Abstract
Over the last decades, losses of bee populations have been observed worldwide. A panoply of biotic and abiotic factors, as well as the interplay among them, has been suggested to be responsible for bee declines, but definitive causes have not yet been identified. Among pollinators, the honeybee Apis mellifera is threatened by various diseases and environmental stresses, which have been shown to impact the insect gut microbiota that is known to be fundamental for host metabolism, development and immunity. Aimed at preserving the gut homeostasis, many researches are currently focusing on improving the honeybee health through the administration of probiotics e.g., by boosting the innate immune response against microbial infections. Here, we review the knowledge available on the characterization of the microbial diversity associated to honeybees and the use of probiotic symbionts as a promising approach to maintain honeybee fitness, sustaining a healthy gut microbiota and enhancing its crucial relationship with the host immune system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal pollinators provide an important ecosystem service helping a various range of plants to reproduce and ensuring the maintenance of plant species diversity and food production (Greenleaf and Kremen 2006; Klein et al. 2007; Winfree et al. 2008; Buchon et al. 2013). Apis mellifera is arguably one of the most considerable insect pollinators (Klein et al. 2007), due to the role it plays in the production of vegetables, fruits, and stimulating crops.
In last decades, abnormal bee mortality has been globally observed, especially in several European and North American countries (Aizen and Harder 2009), posing serious issues to the pollination service and, thus, crop yield (Steffan-Dewenter et al. 2005). Some wild bee species are currently vulnerable (Yeung et al. 2006; Oldroyd 2007), whereas others have suffered large contractions (Goulson et al. 2008). Many factors could be responsible for bee declines, including abiotic and biotic agents, as well as their combination. Among abiotic stressors, we can mention bee environment destruction, pesticide use and climate change (Barnett et al. 2007; Pettis et al. 2012), whereas biotic factors include infections caused by microsporidia parasites, such as Nosema spp. (Higes et al. 2006; Higes et al. 2008; Bromenshenk et al. 2010; Hatjina et al. 2011), spore-forming bacteria, such as Paenibacillus larvae, the causative agent of American foulbrood (AFB) (Bailey 1983; Dobbelaere et al. 2001), pathogenic fungi, such as Ascosphaera apis, parasitic mites, such as Varroa destructor (Le Conte et al. 2010; Rosenkranz et al. 2010) and viruses, such as Deformed Wing Virus (DWV). V. destructor is ultimately a vector of different viruses that can infect Apis mellifera (Rosenkranz et al. 2010). Considering bee importance, research on bee health has recently become a hot topic.
To protect honeybees against diseases, beekeepers can adopt various intervention measures, e.g. the use of the antibiotics oxytetracyclin and fumagilin-B to control P. larvae and Nosema pathogens, respectively (Huang et al. 2013). However, the prolonged use of antibiotics causes several troubles, among which the emergence of antibiotic-resistant pathogens and, consequently, their use to treat honeybee diseases has been banned in many European countries. Another serious concern for honeybee health is the employment of neonicotinoids. These compounds can indeed contribute to the bee decline by acting in synergy with different factors, e.g., the shortage of floral resources and the presence of pathogens and parasites (Goulson et al. 2015). Thus, in 2013, the European Commission has banned the use of the three neonicotinoids clothianidin, imidacloprid, and thiamethoxam on the basis of a risk assessment evaluation (Regulation 2013). Currently, biocontrol research in apiculture is mainly focused on developing alternative strategies to maintain and improve bee health, for example by enhancing the honeybee immune system responsible for fighting against honeybee pathogens (Lourenço et al. 2013). In this review, we summarize the available information on bacterial diversity and interaction with the honeybee gut, focusing on the importance of probiotics’ administration in maintaining and sustaining the health of this host which is crucial for the functioning of both agricultural and natural terrestrial ecosystems.
Diversity and roles of gut microbial communities of honeybees
Honeybee microbiota
Invertebrates are particularly interesting since they generally host simple gut microbial communities if compared to vertebrates. The complexity of human and, in general, mammalian systems has indeed led researchers to consider invertebrates as excellent models for studying the diversity, function, and interactions of the microbiome with the host (Erkosar and Leulier 2014; Engel et al. 2015; Prosdocimi et al. 2015; Saraiva et al. 2015; Kwong and Moran 2016; Zheng et al. 2017). The honeybee gut microbiota shows a number of similarities with the human intestinal microbial community, and thus, it has been recently proposed as an interesting experimental model (Zheng et al. 2018).
As revealed by 16S rRNA gene high-throughput surveys and metagenomics analysis, the adult honeybee gut consists of host-adapted, facultative anaerobic, and microaerophilic bacteria, which encompass nine bacterial species or phylotypes constituting the 95–99.9% of the bacterial community in almost all specimens (Martinson et al. 2011; Engel et al. 2012; Moran et al. 2012; Corby-Harris et al. 2014a; Kwong and Moran 2016; Kwong et al. 2017; Bleau et al. 2020; Callegari et al. 2021; Su et al. 2022) (Fig. 1). Among these, five bacterial phylotypes represent the core microbiota, namely Snodgrassella alvi and Gilliamella apicola (two Gram-negative species from the Proteobacteria phylum), Lactobacillus Firm-4 and Lactobacillus Firm-5 (two Gram-positive species from the Firmicutes phylum) and Bifidobacterium species (from the Actinobacteria phylum) (Babendreier et al. 2006; Martinson et al. 2011; Bottacini et al. 2012; Kwong and Moran 2013). Other four, less abundant, bacterial phylotypes, members of the Proteobacteria phylum, are Frischella perrarra, Bartonella apis, Commensalibacter sp. (previously indicated as Gluconobacter Alpha 2.1; Bonilla-Rosso et al. 2019) and Bombella apis (previously indicated as Acetobacteraceae Alpha2.2, then formerly Parasaccharibacter apium; Smith et al. 2021) (Engel et al. 2013; Corby-Harris et al. 2014b; Kešnerová et al. 2016). These core and non-core bacterial taxa are generally acquired from surrounding (hive components) and transmitted by bee workers (Powell et al. 2014; Kwong and Moran 2016).
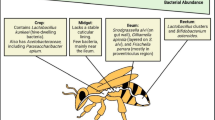
Anatomically, the adult gut is subdivided into various sections, i.e., crop, midgut, ileum and rectum (Fig. 2): these compartments are responsible for storing nectar, digestion and nutrient assimilation, waste excretion and water/salt reuptake, respectively. While few bacteria inhabit the first two compartments, the two distal sections are densely populated. Particularly, the crop harbours members of Enterobacteriaceae family, L. (= Apilactobacillus) kunkeei and Bo. apis (Corby-Harris et al. 2014a), while the midgut is often dominated by G. apicola and Ba. apis (Ludvigsen et al. 2015; Jia et al. 2016; Anderson and Ricigliano 2017). The ileum is instead inhabited by S. alvi, G. apicola and Lactobacillus Firm-5, while the rectum is dominated by the two Firmicutes phylotypes Lactobacillus Firm-5 and Lactobacillus Firm-4 and the Actinobacteria Bifidobacterium spp. (Martinson et al. 2012; Powell et al. 2014; Maes et al. 2016; Anderson and Ricigliano 2017) (Table 1).
Changes in the bacterial community structure and composition have been reported to occur in accordance to age, caste differentiation and season turnover. Dong et al. (2020) assessed the composition of the gut microbiota in workers at different age, reporting that their bacterial community is significantly altered with age progression. For instance, the gut microbiota at 1 day post-emergence is dominated by Gilliamella, Frischella, and Snodgrassella, while along the life cycle the proportions of these bacterial phylotypes change. Considering caste differentiation, the gut of adult workers is inhabited by a relatively stable array of bacterial phylotypes compared with the ones of males or queens (Kapheim et al. 2015; Tarpy et al. 2015).
Conversely to the adult gut, the larval one has been much less explored with regards to the microbiota characterization (Hroncova et al. 2019; Kowallik and Mikheyeva 2021; Daisley et al. 2020a). For instance, using an isolation-based approach, Vojvodic et al. (2013) showed that the larval gut is colonized by a number of bacterial groups previously found in the honeybee adult gut, i.e. Bo. apis, L. kunkeei, Lactobacillus sp. A (Lactobacillus Firm-4), Lactobacillus sp. B (Lactobacillus Firm-5), S. alvi, Bifidobacterium, Fructobacillus fructosus and Bacillus spp. The diversity of the larval bacterial communities changes with the larval age: first and second larval instars are exclusively dominated by Bo. apis, whereas older instars are mainly inhabited by Bo. apis, Lactobacillus Firm-4, Lactobacillus Firm-5, L. kunkeei and S. alvi (Vojvodic et al. 2013) (Table 1).
Honeybee bacterial community also undergoes seasonal variations (Kwong and Moran 2016). Winter honeybees host a more abundant community with lower diversity in comparison with nurses and foragers, showing higher levels of Bartonella and Commensalibacter (Kešnerová et al. 2020). Similarly, it has been found that also in tropical or subtropical climates, i.e. where honeybees emerge constantly along the year, nurses show a higher gut microbiota diversity in spring and a lower one in summer and winter: Lactobacillus spp. dominate in spring, while Gilliamella and Snodgrasella are more abundant in summer and winter (Castelli et al. 2022). Differently from Kešnerová et al. (2020), no variation in the total bacterial abundance was significantly reported through the year in nurses sampled in tropical or subtropical climates (Castelli et al. 2022).
Honeybee gut is also inhabited by yeasts and other fungal partners, on which so far not so many studies have been devoted compared to the ones performed on the bacterial component of the honeybee microbiota (Cox-Foster et al. 2007; Ludvigsen et al. 2020; Callegari et al. 2021). Acquired during food intake, fungal populations are less abundant than bacterial ones in the host gut, but nevertheless, they have been suggested to have a role in food digestion and to help the establishment of spatial and trophic interactions among gut members (Callegari et al. 2021). Yeasts such as Hanseniaspora and Starmerella have been found to dominate the midgut, ileum and rectum compartments of Italian honeybees, whereas Zygosaccharomyces are mainly associated to honeybees collected in Saudi Arabia, displaying a different vegetation in the sampling areas (Callegari et al. 2021).
Microbiota functions in the honeybee gut
The microbiota of adult honeybees plays many crucial functions in the host, e.g. supporting meal digestion, improving the innate immune response against pathogens and parasites, enhancing host development, modulating host behaviour and protecting the honeybee against microplastic contamination (Engel et al. 2015; Kwong and Moran 2016; Zheng et al. 2017; Wang et al. 2021). The presence of the bacterial community is essential for the honeybee, as it promotes the acquisition of insect weight through its metabolism and hormonal signalling, further influencing the insect behaviour (Zheng et al. 2017). The abundance of the core bacterium S. alvi in ileum has been positively related to bee survival and development (Maes et al. 2016), whereas the occurrence of F. perrara in the pylorus area has been correlated with scab formation, highlighting that the host mounts an immune response and a melanisation cascade against this bacterium, thus suggesting its recent acquisition or evolution as a symbiont (Engel et al. 2015). Indeed, when honeybees are fed with aged diets, the increase of F. perrara in the ileum, with a simultaneous decrease of S. alvi, is related to an increased mortality of honeybees (Maes et al. 2016).
Numerous studies have also shown that Gram-positive bacteria isolated from honeybee larvae and adults are active against bee pathogens thanks to the production and release of antibacterial substances. Particularly, Brevibacillus spp., Bacillus, and lactic acid bacteria (L. kunkeei, L. acidophilus and L. crispatus) exhibit inhibitory effects on the growth of P. larvae (Evans et al. 2006; Yoshiyama and Kimura 2009; Kačániová et al. 2021). Finally, both Betaproteobacteria and Gammaproteobacteria have been proven to possess a defensive role towards the invasion of the parasite Chritidia bombi in bumble bees (Koch and Schmid-Hempel 2011).
Biotic and abiotic factors of honeybee gut dysbiosis
Gut dysbiosis refers to the intestine microbial imbalance which leads to negative host physiological and functional changes (Hamdi et al. 2011). To understand this abnormal mechanism, the interaction between the intestinal microbes and host fitness needs to be carefully investigated. So far, only a few works have shown that the loss of host performance or function is directly related to the gut microbial variation, such as an altered proportion among core gut species, or a displacement of the core microbiota by opportunistic/pathogenic microorganisms (Anderson and Ricigliano 2017). Considering that many types of biotic and abiotic stresses, as well as the interplay among them, are known to affect honeybee fitness, it is hence essential to investigate their link with the bee gut dysbiosis (Anderson and Ricigliano 2017).
Abiotic factors
Abiotic factors, such as the lack of food and pollen, pesticide exposure and climate change, can impose a stress to the honeybee, likely increasing its susceptibility to other stressors (Schwarz et al. 2015). Poor pollen diet increases the host sensitivity to pesticides, pathogens (such as viruses) and parasites (such as Nosema) (Huang et al. 2012; Maes et al. 2016). Maes and co-authors (2016) have also revealed that newly emerged bees fed with an aged diet show a bacterial intestinal dysbiosis with the increase of the opportunistic pathogen F. perrara and the decrease of the core bacterium S. alvi, which ultimately results in increased honeybee mortality, reduced thorax weight and increased loads of Nosema spp. (Maes et al. 2016). Recently, Li et al. (2022) have also demonstrated that reduction in pollen consumption, due to seasonal diet shifts, drives a variation of the gut community structure, resulting in the winter dominance of the non-core bacterium Bartonella, which may help the host to survival to winter conditions.
Honeybee habitat disruption, due to hive installation in greenhouses, results in the down regulation of immune-related and antioxidant system genes of the bees, leading to the increase of Nosema spp. loads in these immune-suppressed hosts which also accumulate oxidative damage (Morimoto et al. 2011). Additionally, bee colonies exposed to a low, sub-lethal level of the neonicotinoid imidacloprid experience increased levels of Nosema parasites (Pettis et al. 2012).
Biotic factors
When infected by Nosema parasites or other pathogens, such as the causative agents of AFB or European Foulbrood (EFB), a perturbation of the core gut microbiota associated to honeybees occurs (Hamdi et al. 2011). Obligate intracellular Nosema microsporidia represent a serious risk for the beekeeping sector (Higes et al. 2006, 2007). They can infect honeybees through faecal–oral and oral–oral routes of transmission: spores can be found in bee faeces, but also in pollen, and they can germinate in midgut and infect midgut epithelium cells (Higes et al. 2008; Genersch 2010; Fries 2010; BenVau and Nieh 2017). Nosema-infected honeybees show shortened lifespans, delayed development, and altered physiology, immunity and behaviour (Genersch 2010). Paris et al. (2017) have interestingly reported a decrease of reactive oxygen species (ROS) amounts and ROS damage in honeybees infected by N. ceranae; however, high levels of protein oxidation have been detected in honeybees infected by this parasite and exposed to the insecticide fipronil at the same time, suggesting that Nosema could have a role in increasing the toxicity of the insecticide. Moreover, infections with N. apis have been associated with the presence of several viruses, i.e. the Black Queen Cell Virus (BQCV), the Bee Virus Y (BVY), and the Filamentous Virus (FV) (Fries et al. 2013). Abdi et al. (2018) have also reported a co-infection of honeybees from the same apiary with the three pathogens DWV, N. apis, and N. ceranae. Finally, in worker bees, poor pollen nutrition results in a reduced resistance against DWV and Nosema spp., while good protein supplementation can increase bee resistance to pathogens and parasites (Posada-Florez et al. 2019; Watkins de Jong et al. 2019; Huang 2012).
Affecting apiculture worldwide with severe economic losses for beekeepers, AFB is a honey bee brood disease caused by the highly contagious bacterium P. larvae (Genersch 2008). P. larvae spores, which can be transferred by adult nestmates to larvae during feeding activity, germinate inside the larval midgut within 24 h, after which they breach through the midgut wall (Evans 2004). López et al. (2017) have showed that larvae, co-exposed to P. larvae and to sub-lethal doses of pesticides (dimethoate or clothianidin), have higher mortality levels than larvae exposed solely to the bacterial pathogen. Interactions between pesticides used in beekeeping and pathogens of honeybees could hence have a more marked effect than what previously considered on colony health.
Dysbiosis has been also observed in hives with colony collapse disorder (CCD) symptoms: comparing healthy bee colonies with CCD-suffering ones, higher relative abundance of α-Proteobacteria and Firmicutes have been found in honeybees from the first ones (Cox-Foster et al. 2007). Similar results have been also reported by Cornman et al. (2012). In this light, great efforts need to be carried out to unravel the variation of the honeybee gut microbiota when challenged by different stressors, and combination of them, to understand stress-gut microbiome interplay. Moreover, alternative treatments should take into consideration the possibility to restore the indigenous microbial community perturbed by stressors.
Limitations of available treatments aimed at controlling honeybee pathogens
Chemical treatments against honeybee pathogens
Antibiotics have been used to manage AFB and EFB, since the early 1950’s (Kochansky et al. 2001). Up to 2005, oxytetracycline (OTC), also known as Terramycin (trade name), was the only authorized antibiotic used against these two foulbrood diseases in the United States (Gochnauer 1951; Kochansky et al. 2001). Then, in consequence of recurrent findings and increasing concerns for the appearance of oxytetracycline-resistance strains of P. larvae, in 2005 the use of an alternative antibiotic, i.e. the macrolide tylosin, also known as Tylan (trade name), was formerly accepted by the U.S. Food and Drug Administration (Alippi 2000; Evans 2004; Yoshiyama and Kimura 2009; Tian et al. 2012). Indeed, high incidences of tetracycline/oxytetracycline resistance genes have been reported in the gut bacterial community of American honeybees both using molecular tool, as well as through the isolation of tetracycline-resistant G. apicola and S. alvi isolates (Tian et al. 2012). On the other hand, following a more precautionary approach antibiotics in Europe have been banned for apiculture. Besides the emergence of pathogenic resistant strains, antibiotics have indeed many disadvantages, e.g., the inefficacy against pathogenic spores, the negative effect on bee vitality and longevity, and the presence of chemical residues in pollen, bee wax and honey, affecting consequently honey safety and quality (Genersch 2010; Barrasso et al. 2018). Currently, bacterial resistance to antibiotics, and more general to antimicrobials, is considered a serious risk for human, animal and environmental health (Perry and Wright 2013; Larsson and Flach 2022).
On the other hand, in Canada and USA, beekeepers manage Nosema microsporidia control taking advantage of the only commercially registered antibiotic, i.e. fumagillin bicyclohexyl ammonium, also known as Fumagilin-B (trade name), produced by Medivet Pharmaceuticals Ltd. (Williams et al. 2008). Conversely, antibiotic treatments on Nosema-infected hives are not allowed in Europe (Fries 2010).
Effects of biocidal treatments on honeybee gut
Similarly to humans and livestock, the use of antibiotics has been shown to cause gut dysbiosis in honeybees, resulting in the increase of insect mortality and host susceptibility to opportunistic pathogens’ invasion both in hive and in laboratory experiments (Bulson et al. 2021; Raymann et al. 2017; Soares et al. 2021; Aljedani 2022). Particularly, adult workers treated with tetracycline hosted a disturbed gut microbiota, in terms of size and composition (Raymann et al. 2017; Daisley et al. 2020b). Raymann et al. (2017) have indeed documented smaller bacterial communities in antibiotic-treated bees than in non-treated ones, with a reduction of the core phylotypes Bifidobacterium, Lactobacillus Firm-4, Lactobacillus Firm-5 and Ba. apis, and an increase of the relative abundance of G. apicola. Persisting for long periods of time, antibiotics inducing dysbiosis can lead to the increase of non-bacterial pathogens such as Nosema spp. and viruses (Koch and Schmid-Hempel 2011; Schwarz et al. 2016; Raymann et al. 2017). Moreover, honeybees exposed to pesticides (e.g., chlorothalonil) have perturbed native gut communities (Kakumanu et al. 2016).
The promise of probiotics
The term “probiotic” has been used with different meanings over the years. In 1965, it was formerly used to describe substances secreted by microorganisms, able to stimulate the growth of other microorganisms (Lilly and Stillwell 1965). Currently, it is used to indicate microorganisms with beneficial effects for humans and animals: the commonly used definition indicates probiotics as dietary supplements of “live microorganisms that when administered in adequate quantities confer a health benefit on the host” (Fuller 1992; Schrezenmeir and de Vrese 2001; Texts 2001; Johnston et al. 2016; Perdigon and Maldonado Galdeano 2017; Hill et al. 2014) by enhancing its intestinal microbial balance (Rasic 1983; Johnston et al. 2016). Therefore, to understand probiotics’ functioning, knowledge of the microbial diversity and ecology of the intestinal tract is pivotal (Smoragiewicz et al. 1993).
In humans, probiotics are used for the treatment of clinical conditions characterized by abnormal gut microflora and impaired intestinal mucosal barrier functions, favouring mechanisms of pathogen elimination and, generally, stimulating the immune response (Salminen et al. 1998; Gourbeyre et al. 2011; Johnston et al. 2016). Negative effects of antibiotic treatments can be indeed reduced by the administration of probiotic strains such as Lactobacillus and Bifidobacterium ones (Colombel 1987; Gorbach et al. 1987; Kujawa-Szewieczek et al. 2015; Härtel et al. 2017; Baud et al. 2020). Probiotic bacteria (e.g., Lactobacillus and Bifidobacterium strains) have been also shown to interact with the gut microbiome to strengthen the immune system, being effective in reducing not only bacterial replication, but also viral one (Bozkurt and Quigley 2020; Mirzaei et al. 2021). However, if probiotics have been considered an economical and safe alternative for the cure of human viral diseases, there are no data on the use of probiotics against honeybee viral infections. Further research is thus needed to investigate if probiotics could be used by beekeepers for the treatment of viral diseases.
With regards to insects and in particular to honeybees, recent studies have suggested that non-pathogenic bacteria could be used in probiotic treatments to enhance insect growth and population and to prevent diseases (Crotti et al. 2013; Audisio 2017; Grau et al. 2017). Interestingly, in Drosophila melanogaster, the presence of specific indigenous gut bacteria, such as L. (= Lactiplantibacillus) plantarum (Storelli et al. 2011) and Acetobacter pomorum (Shin et al. 2011; Storelli et al. 2011), regulate larval growth and body size via activation of the insulin pathway (Buchon et al. 2013). Among the bacterial groups frequently found in the honeybee microbiota, lactic acid bacteria (LAB) are attracting a great interest (Olofsson and Vásquez 2008; Crotti et al. 2010; Buchon et al. 2013; Daisley et al. 2020a; Daisley et al. 2020b; Daisley et al. 2022). Lactobacillus and Bifidobacterium are certainly two of the most important genera normally found as commensals and used as probiotics for both humans and animals (Ouwehand et al. 2002; Bozkurt and Quigley 2020).
In last years, great efforts have been devoted to study honeybee response after the exposure to probiotic microorganisms, considering both the elicitation of the innate immune system, which ultimately act to maintain homeostasis in case of a pathogen attack, and the probiotic ability to counteract directly a pathogen (Evans and Lopez 2004; Olofsson and Vásquez 2008; Kwong et al. 2017; Daisley et al. 2022). For instance, experiments carried out with Lactobacillus and Bifidobacterium strains showed a stimulation of the innate immune response of the bee and a positive contribution for the host fitness in presence of a pathogen (Evans and Lopez 2004; Daisley et al. 2020a; Daisley et al. 2020b; Iorizzo et al. 2022).
Evans and Lopez (2004) have orally administered to bees a mix of bacterial species belonging to the genera Bifidobacterium and Lactobacillus (B. infantis, B. longum, L. (= Lacticaseibacillus) rhamnosus, L. acidophilus, and L. (= Limosilactobacillus) reuteri, observing the ability of these non-symbiotic bacteria to induce a strong immune response with high levels of the antimicrobial peptide (AMP) abaecin, which could help the larvae to overcome pathogens’ attacks (Evans and Lopez 2004; Olofsson and Vásquez 2008). Moreover, some native bacterial species (e.g., originating from the honeybee stomach) have shown antagonistic activity against pathogens (Olofsson and Vásquez 2008; Zendo et al. 2020). Strains of Lactobacillus johnsonii (CRL 1647, AJ5, and IG9) and Bacillus subtilis, isolated from honeybee gut and honey samples, showed a beneficial effect on bee colony health agaisnt P. larvae and N. apis (Sabaté et al. 2009; Audisio et al. 2011, 2015; Vásquez et al. 2012; Lazzeri et al. 2020). B. subtilis subsp. subtilis Mori2, obtained from a honey sample, favoured bee performance, reducing the prevalence of Nosema and Varroa (Sabaté et al. 2012). Again, Daisley et al. (2020a) showed that a mix of three selected lactobacilli strains (L. rhamnosus GR-1, L. plantarum Lp39, and L. kunkeei BR-1, namely LX3, including non-symbiotic and healthy bee hive derived bacteria) could improve bee survival against AFB causative agent, inhibiting the pathogen and beneficially modulating the bee innate immune response. Moreover, the administration of these strains have been shown to mitigate the gut dysbiosis and immune dysregulation induced by the application of oxytetracycline (Daisley et al. 2020b).
The reported biological effects of probiotics might be due to several factors e.g. the ability of probiotics to produce active compounds or organic acids against pathogens, to elicit the immune response through AMPs expression, to competitively exclude the pathogens and to suppress collaborative interactions (Lee and Salminen 2009; Endo and Salminen 2013; Kanmani et al. 2013; Muñoz-Atienza et al. 2014; Torres et al. 2016; Daisley et al. 2022). Yoshiyama et al. (2013) have indeed reported that the effect of LAB against P. larvae could be due to the release of organic acids.
Concluding remarks
Honeybee fitness is challenged by different biotic and abiotic stresses, which pose serious threats not only for honeybee individuals, but also for the ecosystem and crop yield. In this context, research interest is currently focused on the exploitation of probiotic formulations aimed at maintaining honeybee fitness and boosting its immune response. Probiotic products thus represent promising solutions for the sustainable management of honeybee health, but proper probiotics must be selected and tested in order to achieve this purpose considering all the different stressors and that, up to now, only limited studies have been performed with managed colonies. Moreover, further investigations should be carried out to decipher the mechanisms of competition and collaboration established between probiotics and native gut strains.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Abdi K et al (2018) Parasites-Iflavirus association and emergence of three master variants of DWV affecting Apis mellifera intermissa in Tunisian apiaries. Bull Insectol 71:273–282
Aizen MA, Harder LD (2009) The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr Biol 19:915–918
Alippi AM (2000) Is Terramycin® losing its effectiveness against AFB. Bee Biz 11:27–29
Aljedani DM (2022) Antibiotic treatment (Tetracycline) effect on bio-efficiency of the larvae honey bee (Apis mellifera jemenatica). Saudi J Biol Sci 29:1477–1486
Anderson KE, Ricigliano VA (2017) Honey bee gut dysbiosis: a novel context of disease ecology. Curr Opin Insect Sci 22:125–132
Audisio MC (2017) Gram-positive bacteria with probiotic potential for the Apis mellifera L. honey bee: the experience in the northwest of Argentina. Probiotics Antimicrob Proteins 9:22–31
Audisio MC, Torres MJ, Sabaté DC, Ibarguren C, Apella MC (2011) Properties of different lactic acid bacteria isolated from Apis mellifera L. bee-gut. Microbiol Res 166:1–13
Audisio MC, Sabate DC, Benítez-Ahrendts MR (2015) Effect of Lactobacillus johnsonii CRL1647 on different parameters of honeybee colonies and bacterial populations of the bee gut. Beneficial Microbes 6:687–695
Babendreier D, Joller D, Romeis J, Bigler F, Widmer F (2006) Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol 59:600–610
Bailey L (1983) Melissococcus pluton, the cause of European foulbrood of honey bees (Apis spp.). J Appl Bacteriol 55:65–69
Barnett EA, Charlton AJ, Fletcher MR (2007) Incidents of bee poisoning with pesticides in the United Kingdom, 1994–2003. Pest Manag Sci 63:1051–1057
Barrasso R, Bonerba E, Savarino AE, Ceci E, Bozzo G, Tantillo G (2018) Simultaneous quantitative detection of six families of antibiotics in honey using a biochip multi-array technology. Veterinary Sciences 6:1
Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E (2020) Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front Public Health 8:186
BenVau LR, Nieh JC (2017) Larval honey bees infected with Nosema ceranae have increased vitellogenin titers as young adults. Sci Rep 7:14144
Bleau N, Bouslama S, Giovenazzo P, Derome N (2020) Dynamics of the honeybee (Apis mellifera) gut microbiota throughout the overwintering period in Canada. Microorganisms 8:1146
Bonilla-Rosso G, Engel P (2018) Functional roles and metabolic niches in the honey bee gut microbiota. Curr Opin Microbiol 43:69–76
Bonilla-Rosso G et al (2019) Acetobacteraceae in the honey bee gut comprise two distant clades with diverging metabolism and ecological niches. bioRxiv 861260:41–50
Bottacini F et al (2012) Bifidobacterium asteroides PRL2011 genome analysis reveals clues for colonization of the insect gut. PLoS ONE 7:e44229
Bozkurt HS, Quigley EM (2020) The probiotic Bifidobacterium in the management of Coronavirus: a theoretical basis. Int J Immunopathol Pharmacol 34:2058738420961304
Bromenshenk JJ et al (2010) Iridovirus and microsporidian linked to honey bee colony decline. PLoS ONE 5:e13181
Buchon N, Broderick NA, Lemaitre B (2013) Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nat Rev Microbiol 11:615–626
Bulson L, Becher MA, McKinley TJ, Wilfert L (2021) Long-term effects of antibiotic treatments on honeybee colony fitness: a modelling approach. J Appl Ecol 58:70–79
Callegari M et al (2021) Compartmentalization of bacterial and fungal microbiomes in the gut of adult honeybees. Npj Biofilms Microbiomes 7:1–15
Castelli L, Branchiccela B, Romero H, Zunino P, Antúnez K (2022) Seasonal dynamics of the honey bee gut microbiota in colonies under subtropical climate. Microb Ecol 83:492–500
Colombel J (1987) Yogurt with Bifidobacterium longum reduces erythromycin-induced gastrointestinal effects. Lancet 2:8549
Corby-Harris V, Maes P, Anderson KE (2014a) The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 9:e95056
Corby-Harris V, Snyder LA, Schwan MR, Maes P, McFrederick QS, Anderson KE (2014b) Origin and effect of alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl Environ Microbiol 80:7460–7472
Cornman RS et al (2012) Pathogen webs in collapsing honey bee colonies. PLoS ONE 7:e43562
Cox-Foster DL et al (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287
Crotti E et al (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol 76:6963–6970
Crotti E et al (2013) Microbial symbionts of honeybees: a promising tool to improve honeybee health. New Biotechnol 30:716–722
Daisley BA et al (2020a) Novel probiotic approach to counter Paenibacillus larvae infection in honey bees. ISME J 14:476–491
Daisley BA et al (2020b) Lactobacillus spp. attenuate antibiotic-induced immune and microbiota dysregulation in honey bees. Commun Biol 3:1–13
Daisley BA, Pitek AP, Mallory E, Chernyshova AM, Allen-Vercoe E, Reid G, Thompson GJ (2022) Disentangling the microbial ecological factors impacting honey bee susceptibility to Paenibacillus larvae infection. Trends Microbiol. https://doi.org/10.1016/j.tim.2022.11.012
de Jong W et al (2019) Effects of diets containing different concentrations of pollen and pollen substitutes on physiology, Nosema burden, and virus titers in the honey bee (Apis mellifera L.). Apidologie 50:845–858
Dobbelaere W, de Graaf DC, Peeters JE (2001) Development of a fast and reliable diagnostic method for American foulbrood disease (Paenibacillus larvae subsp. larvae) using a 16S rRNA gene based PCR. Apidologie 32:363–370
Dong Z-X et al (2020) Colonization of the gut microbiota of honey bee (Apis mellifera) workers at different developmental stages. Microbiol Res 231:126370
Endo A, Salminen S (2013) Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst Appl Microbiol 36:444–448
Engel P, Martinson VG, Moran NA (2012) Functional diversity within the simple gut microbiota of the honey bee. Proc Natl Acad Sci 109:11002–11007
Engel P, Kwong WK, Moran NA (2013) Frischella perrara gen. nov., sp. nov., a gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int J Syst Evol Microbiol 63:3646–3651
Engel P, Stepanauskas R, Moran NA (2014) Hidden diversity in honey bee gut symbionts detected by single-cell genomics. PLoS Genet 10:e1004596
Engel P, Bartlett KD, Moran NA (2015) The bacterium Frischella perrara causes scab formation in the gut of its honeybee host. Mbio 6:e00193-e1115
Erkosar B, Leulier F (2014) Transient adult microbiota, gut homeostasis and longevity: novel insights from the Drosophila model. FEBS Lett 588:4250–4257
Evans JD (2004) Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. J Invertebr Pathol 85:105–111
Evans JD, Lopez DL (2004) Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae). J Econ Entomol 97:752–756
Evans J et al (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol 15:645–656
Fries I (2010) Nosema ceranae in European honey bees (Apis mellifera). J Invertebr Pathol 103:S73–S79
Fries I et al (2013) Standard methods for Nosema research. J Apic Res 52:1–28
Fuller R (1992) History and development of probiotics. probiotics. Springer, Dordrecht, pp 1–8
Genersch E (2008) Paenibacillus larvae and American foulbrood–long since known and still surprising. J Verbr Lebensm 3:429–434
Genersch E (2010) Honey bee pathology: current threats to honey bees and beekeeping. Appl Microbiol Biotechnol 87:87–97
Gochnauer T (1951) Drugs fight foulbrood disease in bees. Minn Home Fam Sci 9:15
Gorbach S, Chang T-W, Goldin B (1987) Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. The Lancet 330:1519
Goulson D, Lye GC, Darvill B (2008) Decline and conservation of bumble bees. Annu Rev Entomol 53:191–208
Goulson D, Nicholls E, Botías C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957
Gourbeyre P, Denery S, Bodinier M (2011) Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol 89:685–695
Grau T, Vilcinskas A, Joop G (2017) Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Zeitschrift Für Naturforschung C 72:337–349
Greenleaf SS, Kremen C (2006) Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc Natl Acad Sci 103:13890–13895
Hamdi C et al (2011) Gut microbiome dysbiosis and honeybee health. J Appl Entomol 135:524–533
Härtel C et al (2017) Lactobacillus acidophilus/Bifidobacterium infantis probiotics are associated with increased growth of VLBWI among those exposed to antibiotics. Sci Rep 7:5633
Hatjina F et al (2011) Polar tube protein gene diversity among Nosema ceranae strains derived from a Greek honey bee health study. J Invertebr Pathol 108:131–134
Higes M, Martín R, Meana A (2006) Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J Invertebr Pathol 92:93–95
Higes M, García-Palencia P, Martín-Hernández R, Meana A (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol 94:211–217
Higes M et al (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10:2659–2669
Hill C et al (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Hroncova Z, Killer J, Hakl J, Titera D, Havlik J (2019) In-hive variation of the gut microbial composition of honey bee larvae and pupae from the same oviposition time. BMC Microbiol 19:1–8
Huang Z (2012) Pollen nutrition affects honey bee stress resistance. Terr Arthropod Rev 5:175–189
Huang Q, Kryger P, Le Conte Y, Moritz RF (2012) Survival and immune response of drones of a Nosemosis tolerant honey bee strain towards N. ceranae infections. J Invertebr Pathol 109:297–302
Huang W-F, Solter LF, Yau PM, Imai BS (2013) Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog 9:e1003185
Iorizzo M et al (2022) Functional properties and antimicrobial activity from lactic acid bacteria as resources to improve the health and welfare of honey bees. InSects 13:308
Jia H-R et al (2016) The effects of Bt Cry1Ie toxin on bacterial diversity in the midgut of Apis mellifera ligustica (Hymenoptera: Apidae). Sci Rep 6:24664
Johnston BC, Goldenberg JZ, Parkin PC (2016) Probiotics and the prevention of antibiotic-associated diarrhea in infants and children. JAMA 316:1484–1485
Kačániová M, Gasper J, Terentjeva M (2021) Antagonistic effect of gut microbiota of honeybee (Apis mellifera) against causative agent of American foulbrood Paenibacillus larvae. J Microbiol Biotechnol Food Sci 2021:478–481
Kakumanu ML, Reeves AM, Anderson TD, Rodrigues RR, Williams MA (2016) Honey bee gut microbiome is altered by in-hive pesticide exposures. Front Microbiol 7:1255
Kanmani P, Satish Kumar R, Yuvaraj N, Paari K, Pattukumar V, Arul V (2013) Probiotics and its functionally valuable products—a review. Crit Rev Food Sci Nutr 53:641–658
Kapheim KM, Rao VD, Yeoman CJ, Wilson BA, White BA, Goldenfeld N, Robinson GE (2015) Caste-specific differences in hindgut microbial communities of honey bees (Apis mellifera). PLoS ONE 10:e0123911
Kešnerová L, Moritz R, Engel P (2016) Bartonella apis sp. nov., a honey bee gut symbiont of the class Alphaproteobacteria. Int J Syst Evol Microbiol 66:414–421
Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P (2020) Gut microbiota structure differs between honeybees in winter and summer. ISME J 14:801–814
Klein A-M et al (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc Lond b Biol Sci 274:303–313
Koch H, Schmid-Hempel P (2011) Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci 108:19288–19292
Kochansky J, Knox DA, Feldlaufer M, Pettis JS (2001) Screening alternative antibiotics against oxytetracycline-susceptible and-resistant Paenibacillus larvae. Apidologie 32:215–222
Kowallik V, Mikheyev AS (2021) Honey bee larval and adult microbiome life stages are effectively decoupled with vertical transmission overcoming early life perturbations. Mbio 12:e02966-e3021
Kujawa-Szewieczek A, Adamczak M, Kwiecień K, Dudzicz S, Gazda M, Więcek A (2015) The effect of Lactobacillus plantarum 299v on the incidence of Clostridium difficile infection in high risk patients treated with antibiotics. Nutrients 7:10179–10188
Kwong WK, Moran NA (2013) Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst Evol Microbiol 63:2008–2018
Kwong WK, Moran NA (2016) Gut microbial communities of social bees. Nat Rev Microbiol 14:374
Kwong WK, Mancenido AL, Moran NA (2017) Immune system stimulation by the native gut microbiota of honey bees. R Soc Open Sci 4:170003
Larsson DG, Flach CF (2022) Antibiotic resistance in the environment. Nat Rev Microbiol 20:257–269
Lazzeri AM, Mangia NP, Mura ME, Floris I, Satta A, Ruiu L (2020) Potential of novel food-borne Lactobacillus isolates against the honeybee pathogen Paenibacillus larvae. Biocontrol Sci Tech 30:897–908
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41:353–363
Lee YK, Salminen S (2009) Handbook of probiotics and prebiotics. John Wiley & Sons
Li C, Tang M, Li X, Zhou X (2022) Community dynamics in structure and function of honey bee gut bacteria in response to winter dietary shift. Am Soc Microbiol. https://doi.org/10.1128/mbio.01131-22
Lilly DM, Stillwell RH (1965) Probiotics: growth-promoting factors produced by microorganisms. Science 147:747–748
López JH, Krainer S, Engert A, Schuehly W, Riessberger-Gallé U, Crailsheim K (2017) Sublethal pesticide doses negatively affect survival and the cellular responses in American foulbrood-infected honeybee larvae. Sci Rep 7:40853
Lourenço AP, Guidugli-Lazzarini KR, Freitas FC, Bitondi MM, Simões ZL (2013) Bacterial infection activates the immune system response and dysregulates microRNA expression in honey bees. Insect Biochem Mol Biol 43:474–482
Ludvigsen J et al (2015) Shifts in the midgut/pyloric microbiota composition within a honey bee apiary throughout a season. Microbes Environ 30:235–244
Ludvigsen J, Andersen Å, Hjeljord L, Rudi K (2020) The honeybee gut mycobiota cluster by season versus the microbiota which cluster by gut segment. Veterinary Sciences 8:4
Maes PW, Rodrigues PA, Oliver R, Mott BM, Anderson KE (2016) Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol Ecol 25:5439–5450
Martinson VG, Danforth BN, Minckley RL, Rueppell O, Tingek S, Moran NA (2011) A simple and distinctive microbiota associated with honey bees and bumble bees. Mol Ecol 20:619–628
Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78:2830–2840
Mirzaei R et al (2021) The emerging role of probiotics as a mitigation strategy against coronavirus disease 2019 (COVID-19). Adv Virol 166:1819–1840
Moran NA, Hansen AK, Powell JE, Sabree ZL (2012) Distinctive gut microbiota of honey bees assessed using deep sampling from individual worker bees. PLoS ONE 7:e36393
Morimoto T et al (2011) The habitat disruption induces immune-suppression and oxidative stress in honey bees. Ecol Evol 1:201–217
Muñoz-Atienza E et al (2014) In vitro and in vivo evaluation of lactic acid bacteria of aquatic origin as probiotics for turbot (Scophthalmus maximus L.) farming. Fish Shellfish Immunol 41:570–580
Oldroyd BP (2007) What’s killing American honey bees? PLoS Biol 5:e168
Olofsson TC, Vásquez A (2008) Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr Microbiol 57:356–363
Ouwehand AC, Salminen S, Isolauri E (2002) Probiotics: an overview of beneficial effects. Antonie Van Leeuwenhoek 82:279–289
Paris L, Roussel M, Pereira B, Delbac F, Diogon M (2017) Disruption of oxidative balance in the gut of the western honeybee Apis mellifera exposed to the intracellular parasite Nosema ceranae and to the insecticide fipronil. Microb Biotechnol 10:1702–1717
Perdigon G, Maldonado Galdeano C (2017) Beneficial effect of probiotics consumption on the immune system. in: annals of nutrition and metabolism. Karger Allschwilerstrasse 10, CH-4009 Basel, Switzerland, pp 20–20
Perry J, Wright G (2013) The antibiotic resistance “mobilome”: searching for the link between environment and clinic. Front Microbiol 4:138
Pettis JS, Johnson J, Dively G (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99:153–158
Posada-Florez F et al (2019) Deformed wing virus type A, a major honey bee pathogen, is vectored by the mite Varroa destructor in a non-propagative manner. Sci Rep 9:1–10
Powell JE, Martinson VG, Urban-Mead K, Moran NA (2014) Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl Environ Microbiol 80:7378–7387
Prosdocimi EM, Mapelli F, Gonella E, Borin S, Crotti E (2015) Microbial ecology-based methods to characterize the bacterial communities of non-model insects. J Microbiol Methods 119:110–125
Rasic JL (1983) The role of dairy foods containing bifido-and acidophilus bacteria in nutrition and health? North Eur Dairy J 48:80–86
Raymann K, Shaffer Z, Moran NA (2017) Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol 15:e2001861
Regulation E (2013) No 485/2013. Off J Eur Union 139:12–26
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:S96–S119
Sabaté DC, Carrillo L, Audisio MC (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160:193–199
Sabaté D, Cruz M, Benitez-Ahrendts M, Audisio M (2012) Beneficial effects of Bacillus subtilis subsp. subtilis Mori2, a honey-associated strain, on honeybee colony performance. Probiotics Antimicrob Proteins 4:39–46
Salminen S et al (1998) Functional food science and gastrointestinal physiology and function. Br J Nutr 80:S147–S171
Saraiva MA et al (2015) Relationship between honeybee nutrition and their microbial communities. Antonie Van Leeuwenhoek 107:921–933
Schrezenmeir J, de Vrese M (2001) Probiotics, prebiotics, and synbiotics—approaching a definition–. Am J Clin Nutr 73:361s–364s
Schwarz RS, Huang Q, Evans JD (2015) Hologenome theory and the honey bee pathosphere. Curr Opin Insect Sci 10:1–7
Schwarz RS, Moran NA, Evans JD (2016) Early gut colonizers shape parasite susceptibility and microbiota composition in honey bee workers. Proc Natl Acad Sci 113:9345–9350
Shin SC et al (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674
Smith EA et al (2021) Reclassification of seven honey bee symbiont strains as Bombella apis. BioRxiv. https://doi.org/10.1099/ijsem.0.004950
Smoragiewicz W, Bielecka M, Babuchowski A, Boutard A, Dubeau H (1993) Les probiotiques. Can J Microbiol 39:1089–1095
Soares KO, Oliveira CJBD, Rodrigues AE, Vasconcelos PC, Silva NMVD, Cunha Filho OGD, Madden C, Hale VL (2021) Tetracycline exposure alters key gut microbiota in Africanized honey bees (Apis mellifera scutellata x spp.). Front Ecol Evol. https://doi.org/10.3389/fevo.2021.716660
Steffan-Dewenter I, Potts SG, Packer L (2005) Pollinator diversity and crop pollination services are at risk. Trends Ecol Evol 20:651–652
Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F (2011) Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 14:403–414
Su Q et al (2022) Significant compositional and functional variation reveals the patterns of gut microbiota evolution among the widespread Asian honeybee populations. Front Microbiol. https://doi.org/10.3389/fmicb.2022.934459
Tarpy DR, Mattila HR, Newton IL (2015) Development of the honey bee gut microbiome throughout the queen-rearing process. Appl Environ Microbiol 81:3182–3191
Texts CAFHB (2001) Food and Agricultural Organization of the United Nations. World Health Organization, Rome:23–22.25
Tian B, Fadhil NH, Powell JE, Kwong WK, Moran NA (2012) Long-term exposure to antibiotics has caused accumulation of resistance determinants in the gut microbiota of honeybees. Mbio 3:e00377-e1312
Tola YH, Waweru JW, Hurst GD, Slippers B, Paredes JC (2020) Characterization of the Kenyan honey bee (Apis mellifera) gut microbiota: a first look at tropical and Sub-Saharan African bee associated microbiomes. Microorganisms 8:1721
Torres M, Brandan CP, Petroselli G, Erra-Balsells R, Audisio M (2016) Antagonistic effects of Bacillus subtilis subsp. subtilis and B. amyloliquefaciens against Macrophomina phaseolina: SEM study of fungal changes and UV-MALDI-TOF MS analysis of their bioactive compounds. Microbiol Res 182:31–39
Vásquez A et al (2012) Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS ONE 7:e33188
Vojvodic S, Rehan SM, Anderson KE (2013) Microbial gut diversity of Africanized and European honey bee larval instars. PLoS ONE 8:e72106
Wang K et al (2021) Gut microbiota protects honey bees (Apis mellifera L.) against polystyrene microplastics exposure risks. J Hazard Mater 402:123828
Williams GR, Sampson MA, Shutler D, Rogers RE (2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J Invertebr Pathol 99:342–344
Winfree R, Williams NM, Gaines H, Ascher JS, Kremen C (2008) Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J Appl Ecol 45:793–802
Yeung T et al (2006) Receptor activation alters inner surface potential during phagocytosis. Science 313:347–351
Yoshiyama M, Kimura K (2009) Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American foulbrood. J Invertebr Pathol 102:91–96
Yoshiyama M, Wu M, Sugimura Y, Takaya N, Kimoto-Nira H, Suzuki C (2013) Inhibition of Paenibacillus larvae by lactic acid bacteria isolated from fermented materials. J Invertebr Pathol 112:62–67
Zendo T, Ohashi C, Maeno S, Piao X, Salminen S, Sonomoto K, Endo A (2020) Kunkecin A, a new nisin variant bacteriocin produced by the fructophilic lactic acid bacterium, Apilactobacillus kunkeei FF30-6 isolated from honey bees. Front Microbiol 11:571903
Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA (2017) Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci 114:4775–4780
Zheng H, Steele MI, Leonard SP, Motta EV, Moran NA (2018) Honey bees as models for gut microbiota research. Lab Anim 47:317–325
Funding
No funding is applied for this current project.
Author information
Authors and Affiliations
Contributions
KA conceived the idea, performed literature search and wrote the review. MBS edited the manuscript, and MBS, EC, ASM and AC finalized it.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdi, K., Ben Said, M., Crotti, E. et al. The promise of probiotics in honeybee health and disease management. Arch Microbiol 205, 73 (2023). https://doi.org/10.1007/s00203-023-03416-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03416-z