700–2707. doi:10.1182/blood-2006-07-025809
Jung YJ, Isaacs JS, Lee SM, Trepel J, Neckers L (2003) IL-1 beta mediated up-regulation of HIF-1 alpha via an NFkB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J 17(12):2115–2117. doi:10.1096/fj.03-0329fje
Jurenka JS (2009) Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research. Altern Med Rev 14(2):141–153
Kamata H, Honda S, Maeda S, Chang LF, Hirata H, Karin M (2005) Reactive oxygen species promote TNF alpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120(5):649–661. doi:10.1016/j.cell.2004.12.041
Kane LP, Mollenauer MN, Xu Z, Turck CW, Weiss A (2002) Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription. Mol Cell Biol 22(16):5962–5974. doi:10.1128/Mcb.22.16.5962-5974.2002
Kane RC, Bross PF, Farrell AT, Pazdur R (2003) Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist 8(6):508–513
Karin M (2006) Nuclear factor-kappa B in cancer development and progression. Nature 441(7092):431–436. doi:10.1038/Nature04870
Karin M, Lin A (2002) NF-kappa B at the crossroads of life and death. Nat Immunol 3(3):221–227. doi:10.1038/Ni0302-221
Karin M, Cao Y, Greten FR, Li ZW (2002) NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2(4):301–310. doi:10.1038/nrc780
Karin M, Yamamoto Y, Wang QM (2004) The IKKNF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov 3(1):17–26. doi:10.1038/Nrd1279
Kawakami H, Tomita M, Matsuda T et al (2005) Transcriptional activation of survivin through the NF-kappa B pathway by human T-cell leukemia virus type I Tax. Int J Cancer 115(6):967–974. doi:10.1002/Ijc.20954
Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N (1998) Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer. Cancer Res 58(22):5071–5074
Keats JJ, Fonseca R, Chesi M et al (2007) Promiscuous mutations activate the noncanonical NF-kappa B pathway in multiple myeloma. Cancer Cell 12(2):131–144. doi:10.1016/j.ccr.2007.07.003
Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS Jr (2001) Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem 276(25):22382–22387. doi:10.1074/jbc.M100938200
Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY (2001) Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem 276(10):7614–7620. doi:10.1074/jbc.M009705200
Kim BY, Gaynor RB, Song K, Dritschilo A, Jung M (2002) Constitutive activation of NF-kappaB in Ki-ras-transformed prostate epithelial cells. Oncogene 21(29):4490–4497. doi:10.1038/sj.onc.1205547
Kirchner D, Duyster J, Ottmann O, Schmid RM, Bergmann L, Munzert G (2003) Mechanisms of Bcr-Abl-mediated NF-kappaB/Rel activation. Exp Hematol 31(6):504–511
Kishi K, Petersen S, Petersen C et al (2000) Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res 60(5):1326–1331
Kobayashi H, Boelte KC, Lin PC (2007) Endothelial cell adhesion molecules and cancer progression. Curr Med Chem 14(4):377–386. doi:10.2174/092986707779941032
Kojima M, Morisaki T, Sasaki N et al (2004) Increased nuclear factor-kappa B activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res 24(2B):675–681
Kornmann M, Arber N, Korc M (1998) Inhibition of basal and mitogen-stimulated pancreatic cancer cell growth by cyclin D1 antisense is associated with loss of tumorigenicity and potentiation of cytotoxicity to cisplatinum. J Clin Invest 101(2):344–352. doi:10.1172/Jci1323
Kornmann M, Danenberg KD, Arber N, Beger HG, Danenberg PV, Korc M (1999) Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res 59(14):3505–3511
Krappmann D, Wegener E, Sunami Y et al (2004) The I kappa B kinase complex and NF-kappa B act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol Cell Biol 24(14):6488–6500. doi:10.1128/Mcb.24.14.6488-6500.2004
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-kappa B: its role in health and disease. J Mol Med 82(7):434–448. doi:10.1007/s00109-004-0555-y
Kyle RA, Rajkumar SV (2004) Multiple myeloma. N Engl J Med 351(18):1860–1873. doi:10.1056/NEJMra041875
LaCasse EC, Baird S, Korneluk RG, MacKenzie AE (1998) The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene 17(25):3247–3259
LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG (2008) IAP-targeted therapies for cancer. Oncogene 27(48):6252–6275. doi:10.1038/Onc.2008.302
Lam LT, Davis RE, Pierce J et al (2005) Small molecule inhibitors of I kappa B kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res 11(1):28–40
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC (2001) Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 20(25):3247–3257. doi:10.1038/sj.onc.1204411
Le Page C, Koumakpayi IH, Lessard L, Mes-Masson AM, Saad F (2005) EGFR and Her-2 regulate the constitutive activation of NF-kappaB in PC-3 prostate cancer cells. Prostate 65(2):130–140. doi:10.1002/pros.20234
Lee H, Herrmann A, Deng JH et al (2009) Persistently activated Stat3 maintains constitutive NF-kappa B activity in tumors. Cancer Cell 15(4):283–293. doi:10.1016/j.ccr.2009.02.015
Levine L, Lucci JA, Pazdrak B et al (2003) Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res 63(13):3495–3502
Li F, Sethi G (2010) Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 1805(2):167–180. doi:10.1016/j.bbcan.2010.01.002
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2(10):725–734. doi:10.1038/nri910
Lind DS, Hochwald SN, Malaty J et al (2001) Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery 130(2):363–369. doi:10.1067/msy.2001.116672
Liu HL, Lo CR, Czaja MJ (2002) NF-kappa B inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35(4):772–778. doi:10.1053/jhep.2002.32534
Loercher A, Lee TL, Ricker JL et al (2004) Nuclear factor-kappa B is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res 64(18):6511–6523. doi:10.1158/0008-5472.Can-04-0852
Lopez-Novoa JM, Nieto MA (2009) Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med 1(6–7):303–314. doi:10.1002/emmm.200900043
Lu CD, Altieri DC, Tanigawa N (1998) Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res 58(9):1808–1812
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M (2004) Inhibition of NF-kappa B in cancer cells converts inflammation-induced tumor growth mediated by TNF alpha to TRAIL-mediated tumor regression. Cancer Cell 6(3):297–305. doi:10.1016/j.ccr.2004.08.012
Luo JL, Tan W, Ricono JM et al (2007) Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 446(7136):690–694. doi:10.1038/nature05656
Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I (1998) The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem 273(50):33508–33516
Madrid LV, Wang CY, Guttridge DC, Schottelius AJG, Baldwin AS, Mayo MW (2000) Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-kappa B. Mol Cell Biol 20(5):1626–1638. doi:10.1128/Mcb.20.5.1626-1638.2000
Madrid LV, Mayo MW, Reuther JY, Baldwin AS (2001) Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-kappa B through utilization of the I kappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem 276(22):18934–18940. doi:10.1074/jbc.M101103200
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKK beta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121(7):977–990. doi:10.1016/j.cell.2005.04.014
Majumdar S, Lamothe B, Aggarwal BB (2002) Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol 168(6):2644–2651
Matsuoka M, Jeang KT (2007) Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer 7(4):270–280. doi:10.1038/Nrc2111
Mayo MW, Wang CY, Cogswell PC et al (1997) Requirement of NF-kappa B activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278(5344):1812–1815. doi:10.1126/science.278.5344.1812
McGivern DR, Lemon SM (2011) Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene 30(17):1969–1983. doi:10.1038/Onc.2010.594
Mishra A, Bharti AC, Varghese P, Saluja D, Das BC (2006) Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int J Cancer 119(12):2840–2850. doi:10.1002/ijc.22262
Mita AC, Mita MM, Nawrocki ST, Giles FJ (2008) Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 14(16):5000–5005. doi:10.1158/1078-0432.Ccr-08-0746
Mitsiades N, Mitsiades CS, Poulaki V et al (2002a) Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA 99(22):14374–14379. doi:10.1073/pnas.202445099
Mitsiades N, Mitsiades CS, Poulaki V et al (2002b) Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood 99(12):4525–4530
Molinolo AA, Amornphimoltham P, Squarize CH, Castilho RM, Patel V, Gutkind JS (2009) Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol 45(4–5):324–334. doi:10.1016/j.oraloncology.2008.07.011
Muller M, Morotti A, Ponzetto C (2002) Activation of NF-kappa B is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Mol Cell Biol 22(4):1060–1072. doi:10.1128/Mcb.22.4.1060-1072.2002
Nakanishi C, Toi M (2005) Nuclear factor-kappa B inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer 5(4):297–309. doi:10.1038/Nrc1588
Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K (2006) Reactive oxygen species mediate crosstalk between NF-kappa B and JNK. Cell Death Differ 13(5):730–737. doi:10.1038/sj.cdd.4401830
Nakashima T, Clayman GL (2000) Antisense inhibition of cyclin D1 in human head and neck squamous cell carcinoma. Arch Otolaryngol 126(8):957–961
Nakata E, Mason KA, Hunter N et al (2004) Potentiation of tumor response to radiation or chemoradiation by selective cyclooxygenase-2 enzyme inhibitors. Int J Radiat Oncol 58(2):369–375. doi:10.1016/j.ijrobp.2003.09.061
Nakayama H, Ikebe T, Beppu M, Shirasuna K (2001) High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer 92(12):3037–3044
Nakshatri H, Bhat-Nakshatri P, Martin DA, Goulet RJ Jr, Sledge GW Jr (1997) Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth. Mol Cell Biol 17(7):3629–3639
Nakshatri H, Rice SE, Bhat-Nakshatri P (2004) Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 23(44):7330–7344. doi:10.1038/sj.onc.1207995
Nam S, Smith DM, Dou QP (2001) Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem 276(16):13322–13330. doi:10.1074/jbc.M004209200
Normanno N, De Luca A, Bianco C et al (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366(1):2–16. doi:10.1016/j.gene.2005.10.018
Olsen LS, Hjarnaa PJV, Latini S et al (2004) Anticancer agent CHS 828 suppresses nuclear factor-kappa B activity in cancer cells through downregulation of ikk activity. Int J Cancer 111(2):198–205. doi:10.1002/Ijc.20255
Ondrey FG, Dong G, Sunwoo J et al (1999) Constitutive activation of transcription factors NF-kappa B, AP-1, and NF-IL6 in human head and neck squamous cell carcinoma cell lines that express pro-inflammatory and pro-angiogenic cytokines. Mol Carcinogen 26(2):119–129. doi:10.1002/(Sici)1098-2744(199910)26:2<119:Aid-Mc6>3.0.Co;2-N
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappa B activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401(6748):82–85
Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD (1999) Constitutive activation of I kappa B kinase alpha and NF-kappa B in prostate cancer cells is inhibited by ibuprofen. Oncogene 18(51):7389–7394. doi:10.1038/sj.onc.1203160
Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G (2004) Linking JNK signaling to NF-kappa B: a key to survival. J Cell Sci 117(22):5197–5208. doi:10.1242/Jcs.01483
Papa S, Bubici C, Zazzeroni F et al (2006) The NF-kappa B-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ 13(5):712–729. doi:10.1038/sj.cdd.4401865
Park KJ, Krishnan V, O’Malley BW, Yamamoto Y, Gaynor RB (2005) Formation of an IKK alpha-dependent transcription complex is required for estrogen receptor-mediated gene activation. Mol Cell 18(1):71–82. doi:10.1016/j.molcel.2005.03.006
Perkins ND (1997) Achieving transcriptional specificity with NF-kappa B. Int J Biochem Cell B 29(12):1433–1448. doi:10.1016/S1357-2725(97)00088-5
Perkins ND (2004) NF-kappa B: tumor promoter or suppressor? Trends Cell Biol 14(2):64–69. doi:10.1016/j.tcb.2003.12.004
Perkins ND (2007) Integrating cell-signalling pathways with NF-kappa B and IKK function. Nat Rev Mol Cell Bio 8(1):49–62. doi:10.1038/Nrm2083
Petersen C, Petersen S, Milas L, Lang FF, Tofilon PJ (2000) Enhancement of intrinsic tumor cell radiosensitivity induced by a selective cyclooxygenase-2 inhibitor. Clin Cancer Res 6(6):2513–2520
Pham CG, Bubici C, Zazzeroni F et al (2004) Ferritin heavy chain upregulation by NF-kappa B inhibits TNF alpha-induced apoptosis by suppressing reactive oxygen species. Cell 119(4):529–542. doi:10.1016/j.cell.2004.10.017
Pianetti S, Arsura M, Romieu-Mourez R, Coffey RJ, Sonenshein GE (2001) Her-2/neu overexpression induces NF-kappa B via a PI3-kinase/Akt pathway involving calpain-mediated degradation of I kappa-B-alpha that can be inhibited by the tumor suppressor PTEN. Oncogene 20(11):1287–1299. doi:10.1038/sj.onc.1204257
Pikarsky E, Porat RM, Stein I et al (2004) NF-kappa B functions as a tumour promoter in inflammation-associated cancer. Nature 431(7007):461–466. doi:10.1038/Nature02924
Plummer SM, Holloway KA, Manson MM et al (1999) Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene 18(44):6013–6020. doi:10.1038/sj.onc.1202980
Poligone B, Baldwin AS (2001) Positive and negative regulation of NF-kappa B by COX-2-Roles of different prostaglandins. J Biol Chem 276(42):38658–38664. doi:10.1074/jbc.M106599200
Qiao L, Zhang H, Yu J et al (2006) Constitutive activation of NF-kappaB in human hepatocellular carcinoma: evidence of a cytoprotective role. Hum Gene Ther 17(3):280–290. doi:10.1089/hum.2006.17.280
Ravaud A, Cerny T, Terret C et al (2005) Phase I study and pharmacokinetic of CHS-828, a guanidino-containing compound, administered orally as a single dose every 3 weeks in solid tumours: an ECSG/EORTC study. Eur J Cancer 41(5):702–707. doi:10.1016/j.ejca.2004.12.023
Ray A, Prefontaine KE (1994) Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA 91(2):752–756
Rayet B, Gelinas C (1999) Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18(49):6938–6947. doi:10.1038/sj.onc.1203221
Reuther JY, Reuther GW, Cortez D, Pendergast AM, Baldwin AS Jr (1998) A requirement for NF-kappaB activation in Bcr-Abl-mediated transformation. Genes Dev 12(7):968–981
Richardson PG, Barlogie B, Berenson J et al (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. New Engl J Med 348(26):2609–2617. doi:10.1056/Nejmoa030288
Ripple MO, Henry WF, Schwarze SR, Wilding G, Weindruch R (1999) Effect of antioxidants on androgen-induced AP-1 and NF-kappa B DNA-binding activity in prostate carcinoma cells. J Natl Cancer I 91(14):1227–1232. doi:10.1093/jnci/91.14.1227
Rius J, Guma M, Schachtrup C et al (2008) NF-kappa B links innate immunity to the hypoxic response through transcriptional regulation of HIF-1 alpha. Nature 453(7196):807-U9. doi:10.1038/Nature06905
Romashkova JA, Makarov SS (1999) NF-kappa B is a target of AKT in anti-apoptotic PDGF signalling. Nature 401(6748):86–90
Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE (2001) Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-kappa B in breast cancer. Cancer Res 61(9):3810–3818
Romieu-Mourez R, Kim DW, Shin SM et al (2003) Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol Cell Biol 23(16):5738–5754
Ross JS, Kallakury BVS, Sheehan CE et al (2004) Expression of nuclear factor-kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res 10(7):2466–2472. doi:10.1158/1078-0432.Ccr-0543-3
Russo SM, Tepper JE, Baldwin AS et al (2001) Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappa B. Int J Radiat Oncol 50(1):183–193. doi:10.1016/S0360-3016(01)01446-8
Sarela AI, Macadam RCA, Farmery SM, Markham AF, Guillou PJ (2000) Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut 46(5):645–650. doi:10.1136/Gut.46.5.645
Sarkar FH, Li YW, Wang ZW, Kong DJ (2008) NF-kappa B signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol 27(5):293–319. doi:10.1080/08830180802276179
Sato Y, Kato J, Takimoto R et al (2006) Hepatitis C virus core protein promotes proliferation of human hepatoma cells through enhancement of transforming growth factor alpha expression via activation of nuclear factor-kappa B. Gut 55(12):1801–1808. doi:10.1136/gut.2005.070417
Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS Jr (1995) Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science 270(5234):283–286
Schramek D, Leibbrandt A, Sigl V et al (2010) Osteoclast differentiation factor RANKL controls development of progestin-driven mammary cancer. Nature 468(7320):98–102. doi:10.1038/Nature09387
Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5(2):138–146
Schrump DS, Chen A, Consoli U (1996) Inhibition of lung cancer proliferation by antisense cyclin D. Cancer Gene Ther 3(2):131–135
Sen R, Baltimore D (1986) Multiple Nuclear Factors Interact with the Immunoglobulin Enhancer Sequences. Cell 46(5):705–716. doi:10.1016/0092-8674(86)90346-6
Senftleben U, Cao YX, Xiao GT et al (2001) Activation by IKK alpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 293(5534):1495–1499. doi:10.1126/science.1062677
Sethi G, Tergaonkar V (2009) Potential pharmacological control of the NF-kappa B pathway. Trends Pharmacol Sci 30(6):313–321. doi:10.1016/j.tips.2009.03.004
Sethi G, Ahn KS, Chaturvedi MM, Aggarwal BB (2007) Epidermal growth factor (EGF) activates nuclear factor-kappa B through I kappa B alpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of I kappa B alpha. Oncogene 26(52):7324–7332. doi:10.1038/sj.onc.1210544
Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V (2012) Multifaceted link between cancer and inflammation. Biosci Rep 32(1):1–15. doi:10.1042/Bsr20100136
Shen HM, Tergaonkar V (2009) NF kappa B signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis 14(4):348–363. doi:10.1007/s10495-009-0315-0
Sherr CJ (1996) Cancer cell cycles. Science 274(5293):1672–1677. doi:10.1126/science.274.5293.1672
Shishodia S, Aggarwal BB (2004) Nuclear factor-KB: a friend or a foe in cancer? Biochem Pharmacol 68(6):1071–1080. doi:10.1016/j.bcp.2004.04.026
Shrivastava A, Manna SK, Ray R, Aggarwal BB (1998) Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J Virol 72(12):9722–9728
Shukla S, MacLennan GT, Fu PF et al (2004) Nuclear factor-kappa B/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia 6(4):390–400. doi:10.1593/Neo.04112
Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S (2005) Constitutive activation of P I3 K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate 64(3):224–239. doi:10.1002/pros.20217
Singh S, Shi Q, Bailey ST et al (2007) Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther 6(7):1973–1982. doi:10.1158/1535-7163.MCT-07-0063
Singhal S, Mehta J, Desikan R et al (1999) Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341(21):1565–1571. doi:10.1056/NEJM199911183412102
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182. doi:10.1146/annurev.biochem.69.1.145
Sovak MA, Bellas RE, Kim DW et al (1997) Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest 100(12):2952–2960. doi:10.1172/JCI119848
Staudt LM (2010) Oncogenic activation of NF-kappa B. Csh Perspect Biol. doi:10.1101/cshperspect.a000109
Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J (1998) Nuclear factor (NF)-kappa B-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med 188(1):211–216. doi:10.1084/jem.188.1.211
Stein B, Baldwin AS (1993) Distinct Mechanisms for Regulation of the Interleukin-8 Gene Involve Synergism and Cooperativity between C/Ebp and Nf-Kappa-B. Mol Cell Biol 13(11):7191–7198
Stein B, Baldwin AS, Ballard DW, Greene WC, Angel P, Herrlich P (1993) Cross-coupling of the Nf-Theta-B P65 and Fos Jun transcription factors produces potentiated biological function. EMBO J 12(10):3879–3891
Subbaramaiah K, Hart JC, Norton L, Dannenberg AJ (2000) Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2—evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J Biol Chem 275(20):14838–14845. doi:10.1074/jbc.275.20.14838
Subbaramaiah K, Marmo TP, Dixon DA, Dannenberg AJ (2003) Regulation of cyclooxgenase-2 mRNA stability by taxanes—evidence for involvement of p38, MAPKAPK-2, and HuR. J Biol Chem 278(39):37637–37647. doi:10.1074/jbc.M301481200
Suh J, Payvandi F, Edelstein LC et al (2002) Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 52(3):183–200. doi:10.1002/pros.10082
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3(10):768–780. doi:10.1038/nrc1189
Surh YJ, Chun KS, Cha HH et al (2001) Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res Fundam Mol M 480:243–268. doi:10.1016/S0027-5107(01)00183-X
Tai DI, Tsai SL, Chang YH et al (2000) Constitutive activation of nuclear factor kappa B in hepatocellular carcinoma. Cancer 89(11):2274–2281. doi:10.1002/1097-0142(20001201)89:11<2274:Aid-Cncr16>3.3.Co;2-U
Tak PP, Firestein GS (2001) NF-kappa B: a key role in inflammatory diseases. J Clin Invest 107(1):7–11. doi:10.1172/Jci11830
Takada Y, Aggarwal BB (2004) Flavopiridol inhibits NF-kappa B activation induced by various carcinogens and inflammatory agents through inhibition of I kappa B alpha kinase and p65 phosphorylation—abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J Biol Chem 279(6):4750–4759. doi:10.1074/jbc.M304546200
Takada Y, Bhardwaj A, Potdar P, Aggarwal BB (2004) Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene 23(57):9247–9258. doi:10.1038/sj.onc.1208169
Tamm I, Wang Y, Sausville E et al (1998) IAP-family protein Survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res 58(23):5315–5320
Tan W, Zhang WZ, Strasner A et al (2011) Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 470(7335):548–553. doi:10.1038/Nature09707
Tang GL, Minemoto Y, Dibling B et al (2001) Inhibition of JNK activation through NF-kappa B target genes. Nature 414(6861):313–317. doi:10.1038/35104568
Terakado N, Shintani S, Yano JY et al (2004) Overexpression of cyclooxygenase-2 is associated with radioresistance in oral squamous cell carcinoma. Oral Oncol 40(4):383–389. doi:10.1016/j.oraloncology.2003.09.005
Tergaonkar V (2006) NF kappa B pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell B 38(10):1647–1653. doi:10.1016/j.biocel.2006.03.023
Terzic J, Grivennikov S, Karin E, Karin M (2010) Inflammation and Colon Cancer. Gastroenterology 138(6):2101-U119. doi:10.1053/j.gastro.2010.01.058
Trifan OC, Durham WF, Salazar VS et al (2002) Cyclooxygenase-2 inhibition with celecoxib enhances antitumor efficacy and reduces diarrhea side effect of CPT-11. Cancer Res 62(20):5778–5784
Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN (1998) Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93(5):705–716. doi:10.1016/S0092-8674(00)81433-6
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappa B transcription factors in the immune system. Annu Rev Immunol 27:693–733. doi:10.1146/annurev.immunol.021908.132641
Van Waes C (2007) Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res 13(4):1076–1082. doi:10.1158/1078-0432.CCR-06-2221
Viatour P, Merville MP, Bours V, Chariot A (2005) Phosphorylation of NF-kappa B and I kappa B proteins: implications in cancer and inflammation. Trends Biochem Sci 30(1):43–52. doi:10.1016/j.tiba.2004.11.009
Voorhees PM, Orlowski RZ (2006) The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol 46:189–213. doi:10.1146/annurev.pharmtox.46.120604.141300
Wagner EF, Nebreda AR (2009) Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9(8):537–549. doi:10.1038/Nrc2694
Wang D, DuBois RN (2006) Prostaglandins and cancer. Gut 55(1):115–122. doi:10.1136/gut.2004.047100
Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS (1998) NF-kappa B antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281(5383):1680–1683. doi:10.1126/science.281.5383.1680
Wang CY, Guttridge DC, Mayo MW, Baldwin AS (1999) NF-kappa B induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol 19(9):5923–5929
Wang ZW, Zhang YX, Banerjee S, Li YW, Sarkar FH (2006) Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer 106(11):2503–2513. doi:10.1002/Cncr.21904
Wang F, Arun P, Friedman J, Chen Z, Van Waes C (2009) Current and potential inflammation targeted therapies in head and neck cancer. Curr Opin Pharmacol 9(4):389–395. doi:10.1016/j.coph.2009.06.005
Waris G, Livolsi A, Imbert V, Peyron JF, Siddiqui A (2003) Hepatitis C virus NS5A and subgenomic replicon activate NF-kappa B via tyrosine phosphorylation of I kappa B alpha and its degradation by calpain protease. J Biol Chem 278(42):40778–40787. doi:10.1074/jbc.M303248200
Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS (2001) The putative oncoprotein Bcl-3 induces cyclin d1 to stimulate G(1) transition. Mol Cell Biol 21(24):8428–8436. doi:10.1128/Mcb.21.24.8428-8436.2001
Wolf JS, Chen Z, Dong G et al (2001) IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin Cancer Res 7(6):1812–1820
Wong ET, Tergaonkar V (2009) Roles of NF-kappa B in health and disease: mechanisms and therapeutic potential. Clin Sci 116(5–6):451–465. doi:10.1042/Cs20080502
Xia CL, Cheshire JK, Patel H, Woo P (1997) Cross-talk between transcription factors NF-kappa B and C/EBP in the transcriptional regulation of genes. Int J Biochem Cell B 29(12):1525–1539. doi:10.1016/S1357-2725(97)00083-6
Xia YF, Ye BQ, Li YD et al (2004) Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173(6):4207–4217
Yamamoto K, Arakawa T, Ueda N, Yamamoto S (1995) Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem 270(52):31315–31320
Yamamoto Y, Yin MJ, Lin KM, Gaynor RB (1999) Sulindac inhibits activation of the NF-kappaB pathway. J Biol Chem 274(38):27307–27314
Yamaoka S, Inoue H, Sakurai M et al (1996) Constitutive activation of NF-kappa B is essential for transformation of rat fibroblasts by the human T-cell leukemia virus type I Tax protein. EMBO J 15(4):873–887
Yang JM, Amiri KI, Burke JR, Schmid JA, Richmond A (2006) BMS-345541 targets inhibitor of kappa B kinase and induces apoptosis in melanoma: involvement of nuclear factor kappa B and mitochondria pathways. Clin Cancer Res 12(3):950–960. doi:10.1158/1078-0432.Ccr-05-1220
Yarden Y (2001) The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer 37:S3–S8
Yeung F, Hoberg JE, Ramsey CS et al (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23(12):2369–2380. doi:10.1038/sj.emboj.7600244
Yin MJ, Yamamoto Y, Gaynor RB (1998) The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396(6706):77–80. doi:10.1038/23948
Yoshida S, Ono M, Shono T et al (1997) Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol 17(7):4015–4023
Young LS, Rickinson AB (2004) Epstein-Barr virus: 40 years on. Nat Rev Cancer 4(10):757–768. doi:10.1038/Nrc1452
Yu HG, Yu LL, Yang YN et al (2003) Increased expression of RelA/nuclear factor-kappa B protein correlates with colorectal tumorigenesis. Oncology-Basel 65(1):37–45. doi:10.1159/000071203
Yu M, Yeh J, Van Waes C (2006) Protein kinase casein kinase 2 mediates inhibitor-kappa B kinase and aberrant nuclear factor-kappa B activation by serum factor(s) in head and neck squamous carcinoma cells. Cancer Res 66(13):6722–6731. doi:10.1158/0008-5472.Can-05-3758
Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 9(11):798–809. doi:10.1038/Nrc2734
Zha S, Yegnasubramanian V, Nelson WG, Isaacs WB, De Marzo AM (2004) Cyclooxygenases in cancer: progress and perspective. Cancer Lett 215(1):1–20. doi:10.1016/j.canlet.2004.06.014
Zhang PL, Pellitteri PK, Law A et al (2005) Overexpression of phosphorylated nuclear factor-kappa B in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Mod Pathol 18(7):924–932. doi:10.1038/modpathol.3800372
Zhang LY, Altuwaijri S, Deng FM et al (2009) NF-kappa B regulates androgen receptor expression and prostate cancer growth. Am J Pathol 175(2):489–499. doi:10.2353/ajpath.2009.080727
Zhou P, Jiang W, Zhang YJ et al (1995) Antisense to Cyclin D1 inhibits growth and reverses the transformed phenotype of human esophageal cancer-cells. Oncogene 11(3):571–580
Zhou BP, Hu MC, Miller SA et al (2000) HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem 275(11):8027–8031
Zong WX, Edelstein LC, Chen CL, Bash J, Gelinas C (1999) The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappa B that blocks TNF alpha-induced apoptosis. Genes Dev 13(4):382–387. doi:10.1101/Gad.13.4.382
Acknowledgments
This work was supported by NUHS Bench-to-Bedside grant to GS. Deanship of Scientific Research, College of Science Research Centre, King Saud University, Kingdom of Saudi Arabia, is also acknowledged. GS also thanks King Saud University, Riyadh, Kingdom of Saudi Arabia, for the Visiting Professorship. APK was supported by Grants from Singapore Ministry of Education Tier 2 [MOE2012-T2-2-139], Academic Research Fund Tier 1 [R-184-000-228-112], and Cancer Science Institute of Singapore, Experimental Therapeutics I Program [Grant R-713-001-011-271]. KSA was supported by the Korea Science and Engineering Foundation (KOSEF) Grant funded by the Korean Ministry of Education, Science and Technology (MoEST) (No. 2011-0006220).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, F., Zhang, J., Arfuso, F. et al. NF-κB in cancer therapy. Arch Toxicol 89, 711–731 (2015). https://doi.org/10.1007/s00204-015-1470-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1470-4