Abstract
Honeybee is certainly one of the most familiar flying insects of terrestrial habitats. Honeybees are critically important in the environment, sustaining biodiversity and providing essential pollination for a wide range of crops and wild plants. Extensive losses of honeybee colonies in recent years are becoming a major cause of concern. These social insects continuously face threats (diseases, climate change, and management practices) that weaken their health. In this chapter, we will focus on the western honeybee A. mellifera, focusing on gut symbionts, their functions and role on honeybee health
Honey bees are social insects and their activities within and outside the hive have been described over the centuries since they are a combination of organization, intelligence and sensitivity, starting from the ritualized body movements to the their capacity to “sampling” the environment and smell the odour of the food source.
Menzel (1993)
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
9.1 The Importance of Honeybee
Honeybee is certainly one of the most familiar flying insect of terrestrial habitats. Honeybee belongs to the order Hymenoptera, family Apidae, and is a member of the genus Apis. The center of origin is presumably Southeast Asia where most of the species are found. Mainly, they are limited in range to tropical and montane zones in Southeast and South Asia, but two species have far broader ranges, e.g., A. mellifera and A. ceranae. Ten species are generally recognized within the genus Apis (Engel 1999; Arias and Sheppard 2005). Phylogenetic analyses based on nuclear and mitochondrial DNA markers strongly support a cluster into three distinct groups: cavity-nesting bees (A. mellifera, A. cerana, A. koschevnikovi, A. nulensis), giant bees (A. dorsata, A. laboriosa, A. binghami, A. nigrocincta), and dwarf bees (A. florea, A. andreniformis) (Arias and Sheppard 2005; Raffiudin and Crozier 2007). In this chapter, we will focus on the western honeybee A. mellifera, which is the most widely distributed honeybee in the world because of its great honey-harvesting potential. The native distribution of A. mellifera includes Africa, Europe, and Western Asia, and molecular dating suggests that the population expanded into this range around one million years ago. Conflicting hypotheses have been proposed for the origin of this expansion (Middle East and Africa), although a recent work put A. mellifera closer to the only other Apis species, which are all restricted to Asia (Wallberg et al. 2014). The species includes 25 subspecies or geographic races described by morphometric and molecular analysis and grouped into evolutionary branches based on their morphological similarities.
It expanded its range into Europe and Asia as the Ice Age glaciers retreated, and it has been spread by humans to the Americas, Australia, and Hawaii. A. mellifera has also been introduced through much of the range occupied by A. cerana, including Japan and China.
Honeybees have an extremely elaborate social life, fulfilling the requirement of the “superorganism”; the honeybee colony “superorganism” consists of individual, groups, and hive components, complete with a large repertoire of socially interactive and homeostatic behaviors (Hölldobler et al. 2009). They typically live in colonies with intra-colonial homeostasis, consisting of a single queen, approximately 10–30 thousand “sterile” female workers, and from zero to a few thousand males, depending on the time of year (Page and Peng 2001). Food is stored in designated areas of the nest, and the workers use glandular secretions to feed the brood. Division of labor is well developed and pheromone regulated (Moritz and Southwick 1992).
Honeybees are critically important in the environment, sustaining biodiversity and providing essential pollination for a wide range of crops and wild plants (EFSA 2017). The Food and Agriculture Organization of the United Nations (FAO) estimates that of the 100 crop species that provide 90% of food worldwide, 71 are pollinated by bees (Copping 2013). The majority of crops grown in the European Union depend on insect pollination. The annual monetary value of pollination has been estimated to be billions of dollars (Hedtke et al. 2015). They contribute to human health and well-being directly through the production of honey, which is produced by honeybees from the nectar they gather, and other food and feed supplies such as pollen, wax, propolis, and royal jelly, as dietary supplements and ingredients in food (Ajibola et al. 2012). They can also be considered important bioindicators of environmental pollution (Celli and Maccagnani 2003).
Beekeeping is the art and science of rearing, breeding, and managing honeybee colonies in artificial hives for economic benefits (Ikediobi et al. 1985; Morse 1989). The most common species utilized for this purpose is Apis mellifera of which about 25 subspecies of economic importance occur in Europe, Middle East of Asia, and Africa (Leven et al. 1997).
Beekeeping is an ancient tradition, and honeybees have been kept in Europe for several millennia.
In recent years, a growing interest has been reported for the urban beekeeping practice as a fascinating rewarding pastime, which allows people to increase biodiversity, produce local foods, and reconnect with nature (Moore and Kosut 2013).
Given the importance of honeybees in the ecosystem and the food chain, and given the multiple services they provide to humans, their protection is pivotal. Beyond the essentiality of honeybee for a balanced vitamin and antioxidant-rich diet, honeybee is vital for the mankind for their contribution to biodiversity and to some extent to human survival.
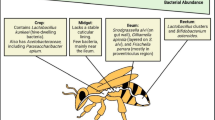
Extensive losses of honeybee colonies in recent years are becoming a major cause of concern. Unfortunately, they continuously face threats (diseases, climate change, and management practices); managed honeybees are highly social, frequent a multitude of environmental niches, and continually share food, conditions that promote the transmission of parasites and pathogens (Fig. 9.1).
9.2 Biotic and Abiotic Stresses
Although managed honeybee colonies are continuously increasing over the last 55 years, colony populations have significantly decreased in many European and North American countries (Aizen and Harder 2009), as a result of several incoming stressors (agrochemicals, pathogens, climate change) and socioeconomic reasons (Potts et al. 2010; VanEngelsdorp and Meixner 2010).
There is still a huge gap between pollinator demand and honeybee colony supply because the area planted with insect-pollinated crops increased more than the number of honeybee colonies (Aizen and Harder 2009; Breeze et al. 2014). At the same time, cultivation of crops, relying on insects for pollination, has increased (Aizen and Harder 2009).
In the last decade, a special attention has arisen toward “colony collapse disorder” (CCD) in the USA with the alarming claims of media, describing the dramatic demise of honeybee colonies, a world pollinator crisis, and the spectra of massive human starvation. Colony losses have exceeded 90% in some locations, and loss of pollination services has had major impacts on some fruit and vegetable production. Nevertheless, in the twentieth century, several honeybee losses were already registered (Oldroyd 2007). Symptoms were very close to those observed in the USA, and consequent losses of colonies were also traced throughout the world, but a clear explanation of the main causes was never found. Surely, viruses (i.e., Israeli acute paralysis virus) and the mite Varroa destructor were involved since the broad patterns of CCD coincide with continents with different pressures from V. destructor. In addition, during the same period of the CCD, a new parasite was moving all over the world, Nosema ceranae, jumping from its host, the Asian honeybee Apis cerana, to the western A. mellifera, causing gradual depopulation and copious colony death (Higes et al. 2008). Moreover, in such a dramatic moment, the attention was also addressed to the agrochemicals, above all the neonicotinoid insecticide imidacloprid, which are over employed in the American agriculture system. Overall, researchers concluded that no single driver could emerge as the definitive cause of the phenomenon and that CCD was a multifactorial syndrome. Bees were all sick, but each colony seemed to suffer from a different combination of such diseases.
As reported by Vanengelsdorp and Meixner (2010), honeybee can die in many ways, and CCD is just one of them. Finally, since a great genetic variability exists both in honeybee host and pathogens, the symptoms and causes of colony losses may well be different in different regions (Neumann and Carreck 2010).
Concerning the abovementioned pesticides, in 2013, the EU imposed a temporary ban on the use of the three key neonicotinoids on some crops. However, the new proposals are for a complete ban on their use in fields, with the only exception being for plants entirely grown in greenhouses (EFSA 2013). Monitoring schemes on pesticide effects are currently ongoing in some member states to provide more insights into the acute effects of pesticides on honeybees. The effects of pesticide drifting during treatment were addressed in the “APEnet” project (Apenet, 2011), which mentioned the case of the fatal powdering of bees in flight with particulates of neonicotinoid seed coating, the implications of humidity (Girolami et al. 2012), and the lethal aerial powdering of honeybees with neonicotinoids from fragments of maize seed coat (Marzaro et al. 2011). Moreover, some reports of experimental studies describe an interaction between N. ceranae and other stressors (e.g., chronic bee paralysis virus (CBPV), black queen cell virus (BQCV), or imidacloprid) that can lead to elevating honeybee mortality (Alaux et al. 2010; Doublet et al. 2015).
The situation is different with honeybee colony losses (i.e., the death of colonies), which mainly occur during the winter season (winter losses of honeybee colonies). These winter losses do not follow a general pattern. In some countries and some winters, losses are high (above 15%), sometimes even catastrophic (above 30%), but they are not always and everywhere high and unusual and catastrophic. The emerging picture is that the losses reported by beekeepers to the media are always much higher than the losses counted by official inspectors in the course of nationwide monitoring programs or surveys (see the official reports available under http://ec.europa.eu/food/animals/live_animals/bees/study_on_mortality/index_en.htm). While in the winter 2012–2013, at least the Northern part of Europe experienced high winter losses, in the winter 2013–2014, the losses were below 15% in all participating member states except for Sweden (15.4%) and in some member states even below 10% or 5%. This is far from being an alarming situation. In addition, such losses are not a problem for a normal beekeeper who replaces lost colonies easily by nucs made during the bee season.
While it is impossible to identify a single factor, which can account for all colony losses in all regions of the world over a given time period, it is clear that several biological and environmental factors acting alone or in combination have the potential to cause premature colony mortality by adversely affecting colony health and life span. Among these factors, certain honeybee diseases and parasites have been shown to play a significant role in increased honeybee colony mortality and in the described colony losses.
In the following paragraph, a list and a brief description of the main pathogens, affecting honeybee health, will be listed.
9.3 Pathogens Affecting Honey Bee
9.3.1 Brood Pathogens
Melissococcus plutonius is the causative agent of the European foulbrood (EFB) affecting honeybee larvae in the western Apis mellifera. However, the bacterium can also infect and kill the brood of the Eastern honeybee (Apis ceranae) and the Himalayan honeybee (Apis laboriosa) (Bailey 1974; Allen et al. 1990). M. plutonius is a lanceolate non-spore-forming coccus with a close phylogenetic relationship to the genus Enterococcus (Cai and Collins 1994). Bacterial cells are ingested with contaminated food and invade the midgut where they reproduce, assimilating the larval food. Infected larvae can die before or after capping from starvation (Bailey 1983), or they may successfully pupate and form normal or undersized adults. Following infection, secondary invaders, like Paenibacillus alvei and Enterococcus faecalis, are involved in the decomposition of the larval remains. Dead larvae are found twisted around the walls of the cell or stretched out lengthways. These larvae turn yellow and then brown and finally decompose, adopting a grayish black color (Forsgren 2010). Although symptomatology is rather well described, many aspects of the pathogenesis, transmission and control of M. plutonius are poorly understood and remain elusive (Genersch 2010). In a recent work performed in our laboratory, we evidenced that honeybee larvae were affected by EFB, with the presence of an atypical Paenibacillus strain (P. dendritiformis) as a new putative second invader, which presumably conferred a different symptomatology to the diseased brood (Gaggia et al. 2015).
EFB did not create serious problems in many European countries since many infected and diseased colonies spontaneously recovered from the disease (Bailey 1968). Nevertheless, a dramatic increase in the incidence of EFB has been recently observed, in particular in the United Kingdom, Switzerland (Wilkins et al. 2007; Roetschi et al. 2008), and Norway (Dahle et al. 2011).
Paenibacillus larvae is a Gram-positive, spore-forming bacillus that causes the American foulbrood (AFB) (Genersch et al. 2006), which contaminate the first instar larvae leading to its death after the cell capping. AFB is not only fatal to single honeybee larvae, but leads to the collapse of the entire colonies. In addition, AFB is highly contagious, and the spores are extremely tenacious.
As for the EFB, the infection originates from the ingestion of food contaminated with spores; once in the midgut, spores germinate, and the vegetative cells reproduce and invade the hemocoel (Davidson 1973; Bailey and Ball 1991), by synthesizing highly active extracellular proteases (Hrabák and Martínek 2007). In the second stage, the larvae become a brownish, semifluid, glue-like colloid (ropy stage) releasing a putrid smell. The ropy aspect (dead larvae adhere and form a thread span when touched with a wooden stick) confirmed the presence of AFB. Finally, the larva remains dry down to a hard scale (foulbrood scale), which tightly adheres to the lower cell wall. The scales contain millions of spores, which could distribute the infection for many years within and between colonies (Bailey and Ball 1991).
For both foulbroods, antibiotics are used by some beekeepers (especially in the USA and other non-European countries), leading to concerns over antibiotic resistance, collateral losses of beneficial microbes, and the risks of antibiotic residues in honey and pollen destined for human consumption.
The fungus Ascosphaera apis is responsible for the chalkbrood disease; larvae are infected by ingesting fungal spores that germinate in the digestive tract. The subsequent mycelial growth is lethal to the larvae. Dead larvae and pupae desiccated, forming mummies that contain millions of spores and that are highly infectious (Aronstein and Murray 2010). A. apis is responsible for large economic losses, particularly in combination with other pathogens such as Nosema apis (Aydin et al. 2006), N. ceranae, and V. destructor (Hedtke et al. 2008).
9.3.2 Nosema apis and Nosema ceranae
Adult honeybees host two parasites belonging to the fungal phylum Microsporidia—Nosema apis and Nosema ceranae—both of which have received extensive attention, in particular N. ceranae, which moved, in the last decades, from their natural Asiatic host (Apis cerana) to the European one, finding fertile ground for its development (Higes et al. 2008; Rosenkranz et al. 2010). Recently, it became evident that N. ceranae is also widespread in the A. mellifera population throughout the world, particularly in countries with temperate climate (Paxton et al. 2007; Giersch et al. 2009; Higes et al. 2007). Due to its distribution, and severity, it is now considered one of the major health problems both in individual honeybees (Paxton et al. 2007; Antúnez et al. 2009) and in whole colonies (Higes et al. 2008).
As obligate intracellular parasites, the Microsporidia invade epithelial cells of the adult midgut and undergo repeated cell divisions to produce new infectious spores. These infections often result in heavy parasite loads, tens of millions of spores per bee (Forsgren and Fries 2010), which lead to an increase of the nutritional requirement, morbidity, and mortality of the bee host (Martín-Hernández et al. 2011).
N. apis is mainly characterized by dysentery, dilated abdomens, brown fecal marks on combs and the front of the hives, sick or dead bees in the vicinity of the hives, and a decrease in brood production and in the size of bee colony, particularly in spring. N. ceranae caused death of individuals and colonies not preceded by any visible symptoms. The microsporidium develops exploiting the host cell mitochondria (Chen et al. 2009; Higes et al. 2007), inducing a severe energetic stress and competing directly for key nutrients and energy resources. The infection firstly causes increased food consumption (Martín-Hernández et al. 2011), immune suppression (Antúnez et al. 2009), degeneration of gut epithelial cells, shortened life spans (Higes et al. 2007) and a decrease on population size and loss of adult bees. It has also been suggested that N. ceranae induces significantly higher mortality than N. apis (Paxton et al. 2007; Martín-Hernández et al. 2009; Higes et al. 2010). Considering the different symptomatology, the members of a recent international meeting assigned two different clinical patterns: nosemosis type A caused by N. apis and nosemosis type C caused by N. ceranae (COLOSS Workshop 2009).
Evidences show that N. ceranae, due to epithelial lesions, increases the susceptibility to other pathogens, in particular viruses (Higes et al. 2008). In addition, the exposure to sublethal concentration of neonicotinoids in immature bees significantly enhanced the number of spore production per bee (Vidau et al. 2011). Nowadays, the antibiotic Fumagilin-B (dicyclohexylammonium salt) is the only available compound to treat N. ceranae infection; however, it is no longer licensed in the EU states, and recent reports provide controversial results about its efficacy and its effects related to residues in honey (Lopez et al. 2008; Williams et al. 2008).
9.3.3 Spiroplasmosis
Spiroplasmas are small, helical, and motile eubacteria and are descendants of Gram-positive bacteria that lack a cell wall (Regassa and Gasparich 2006). Spiroplasma melliferum and Spiroplasma apis are two pathogens of adult honeybee that have been identified in Western honeybees (Clark 1977; Mouches et al. 1982), but infection has been also reported in Asia and the USA. Pathogenesis occurs when the organisms breach the gut barrier and invade the hemolymph, causing a systemic infection that can ultimately lead to fatal disease in the bee. Spiroplasma infections are much more difficult to recognize and diagnose than the foulbrood diseases, hindering the ability to monitor bacterial abundance and impact on the beekeeping industry. They remain interesting targets for study, owing to their seasonal abundance in honeybee colonies, which is presumably tied to flowering cycles of specific plants that act as transmission sites (Clark 1982).
The main groups of protists infecting honeybee have been neglected for many years due to different reasons, e.g., obscure pathology, low detectability, difficulty in culturing, and absence of genetic markers. Nowadays, the research community is focusing its attention on trypanosomes (Crithidia mellificae and the recent strain San Francisco), gregarines (Apycystis bombi), and amoeba (Malpighamoeba mellificae).
C. mellificae and gregarines colonize the hindgut and midgut, respectively. C. mellificae produces encrustations on the gut epithelia surface, and gregarines attach to the epithelia and absorb nutrients, creating tissue damage and reducing nutrient absorption by the bee. However, their role in honeybee health and distribution in the world is not well understood; colonies seem more susceptible in tropical climates. Trypanosomes have probably a cosmopolitan distribution since C. mellificae has been reported in Australia, China, France, Japan, Switzerland, and the USA (Ravoet et al. 2013). The related species C. bombi, also reported from Asian honeybees, has seriously affected the survival of bumble bees under stress conditions (Brown et al. 2000; Li et al. 2012). Recently, complex dynamic immune responses to C. mellificae infection were reported, with a distinct response when individuals were infected with C. mellificae and N. ceranae simultaneously (Schwarz and Evans 2013). In addition, an association between both pathogens was reported in the USA (Runckel et al. 2011). Gregarines infecting other bees and social wasps inhibit foraging, reduce fecundity, and increase queen mortality. After its detection in honeybees in Finland (Lipa and Triggiani 1996), A. bombi was also reported in honeybees in Japan (Morimoto et al. 2013) and Argentina (Plischuk et al. 2011).
The amoeba Malpighamoeba mellificae infects adult bees in temperate to tropical regions. The ingested cysts develop into trophozoites and invade the Malpighian tubules, degrading their tissues. As the amoebae replicate, they pack the lumen of the tubules, forming up to 500,000 cysts per bee that are shed through the feces. The damaged tubules are unable to carry out their physiological function bringing bees to death (Lipa and Triggiani 1996). Associated with spring dwindling of bee colonies, M. mellificae is also linked with dysentery symptoms in adult bees and the tendency of infected bees to “disappear inexplicably” from the hive (Prell 1926).
9.3.4 Varroa Destructor
Varroa destructor is a mite parasite of honeybees. Originally, a parasite of the Asiatic honeybee Apis cerana performed a host shift in the early 1970s to the European honeybee Apis mellifera. Where and how this switch occurred is unclear (Rosenkranz et al. 2010), anyhow since then the parasite has crossed the globe, and it is considered endemic in all the beehives of the globe. To date only Australia and few north European territories (Åland Islands and Isle of Man) result as V. destructor-free areas.
Varroa is feeding on the hemolymph of larvae and adult bees, thus weakening the insect. But this doesn’t seem to be the determinant factor leading to the colony collapse. Indeed, varroa infect bees with a relevant number of viruses like deformed wing virus (DWV), chronic bee paralysis virus (CBPV), black queen cell virus (BQCV), and sacbrood virus (SBV). To date 16–18 truly unique viruses (24 if considering the variants) have been identified as pathogenic for bees (De Miranda et al. 2013).
Different approaches have been used to eliminate the varroa parasite from the hives. Upon its arrival in Europe, several acaricides were used to control its proliferation, but an inevitable development of multiple resistances led to commercial withdrawal of the majority of them. Nowadays, only few active ingredients result active like amitraz, coumaphos, and fluvalinate. More recently beekeepers focused their efforts on organic approaches, using organic acids like oxalic, formic, and lactic acids together with comb trapping methods. Also, essential oils and physical approaches like drone brood excision or brood heating are playing a relevant role. As the last approach in the parasite control, a number of research centers and beekeepers tried to develop varroa-resistant bees, with different approaches. Worthy to mention here is the development of the varroa-sensitive hygiene (VSH) behavior.
Nevertheless, even if eradication of the parasites from a beehive is possible, a free colony status does not last long. Indeed, varroa reinfestation occurs due to a permanent exchange of mites between foragers, or drones enter foreign colonies, voluntarily, by drifting or by robbing (Goodwin et al. 2006). Still nowadays, varroosis can be classified in the top list of destabilizing biotic factors for honeybees.
9.4 Digestive and Excretory Systems in Apis mellifera
The alimentary canal (Fig. 9.2) of honeybee extends from the mouth to the anus where the waste material is excreted. The esophagus is the connection between the mouth and the rest of the digestive system in the abdomen (through the thorax).
The posterior end opens into the crop or honey stomach an expandable bag holding (a) honey ingested in the hive and used for energy during the flight and (b) nectar and/or water collected in the field for transport back to the nest. More generally, the crop represents the microbial intersection of food sharing, food storage, and the pollination environment. The pH of the crop is highly acidic but also varies in accordance with the pH of ingested food products. There is a special structure called proventriculus near the end of the crop, which has sceleritized toothlike structures, and also muscles and valves. These structures prevent most of the liquid crop contents from passing through the ventriculus or midgut and allow the removal of pollen grains in the nectar. The proventriculus also allows filtering out particles from 0.5 to 100 μm in diameter, resulting in the partial stop of spores of Nosema sp. and Paenibacillus larvae (Peng and Marston 1986). Moreover, it prevents the contamination of the crop with enzymes and microbes from the more posterior midgut. The valve is open during the feeding, thus allowing the honey to go from the honey stomach to the ventriculus.
The contents of the crop can be spit back into cells, or fed to other workers (trophallaxis), as the case of nectar collected by foragers. Most of the nutrients from digested feed are absorbed through the walls of the ventriculus (midgut), which is the functional stomach of bees, where most of the digestion and adsorption take place. Digestive enzymes work across a range of pH, but the optimum is pH 8. Thus, the proventriculus and the drastic change in pH between the crop and the midgut define two major microbial niches, one coevolved with liquid transfer and food storage and the other coevolved to reside in the enzymatically active and relatively nutrient-rich midgut. Malpighian tubules are small strands of tubes attached near the end of ventriculus and function as the kidney, by removing the liquid nitrogenous waste (in the form of uric acid, not as urea as in humans) from the hemolymph, and the uric acid forms crystals. The undigested material (pollen husks, dead cells, and fat globules) moves through the pyloric valve into the hindgut for excretion; the hindgut is divided into two compartments: the anterior ileum, a narrow tube with six longitudinal invaginations, and the rectum, a larger saclike compartment. During winter, the rectum expands considerably to hold waste material since bees do not defecate in the hive and wait for warm flying weather in the spring.
9.5 Composition of the Honeybee Gut Microbiota
The molecular tools and the new methods of DNA sequencing allowed researchers to investigate the gut microbiota of A. mellifera, giving a more consistent picture of its composition and role in insect health compared to culture-dependent methodologies.
In the past several microorganisms (Bacillus spp., Enterobacteriaceae, Bifidobacterium spp.), together with molds and yeasts, were identified from honeybee guts by using culture-based techniques (Gilliam and Valentine 1976; Gilliam 1997; Scardovi and Trovatelli 1969). Molds, particularly the genera Penicillium and Aspergillus, were commonly found in the alimentary canal of worker honeybees (Gilliam et al. 1974, 1977), and yeast presence appeared to be an indicator of stress conditions in honeybees (Gilliam 1997). Still today, plate count isolation and further identification allow the recovery of new species which could only be detected by traditional microbiology. This is the case of recently characterized gut bacterial species such as Gilliamella apicola, Snodgrassella alvi, Frischella perrara, Lactobacillus kullabergensis, L. kimbladii, L. helsingborgensis, L. mellis, L. mellifer, and L. melliventris (Kwong and Moran 2013; Olofsson et al. 2014).
Globally, the composition and function of the microbial community inhabiting the alimentary tract are closely related to the physiological changes and nutritional regimes associated with honeybee age and tasks. Foragers consume almost exclusively nectar and honey to meet the metabolic demands of flying (Winston 1987), while nurse bees eat large quantities of stored pollen to meet the nutritional demands for synthesizing and secreting royal jelly for larvae and other adults (Anderson et al. 2011). Investigations allowed establishing that a “core” bacterial community has coevolved with the honeybee over millions of years and now represents a relatively stable and constitutive component of healthy bees independent of geography.
A recent study showed that honey crop (honey stomach) of foragers was dominated by Lactobacillus with the dominance of L. kunkeei and Alpha 2.2 (Acetobacteraceae) but also contained a small number of less abundant Enterobacteriaceae that likely have their origins in the pollination environment (Corby-Harris et al. 2014). Other studies based on culture-dependent methods evidenced a crop microbiota composed of several bacterial species within the genera Lactobacillus and Bifidobacterium (Vásquez et al. 2012) with new identified lactobacilli species (Olofsson et al. 2014). The probiotic properties of these bacteria are notably recognized in vertebrates where Lactobacillus and Bifidobacterium strains exert beneficial activities within the gut microbiota (Gaggìa et al. 2010).
The estimates counts ranks from 102 to 105; however, the number varies numerically across seasons with the flowers visited and with the health status of bees. Acetobacteraceae and L. kunkeei thrive in sugar-rich, acidic environments such as the crop, beebread, and honey and are considered core hive bacteria, as they are associated with nurse workers and developing larvae (Anderson et al. 2013). The crop is a central organ in the honeybee’s food production (beebread and honey) and food storage, and all the isolated bacteria exert important function, e.g., exopolysaccharide and antimicrobial compound production, biofilm formation, fermentation activities, and inhibition of spoilage microorganisms (Olofsson and Vásquez 2008; Forsgren 2010).
Finally, crop samples were also found to contain the core gut microbiota to some degree; on average, the gut-specific taxa in the greatest abundance in the forager crop corresponded to Lactobacillus (Firm 5), Gilliamella apicola (Gamma1), and Snodgrassella alvi (Beta).
The midgut contains relatively few bacteria, which are most concentrated at the distal region, adjoining the hindgut. Unlikely, the hindgut houses a large bacterial community dominated by eight major bacterial groups (Moran 2015): two Alphaproteobacteria (Bartonellaceae and Acetobacteraceae), two Gammaproteobacteria (Gilliamella apicola and Frischella perrara), two members of the phylum Firmicutes with different species of lactobacilli (Firm 4, Firm 5), one Betaproteobacteria (Snodgrassella alvi), and one species of the genus Bifidobacterium (B. asteroides).
9.6 The Importance of the Gut Microbiota on Bee Health
The importance of gut-dwelling microbial communities in bees has become appreciated only recently, following the repetitive colony losses registered worldwide due to abiotic and biotic stressors, which led researchers to better understand the role of both gut symbiotic and pathogenic microbial interactions, since they are strictly related to food storage and the pollination environment. As for humans and animals, the understanding of the beneficial nature of insect-microbial systems is fundamental to investigate the effect of the microbial communities on host nutrition and pathogen defense. Thanks to the advanced molecular techniques and metagenomics, the human gut microbiota has revealed a huge number of bacterial genes (100 times more the number of genes found in the host), which strongly influence the physiological and biochemical activities of the host. The works on Drosophila melanogaster have given a picture of the molecular dialog between the microbiota and the insect gut. There is evidence of the role of gut microorganisms in supporting the immune system, influencing the epithelial homeostasis, promoting life span and larval growth upon food scarcity, and driving the host mating preference (Brummel et al. 2004; Ryu et al. 2008; Buchon et al. 2009; Sharon et al. 2010; Storelli et al. 2011). Shin et al. (2011) showed that acetic acid from the gut commensal bacterium, Acetobacter pomorum, modulates insulin/insulin-like growth factor signaling (IIS) in Drosophila to regulate host homeostatic programs controlling developmental rate, body size, energy metabolism, and intestinal stem cell activity. Among studies performed on insects, Dillon and Charnley (2002) reported in the desert locust Schistocerca gregaria the contribution of gut microbiota to host defense against pathogens by producing antimicrobial phenolic compounds and synthesizing key components of the locust cohesion pheromone. In healthy individuals of D. melanogaster, the immune system allows the dominance of two acetic acid bacteria (AAB) strains (Acetobacter pomorum and Commensalibacter intestini), which suppress the proliferation of the gut pathogen Gluconobacter morbifer by competition, which is a gut apoptosis inducer (Ryu et al. 2008; Crotti et al. 2010). A decreased presence of potentially pathogenic Pseudomonads spp. and a higher mating fitness were observed in the Mediterranean fruit fly males Ceratitis capitata fed with a diet enriched of Klebsiella oxytoca live cells, following irradiation (Ben-Ami et al. 2010).
A balanced gut microbiota constitutes an efficient barrier against pathogen colonization, produces metabolic substrates (e.g., vitamins and short-chain fatty acids), and actively exchanges regulatory signals with the host that primes and instructs mucosal immunity (Gaggìa et al. 2010). Although insects harbor a smaller number of symbionts, the honeybee gut microbiota displays high affinity with that of mammals (Kwong and Moran 2013). The huge number of bacterial symbionts, inhabiting selected niches along the whole tract of the gut (from honey crop to the rectum) are host-adapted species, which contribute to host defense, nutrition, and physiology (Hamdi et al. 2011).
Additionally, the honeybee gut microbiota exists at two major levels: within the relatively digestive tract and throughout the hive that houses the developing young and food stores. The majority of commensal gut bacteria are vital for the maintenance of homeostasis and health both in the single insect and into the hive, considering that activities such as trophallaxis and cleaning behavior led bees to partially share their microbial consortium.
The concept of symbiosis, in which both microbial and host elements work synergistically to maintain proper nutrition, health, and immunity, may be more important in social insects where both elements, compared to solitary insects, are often highly coevolved (Vásquez et al. 2012). The most explicative example of coevolution derives from the significant contribution to host protection provided by the interaction of the gut microbiota with the humoral and systemic immunity that is associated to the defense strategies in eusocial insects, whose genome has significantly fewer immune genes than expected (Evans et al. 2006). A balanced gut microbiota is necessarily associated with bee health since it provides countless enzymatic activities to break down the complex sugars of the honeybees’ diet. Some studies evidenced that the lactobacilli and bifidobacteria community (LAB) in the crop vary numerically across seasons with the flowers visited by bees and with the health status of bees (Olofsson and Vásquez 2008). Cox-Foster et al. (2007) demonstrated a high relative abundance of the γ-proteobacterial taxa in the bees from CCD-affected hives than in the healthy ones, while the presence of Firmicutes and Alphaproteobacteria, mainly represented by taxa related to the genus Lactobacillus and AAB, respectively, was dramatically reduced in diseased bees. In three species of wild bumble bees, a low presence of S. alvi and G. apicola strains was associated with a higher incidence of the pathogen Crithidia spp. (Cariveau et al. 2014). Snodgrassella and Gilliamella form biofilm-like layers on the epithelium of the longitudinal invaginations of the ileum; Snodgrassella is in direct association with the host tissue followed by a thick layer of Gilliamella. Studies on gene functions showed significant enrichment in the categories of several activities associated with the formation of the biofilm on the gut epithelial surface and with the host interaction (Engel et al. 2012). The microbial community of Bombus, which is dominated by Gilliamella and Snodgrassella, seems to protect the insect against a trypanosome (Koch and Schmid-Hempel 2011), suggesting a possible role of the biofilm as a protective layer against parasite invasion. Gut symbionts are continuously involved in the bioconversion and preservation of pollen material, nectar, honey, and beebread. Vásquez and Olofsson (2009) suggested that LAB from the honeybee stomach belonging to the genera Lactobacillus and Bifidobacterium are involved in the fermentation process of beebread and may be responsible for improving the nutritive value by vitamin production. As reported by Engel et al. (2012), a wide genetic variation can be observed within different bacterial species involved in food processing, e.g., carbohydrate metabolism and pollen wall demolition, thus reflecting divergent niche adaptation within the gut of honeybees.
In conclusion, the above mentioned findings showed that the interaction host-symbionts goes beyond a mere nutritional complementation of the host diet. Honeybee gut symbionts counteract bee pathogens and parasites, enhance bee immunity, and improve aspects related to host physiology, behavior, reproduction, and evolution. Consequently, microorganisms could be a key element in managing and preserving honeybee health status toward biotic and abiotic stressors.
9.7 Beneficial Bacteria or Probiotic Bacteria?
LAB has been widely studied in animals and humans because of their probiotic properties, which have led to their well-built commercial exploitation in food, feed, and pharmaceutical market (Gaggìa et al. 2010, 2011; Tontou et al. 2015). The findings that a component of the honeybee gut microbiota was represented by lactobacilli and bifidobacteria have increased the interest of scientists in looking for similarity and analogy with the probiotic bacteria widely investigated in humans and animals. They are Gram-positive, acid-tolerant, facultative, and/or strictly anaerobic bacteria and produce lactic and acetic acid as the major metabolic end product of carbohydrate fermentation. LAB are well known for the production of antimicrobial peptide. They are normal inhabitants of the gastrointestinal tract of many insects, and their presence in the honeybee digestive system has been consistently reported in the literature (Olofsson and Vásquez 2008; Baffoni et al. 2016; Gaggia et al. 2015; Moran 2015). The bee’s digestive system represents an optimal niche for LAB, which obtained from the bee’s diet suitable substrates for their growth. The in vitro antagonistic activity toward bee pathogens due to organic acids and antimicrobial peptides (M. plutonius, P. larvae, N. ceranae) is well documented (Audisio et al. 2011b; Wu et al. 2013; Yoshiyama and Kimura 2009; Maggi et al. 2013; Baffoni et al. 2016). Saraiva et al. (2015) found in the gut microbiota of honeybee a relative high presence of genes involved in the biosynthesis of streptomycin and secondary metabolites, which could be associated with protection functions against exogenous microorganisms. Moreover, among Lactobacillus, novel species has been recently identified (Olofsson et al. 2014), thus extending the beneficial potentiality of these bacteria.
Another group of interesting bacterial species is represented by the acetic acid bacteria (AAB) that are a large group of obligate aerobic Gram-negative bacteria within the Alphaproteobacteria clade, commonly found in association with various kinds of sugar matrices. AAB of the genera Gluconobacter, Acetobacter, Gluconacetobacter, and Saccharibacter have been reported as symbionts of bees (Crotti et al. 2010). Among these, the sugar-loving and flower-associated Gluconobacter spp. are among the predominant bacterial groups in bees. Mohr and Tebbe (2006) isolated from the honeybee’s gut about 100 bacterial strains belonging to different bacterial divisions. All isolates of the Alphaproteobacteria were AAB, closely related to Gluconobacter oxydans or Saccharibacter floricola, an osmophilic bacterium previously isolated from pollen.
Lactic acid bacteria and AAB show interesting properties like the capability to grow and tolerate acidic pH, to produce organic acids, and to metabolize different sugars. These features explain the effectiveness of LAB and AAB in colonizing the sugar-rich digestive system of bees and suggest a potential for inhibiting the growth of acid-sensitive pathogenic bacteria. Taking into account that treatments with formic, lactic, and acetic acids are widely employed by beekeepers to prevent pathogen infections, and, in the light of the final products of their metabolism, LAB and AAB may represent natural protecting bee symbionts of considerable importance (Olofsson and Vásquez 2008). It is of common use to describe these microorganisms as “probiotics”; however, the scientific community and authorities of the field have still not yet drawn up a list of properties/characteristics that identified the probiotic concept as in humans and animals. The transfer of the probiotic concept from vertebrates to invertebrates still requires further considerations, and several questions still need to be investigated and debated; in particular, we referred to the origin of the strains and the knowledge of their genome since their diffusion in the environment could be considered a risk.
Modulation of the honeybee gut microbiota by supplementation of selected bacterial strains has risen a special attention since it represents strategies to improve the health status of colonies, in terms of productivity and boosting the presence of beneficial microorganisms within the bee gut of new-generation bees. In the next section, an overview of the main published applications will be reported (Table 9.1).
9.8 Application of Beneficial Microorganisms
Experiments envisaging the administration of beneficial bacteria to honeybees are diverse and sometime confusing. The main target is often to counteract the most widespread pathogens affecting both larvae and adults since in vitro tests evidenced interesting host protection properties by directly stimulating the bee’s immune system and inhibiting pathogens through competitive exclusion and antimicrobial compound production (organic acids and secondary metabolites, e.g., bacteriocins and lipopeptides). Strains are usually isolated from honeybee crop/gut or from the environment; the use of formulations for animal and human consumption is also considered but disputable.
Applications addressed to infected larvae showed a significant reduction of larvae mortality after supplementation of different beneficial bacteria. However, data could result in misleading conclusions, since a reduction of larvae mortality, although statistically significant, is of little biological relevance because the colony will probably succumb to the disease, although it might take 1 or 2 weeks longer. Artificial infections with pathogens at high concentrations have a strong impact on the colony, and it would be preferable to observe lower doses, which simulate a natural infection process.
Forsgren 2010 applied a mixture of beneficial bacteria isolated from honey crop—L. kunkeei, L. mellis, L. kimbladii, L. kullabergensis, L. helsingborgensis, L. melliventris, L. apis, L. mellifer, B. asteroides, and B. coryneforme—with a final concentration of 107 bacteria/mL. Infection in honeybee larvae was performed with two different spore concentrations of P. larvae.
In the detail, the LAB mixture was supplemented with sugar syrup, both in combination with P. larvae at the time of spore inoculum and 48 h postinfection. Results showed the positive effect of LAB supplementation only in the group challenged with the highest dose of P. larvae with a significant reduction of larvae mortality, from 70 to 55%. Hamdi and Daffonchio (2011) used a probiotic mixture composed by Bacillus thuringiensis HD110, Brevibacillus laterosporus BMG65, and Saccharibacter spp. The efficacy was proved on P. larvae-infected larvae, and the experiments showed that the addition of the bacterial mix to the diet decreased the mortality level from 70% in the control to 22% in larvae fed with the microorganism mix.
A single laboratory assay was performed in Apis mellifera (Vásquez et al. 2012) to evaluate the impact of beneficial bacteria against EFB. The LAB strains tested by Forsgren 2010 were orally administered to honeybee larvae challenged with M. plutonius at three concentrations (107, 106, and 105 bacteria/mL). Likewise, the obtained results do not prove the efficacy of the strategy since the reduced mortality between 10 and 20%, although significant, does not resolve the disease. Moreover, if to some extent an efficacy could be demonstrated in laboratory conditions, the “natural open field” situation could display different results, since multiple variables influence the life within the hive. Therefore, it could be interesting to investigate the efficacy of the LAB mixture in infected larvae with a lower dose of the pathogen and perform the treatments as preventive measure before the infection step to better simulate a natural infection process.
In adult honeybees an emergent pathogen affecting bee health is Nosema ceranae, identified as a microsporidium multiplying within gut cells without relevant symptoms during infection (see details in Higes et al. 2010). It has been associated with reduced honeybee life span and colony weakening (Goblirsch et al. 2013). Application of beneficial bacteria is mainly performed in plastic cages under laboratory conditions with newly emerging honeybees infected with the pathogen. Many issues can be argued about the use of cage experiments. Although the laboratory assessment allows the standardization of the variables and the direct observation of the introduced perturbations (e.g., diet change, pathogen inoculation, beneficial microorganisms, pesticides), most of the behavioral and social interactions both inside and outside the hive are lacking. Moreover, this confinement can also introduce stress factors and influence the experiment itself.
In all reported experiments, the biological relevance of spore reduction (less than 1 log) is questionable since the spore numbers remain high. Sabaté et al. (2012) and Audisio et al. (2015) observed a decrease in the amount of spores in field conditions in honeybees orally fed for several months with strains isolated from the gut of healthy insects, namely, B. subtilis Mori2 and L. johnsonii CRL1647. The decrease in Nosema incidence observed by Sabaté et al. (2012) was only evident in September and October when a slight spore increase was observed in the control group. When the control group showed a physiological decrease in the spore number, no relevant reduction was observed in the treated groups. Corby-Harris et al. (2014) observed a reduction of the spore load in honeybee adults originating from larvae fed with pollen patty mixed with an inoculum of Parasaccharibacter apium C6, but it can be argued if this observed reduction could be effective. Moreover, the authors did not specify the species used for the infection step, which is of pivotal importance since the infection process and symptomatology are different. Similarly, Baffoni et al. (2016) observed a significant decrease of N. ceranae in infected honeybees orally fed with a mixture of Lactobacillus and Bifidobacterium strains. However, the ~1 log reduction observed in challenged and treated insects is irrelevant since the spore number remained high and honeybees would surely die. However, in the same experiments, the authors also evidenced a significant reduction in spore load in honeybees exposed to a low natural infection. In this particular case, a hypothetical protective effect, contrasting the low infection rate, might be considered of biological relevance since it could be useful to contain the advance of the infection. Unfortunately, the experiment was performed in cages, and it should be envisaged to confirm the hypothetical effect of the beneficial bacteria also in open field.
An interesting approach to study N. ceranae-host interactions comes from Gisder and Genersch (2015). The authors developed a cell culture model by using the lepidopteran cell line IPL-LD 65Y, from Lymantria dispar, which was susceptible to N. ceranae infection and could support the entire microsporidium life cycle. By this approach, the authors tested several molecules for cytotoxicity and inhibition of N. ceranae intracellular development and demonstrated the efficacy of some of them.
Beneficial microorganisms are also applied to positively influence the hive productivity, and some results showed the significant increase of the brood area, honeybee numbers, and honey production (Audisio and Benítez-Ahrendts 2011a; Sabaté et al. 2012; Alberoni et al. 2015). In particular, Alberoni et al. (2015) also analyzed by NGS the change of the gut microbiota, and a clear increase of bifidobacteria and Acetobacteraceae was evidenced in treated honeybees after supplementation of lactobacilli and bifidobacteria. Both bacterial groups are important endosymbionts of the bee gut and have significant implications related to host nutrition physiology and protection. However, further investigations are necessary to better focus at gut level how this modulation would affect the host-gut microbe interaction.
As already mentioned, the use of beneficial bacteria commercially exploited in humans and animals has also been tested. An improved wax gland cell development was observed by Pătruică et al. (2012), following the supplementation of organic acids and a probiotic product containing Lactobacillus and Bifidobacterium spp. Both individually and in combination, they positively influenced the number, the morphology, and the diameter of the wax cells. Surprisingly, Andrearczyk et al. (2014) found an increase of Nosema spp. infection, following administration in both winter and summer bees of a probiotic product recommended for animals. Ptaszyńska et al. (2016) observed an increased mortality rate in Nosema-infected honeybees fed with the human probiotic Lactobacillus rhamnosus, both as preventive measure and along the infection. The authors argued that the increased infection was associated with a pH reduction of the honeybee midgut, because of the metabolic activity of the supplemented microorganism. However, this consideration relies on previous data (Ptaszyńska et al. 2013), where this association is not clearly and statistically demonstrated and further investigations are necessary to better understand such interactions. However, the use of these strains is controversial since it is preferable to select and use microorganisms from the honeybee gut, possessing the immense pool of genes for host interaction.
The production of antimicrobial compounds by gut symbionts for host protection is another interesting topic. A recent genomic analysis of 13 LAB strains, isolated from the honey crop, put in evidence that most of them produced extracellular proteins of known/unknown function related with antimicrobial action, host interaction, or biofilm formation. In particular, a putative novel bacteriocin with 51% homology with helveticin J was detected in L. helsingborgensis Bma5N (Butler et al. 2013). At the same time, it has to be said that some strains did not evidence any “antimicrobial function,” thus confirming the high variability among the gut microorganisms inhabiting the same niches. Vásquez et al. (2012) analyzed the interaction of some LAB symbionts with the honey crop by SEM and fluorescence microscopy. The resulting images evidenced biofilm formation and structures resembling extracellular polymeric substances (EPS), which are known to be involved in host protection/colonization and cellular recognition (Flemming and Wingender 2010). A further support comes from the work of Ellegaard et al. (2015), which evidences at genome level the presence of gene clusters associated with the biosynthesis of cell wall polysaccharides in both “Firm 4” and “Bifido” groups (Ellegaard et al. 2015). Martinson et al. (2012) reported, in honeybee workers, the presence of genes in G. apicola and S. alvi encoding a relevant number of functions related to biofilm formation and host interaction (type IV pili, outer membrane proteins, and secretion), whose expression could be relevant for the establishment of a micro-niche harsh to pathogen colonization. Finally, the Bacillaceae family includes several spore-forming bacteria, isolated from the bee gut and from the hive environment, showing a strong antibacterial activity against bee pathogens. In this case inhibition activity was mainly due to the production of different classes of lipopeptides (Alippi and Reynaldi 2006; Lee et al. 2009; Sabaté et al. 2009; Yoshiyama and Kimura 2009).
Applications of antimicrobials that could be active against different pathogens are emerging, since it has the advantage of being less invasive. One of the first attempts, performed by Porrini et al. (2010), assessed the effect of four different antimicrobial metabolites: two surfactins (S1 and S2) from B. subtilis Mori4 and B. subtilis C4 and two bacteriocins from Enterococcus avium DSMZ17511 and Enterococcus faecium CRL1385 (B1 and B2). The performed trials – divergent for N. ceranae spore inoculum, metabolites concentration, and administration period—revealed a significant reduction of spore concentration only for surfactin S2. Likely, Maggi et al. (2013) successfully tested, in hives naturally infected with N. ceranae, a pure metabolite from L. johnsonii CRL1647, mainly composed by lactic acid (five times at intervals of 5 days) and coupled in the last treatment with fumagillin. The analysis per individual bee showed a significant decrease of spore counts in treated hives compared to a control, where a regular increase along the experiment was observed. The decrease was observed both before and after the fumagillin application, thus showing a synergistic effect with the antibiotic treatment. Irrespective of the results, the partial standardization of the experiment by choosing sister queens has to be positively pointed out.
Research in this topic is still far to conclude that beneficial microorganisms could actually limit pathogen widespread and support honeybee health and the hive productivity, even if the preliminary results are promising. Nowadays, beekeepers too often rely on subspecies hybrids, with the false hope to increase disease resistance, but the resistance mechanisms against bee pathogens/parasites are usually a result of a coevolution in local ecosystems (Ruottinen et al. 2014). The available applications offer to some extent a picture of the positive influence of these microorganisms on bee health. However, the main issue is how the modulation of the honeybee gut microbiota could influence the composition of the gut microbiota itself and also host immunity and physiology. The widespread of microorganisms into the environment is undoubtedly a dangerous terrain that needs to be deeply investigated to minimize risk associated with bio-treatments.
References
Aizen MA, Harder LD (2009) The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr Biol 19(11):915–918. https://doi.org/10.1016/j.cub.2009.03.071
Ajibola A, Chamunorwa JP, Erlwanger KH (2012) Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr Metab (Lond) 20:9–61. https://doi.org/10.1186/1743-7075-9-61
Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP, Le Conte Y (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12(3):774–782. https://doi.org/10.1111/j.1462-2920.2009.02123.x
Alberoni D, Baffoni L, Gaggia F, Ryan P, Murphy K, Ross RP, Biavati B, Stanton C, Di Gioia D (2015) Administration of lactobacilli and bifidobacteria on Apis mellifera L. beehives to increase health of the bee super-organism. In: Microbial diversity 2015, the challenge of complexity, Perugia, pp 107–108
Alippi AM, Reynaldi FJ (2006) Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J Invertebr Pathol 91(3):141–146. https://doi.org/10.1016/j.jip.2005.12.002
Allen MF, Ball BV, Underwood BA (1990) An isolate of Melissococcus pluton from Apis laboriosa. J Invertebr Pathol 55:439–440. https://doi.org/10.1016/0022-2011(90)90090-S
Anderson KE, Sheehan TH, Eckholm BJ, Mott BM, DeGrandi-Hoffman G (2011) An emerging paradigm of colony health: microbial balance of the honey bee and hive (Apis mellifera). Insectes Sociaux 58(4):431–444
Anderson KE, Sheehan TH, Mott BM, Maes P, Snyder L, Schwan MR, Walton A, Jones BM, Corby-Harris V (2013) Microbial ecology of the hive and pollination landscape: bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS One 8(12):e83125. https://doi.org/10.1371/journal.pone.0083125
Andrearczyk S, Kadhim MJ, Knaga S (2014) Influence of a probiotic on the mortality, sugar syrup ingestion and infection of honeybees with Nosema spp. under laboratory assessment. Med Weter 70:762–765
Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290. https://doi.org/10.1111/j.1462-2920.2009.01953.x
APENET (2011) Effects of coated maize seed on honey bees. Report based on results obtained from the second year (2011) activity of the APENET project. http://www.reterurale.it/apenet
Arias MC, Sheppard WS (2005) Phylogenetic relationships of honey bees (Hymenoptera:Apinae:Apini) inferred from nuclear and mitochondrial DNA sequence data. Mol Phylogenet Evol 37(1):25–35. Epub 2005 Apr 19. Erratum in: 2006 Jul;40(1):315.
Aronstein KA, Murray KD (2010) Chalkbrood disease in honey bees. J Invertebr Pathol 103(1):S20–S29. https://doi.org/10.1016/j.jip.2009.06.018
Audisio MC, Benítez-Ahrendts MR (2011a) Lactobacillus johnsonii CRL1647, isolated from Apis mellifera L. bee-gut, exhibited a beneficial effect on honeybee colonies. Benef Microbes 2(1):29–34. https://doi.org/10.3920/BM2010.0024
Audisio MC, Torres MJ, Sabaté DC, Ibarguren C, Apella MC (2011b) Properties of different lactic acid bacteria isolated from Apis mellifera L. bee-gut. Microbiol Res 166(1):1–13
Audisio MC, Sabaté DC, Benítez-Ahrendts MR (2015) Effect of CRL1647 on different parameters of honeybee colonies and bacterial populations of the bee gut. Benef Microbes 6(5):687–695
Aydin L, Gulegen E, Cakmak I, Girisgin AO, Wells H (2006) Relation between Nosema and Chalkbrood diseases, and its implication for an apiary management model. Bull Vet Inst Pul 50(4):471
Baffoni L, Gaggìa F, Alberoni D, Cabbri R, Nanetti A, Biavati B, Di Gioia D (2016) Effect of dietary supplementation of Bifidobacterium and Lactobacillus strains in Apis mellifera L. against Nosema ceranae. Benef Microbes 7:45–51. https://doi.org/10.3920/BM2015.0085
Bailey L (1968) Honey bee pathology. Annu Rev Entomol 13:191–212
Bailey L (1974) An unusual type of Streptococcus pluton from the Eastern Hive bee. J Inv Pathol 23:246–247
Bailey L (1983) Melissococcus pluton, the cause of European foulbrood of honeybees (Apis ssp.) J Appl Bacteriol 55:65–69
Bailey L, Ball BV (1991) Honey bee pathology, 2nd edn. Academic Press, London
Benitez LB, Velho RV, da Motta AS, Segalin J, Brandelli A (2012) Antimicrobial factor from Bacillus amyloliquefaciens inhibits Paenibacillus larvae, the causative agent of American foulbrood. Arch Microbiol 194(3):177–185
Ben-Ami E, Yuval B, Jurkevitch E (2010) Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J 4:28–37
Breeze TD, Vaissière BE, Bommarco R, Petanidou T, Seraphides N, Kozák L, Scheper J, Biesmeijer JC, Kleijn D, Gyldenkærne S, Moretti M, Holzschuh A, Steffan-Dewenter I, Stout JC, Pärtel M, Zobel M, Potts SG (2014) Agricultural policies exacerbate honeybee pollination service supply-demand mismatches across Europe. PLoS One 9(1):e82996. https://doi.org/10.1371/journal.pone.0082996
Brown M, Loosli R, Schmid-Hempel P (2000) Condition dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91:421–427
Brummel T, Ching A, Seroude L, Simon AF, Benzer S (2004) Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci U S A 101(35):12974–12979
Buchon N, Broderick NA, Chakrabarti S, Lemaitre B (2009) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Gen Dev 23(19):2333–2344
Butler È, Alsterfjord M, Olofsson TC, Karlsson C, Malmström J, Vásquez A (2013) Proteins of novel lactic acid bacteria from Apis mellifera mellifera: an insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol 13:235. https://doi.org/10.1186/1471-2180-13-235
Cai J, Collins MD (1994) Evidence for a close phylogenetic relationship between Melissococcus pluton, the causative agent of European foulbrood disease, and the genus Enterococcus. Int J Syst Bacteriol 44:365–367
Cariveau DP, Powell JE, Koch H, Winfree R, Moran NA (2014) Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus). ISME J 8(12):2369–2379
Celli G, Maccagnani B (2003) Honey bees as bio-indicators of environmental pollution. Bull Insectol 56(1):137–139
Chen YP, Evans JD, Murphy C, Gutell R, Zuker M, Gundensen-Rindal D, Pettis JS (2009) Morphological, molecular and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee Apis mellifera. J Eukaryot Microbiol 56:142–147. https://doi.org/10.1111/j.1550-7408.2008.00374.x
Clark TB (1977) Spiroplasma sp., a new pathogen in honey bees. J Invertebr Pathol 29:112–113
Clark TB (1982) Spiroplasmas: diversity of arthropod reservoirs and host–parasite relationships. Science 217:57–59. https://doi.org/10.1126/science.217.4554.57
COLOSS Workshop (2009) Conclusions. In: Proc. Workshop “Nosema disease: lack of knowledge and work standardization” (COST Action-FA0803) Guadalajara, http://www.coloss.org/ news/nosema-workshop-proceedings-online. Accessed 20 Nov 2009
Copping L (2013) Bees and neonicotinoids: the story continues. Outlooks Pest Manag 24(3):109–119. https://doi.org/10.1564/v24_jun_05
Corby-Harris V, Maes P, Anderson KE (2014) The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS One 9(4):e95056. https://doi.org/10.1371/journal.pone.0095056
Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, Quan P-L, Briese T, Hornig M, Geiser DM, Martinson V, vanEngelsdorp D, Kalkstein AL, Drysdale A, Hui J, Zhai J, Cui L, Hutchison SK, Simons JF, Egholm M, Pettis JS, Lipkin WI (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287. https://doi.org/10.1126/science.1146498
Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, Bandi C, Daffonchio D (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol 76(21):6963–6970. https://doi.org/10.1128/AEM.01336-10
Dahle B, Sørum H, Weideman JE (2011) How to get rid of EFB in Norway. Mellifera 11:22–25
Davidson EW (1973) Ultrastructure of American foulbrood disease pathogenesis in larvae of the worker honey bee, Apis mellifera. J Inv Pathol 21:53–61
De Miranda JR, Bailey L, Ball BV, Blanchard P, Budge G, Chejanovsky N, Chen YP, Gauthier L, Genersch E, De GFD, Bibière M, Ryabov E, De Smet L, Van Der Steen JJM (2013) Standard methods for virus research in Apis mellifera. In V Dietemann; J D Ellis; P Neumann (Eds) The COLOSS BEEBOOK, Volume II: standard methods for Apis mellifera pest and pathogen research. J Apicul Res 52(4):1. https://doi.org/10.3896/IBRA.1.52.4.22
Dillon R, Charnley K (2002) Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res Microbiol 153:503–509. https://doi.org/10.1016/S0923-2508(02)01361-X
Doublet V, Labarussias M, de Miranda JR, Moritz RF, Paxton RJ (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol 17(4):969–983. https://doi.org/10.1111/1462-2920.12426
EFSA (European Food Safety Authority) (2013) Towards holistic approaches to the risk assessment of multiple stressors in bees. pp 1–76. ISBN: 978-92-9199-573-8. https://doi.org/10.2805/53269
Ellegaard KM, Tamarit D, Javelind E, Olofsson TC, Andersson SG, Vásquez A (2015) Extensive intra-phylotype diversity in lactobacilli and bifidobacteria from the honeybee gut. BMC Genomics 16:284. https://doi.org/10.1186/s12864-015-1476-6
Engel MS (1999) The taxonomy of recent and fossil honey bees (Hymenoptera: Apidae; Apis). J Hymen Res 8(2):165–196
Engel P, Martinson VG, Moran NA (2012) Functional diversity within the simple gut microbiota of the honey bee. Proc Natl Acad Sci U S A 109(27):11002–11007. https://doi.org/10.1073/pnas.1202970109
European Food Safety Authority (EFSA), Kass G, Moon J, Robinson T (2017) Horizon 2020: EFSA’s priority research topics. EFSA Supporting Publ 14:1–11. https://doi.org/10.2903/sp.efsa.2017.EN-1166
Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Kanost M, Thompson GJ, Zou Z, Hultmark D (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Bio 15:645–656. https://doi.org/10.1111/j.1365-2583.2006.00682.x
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633. https://doi.org/10.1038/nrmicro2415
Forsgren E (2010) European foulbrood in honey bees. J Inv Pathol 103:5–9. https://doi.org/10.1016/j.jip.2009.06.016
Forsgren E, Fries I (2010) Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet Parasitol 170(3–4):212–217. https://doi.org/10.1016/j.vetpar.2010.02.010
Gaggìa F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141(1):S15–S28. https://doi.org/10.1016/j.ijfoodmicro.2010.02.031
Gaggìa F, Di Gioia D, Baffoni L, Biavati B (2011) The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci Tech 22:58–66. https://doi.org/10.1016/j.tifs.2011.03.003
Gaggia F, Baffoni L, Stenico V, Alberoni D, Buglione E, Lilli A, Di Gioia D, Porrini C (2015) Microbial investigation on honey bee larvae showing atypical symptoms of European foulbrood. Bull Insectol 68(2):321–327. ISSN: 1721-8861
Genersch E (2010) Honey bee pathology: current threats to honey bees and beekeeping. Appl Microbiol Biotechnol 87:87–97. https://doi.org/10.1007/s00253-010-2573-8
Genersch E, Forsgren E, Pentikäinen J, Ashiralieva A, Rauch S, Kilwinski J, Fries I (2006) Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. Int J Syst Evol Microbiol 56:501–511
Giersch T, Berg T, Galea F, Hornitzky M (2009) Nosema ceranae infects honey bees (Apis mellifera) and contaminates honey in Australia. Apidologie 40(2):117–123
Gilliam M, Valentine DK (1976) Bacteria isolated from the intestinal contents of foraging worker honey bees, Apis mellifera: the genus Bacillus. J Invertebr Pathol 28(2):275–276
Girolami V, Marzaro M, Vivan L, Mazzon L, Greatti M, Giorio C, Marton D, Tapparo A (2012) Fatal powdering of bees in flight with particulates of neonicotinoids seed coating and humidity implication. J Appl Entomol 136:17–26. https://doi.org/10.1111/j.1439-0418.2011.01648.x
Gisder S, Genersch E (2015) Identification of candidate agents active against N. ceranae infection in honey bees: establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS One 10(2):e0117200. https://doi.org/10.1371/journal.pone.0117200
Goblirsch M, Huang ZY, Spivak M (2013) Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS One 8(3):e58165. https://doi.org/10.1371/journal.pone.005816
Goodwin RM, Taylor MA, Mcbrydie HM, Cox HM (2006) Drift of Varroa destructor-infested worker honey bees to neighbouring colonies. J Apicult Res 45(3):155–156
Hamdi C, Daffonchio D (2011) Methods for the prevention and control of pathogenic infections in bees and relative composition. Patent Application WO/2011/138310
Hamdi C, Balloi A, Essanaa J, Crotti E, Gonella E, Raddadi N, Ricci I, Boudabous A, Borin S, Manino A, Bandi C, Alma A, Daffonchio D, Cherif A (2011) Gut microbiome dysbiosis and honeybee health. J Appl Entomol 135:524–533. https://doi.org/10.1111/j.1439-0418.2010.01609.x
Hedtke K, Jensen PM, Jensen AB, Genersch E (2008) Evidence for emerging parasites and pathogens influencing outbreaks of stress-related diseases like chalkbrood. J Invertebr Pathol 108(3):167–173. https://doi.org/10.1016/j.jip.2011.08.006
Hedtke SM, Blitzer EJ, Montgomery GA, Danforth BN (2015) Introduction of non-native pollinators can lead to trans-continental movement of bee-associated fungi. PLoS One 10(6):e0130560. https://doi.org/10.1371/journal.pone.0130560
Hider RC (1988) Honeybee venom: a rich source of pharmacologically active peptides. Endeavour 12:60–65
Higes M, Garcia-Palencia P, Martin-Hernandez R, Meana A (2007) Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J Invertebr Pathol 94:211–217. https://doi.org/10.1016/j.jip.2006.11.001
Higes M, Martín-Hernández R, Botías C, Bailón EG, González-Porto AV, Barrios L, Del Nozal MJ, Bernal JL, Jiménez JJ, Palencia PG, Meana A (2008) How natural infection by Nosema ceranae causes honeybee colony collapse. Environ Microbiol 10(10):2659–2669. https://doi.org/10.1111/j.1462-2920.2008.01687.x
Higes M, Martin-Hernandez R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidologie 41:375–392
Hölldobler B, Wilson EO, Nelson MC (2009) Chapter 1: the construction of a superorganism. In: The superorganism: the beauty, elegance, and strangeness of insect societies. W. W. Norton & Company, New York. pp 3–10 (522 pp)
Hrabák J, Martínek K (2007) Screening of secreted proteases of Paenibacillus larvae by using substrate-SDS-polyacrylamide gel electrophoresis. J Apicul Res 46:160–164
Ikediobi CO, Obi VC, Achoba IA (1985) Beekeeping and honey production in Nigeria. Nigeria Field 50:59–70
Koch H, Schmid-Hempel P (2011) Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc Natl Acad Sci U S A 108:19288–19292
Kwong WK, Moran NA (2013) Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst Evol Microbiol 63(6):2008–2018. https://doi.org/10.1099/ijs.0.044875-0
Lee H, Churey JJ, Worobo RW (2009) Isolation and characterization of a protective bacterial culture isolated from honey active against American Foulbrood disease. FEMS Microbiol Lett 296(1):39–44. https://doi.org/10.1111/j.1574-6968.2009.01615.x
Leven LV, Boot WB, Mutsaers M, Segeren P, Velthuis H (1997) Beekeeping in the tropics. In: Leen van’t Leven LV, Segeren P (eds) Agrodok 32 publication. The Netherlands. ISBN Agromisa: 90-8573-043-0
Li J, Qin H, Wu J, Sadd BM, Wang X, Evans JD, Peng W, Chen Y (2012) The prevalence of parasites and pathogens in Asian honeybees Apis cerana in China. PLoS One 7(11):e47955. https://doi.org/10.1371/journal.pone.0047955
Lipa JJ, Triggiani O (1996) Apicystis gen nov and Apicystis bombi (Liu, Macfarlane & Pengelly) comb nov (Protozoa: Neogregarinida), a cosmopolitan parasite of Bombus and Apis (Hymenoptera: Apidae). Apidologie 27:29–34
Lopez MI, Pettis JS, Smith IB, Chu PS (2008) Multiclass determination and confirmation of antibiotic residues in honey using LC–MS/MS. J Agric Food Chem 56:1553–1559. https://doi.org/10.1021/jf073236w
Maggi M, Negri P, Plischuk S, Szawarski N, De Piano F, De Feudis L, Eguaras M, Audisio C (2013) Effects of the organic acids produced by a lactic acid bacterium in Apis mellifera colony development, Nosema ceranae control and fumagillin efficiency. Vet Microbiol 167:474–483. https://doi.org/10.1016/j.vetmic.2013.07.030
Martín-Hernández R, Botías C, Barrios L, Martínez-Salvador A, Meana A, Mayack C, Higes M (2011) Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res 109(3):605–612. https://doi.org/10.1007/s00436011-2292-9
Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78(8):2830–2840. https://doi.org/10.1128/AEM.07810-11
Marzaro M, Vivan L, Mazzon L, Mori N, Greatti M, Petrucco E, Bernardo A, Giorio C, Marton D, Tapparo A, Girolami V (2011) Lethal aerial powdering of honey bees with neonicotinoids from fragments of maize seed coat. Bull Insectol 64(1):119–126. ISSN: 1721-8861
Menzel R (1993) Associative learning in honey bees. Apidologie 24(3):157–168
Mohr KI, Tebbe CC (2006) Diversity and phylotype consistency of bacteria in the guts of three bee species (Apoidea) at an oilseed rape field. Environ Microbiol 8(2):258–272
Moore L, Kosut M (2013) Buzz: urban beekeeping and the power of the bee. New York University Press, New York. http://www.jstor.org/stable/j.ctt9qfrd1. ISBN: 9780814763063
Moran NA (2015) Genomics of the honey bee microbiome. Curr Opin Insect Sci 10:22–28. https://doi.org/10.1016/j.cois.2015.04.003
Morimoto T, Kojima Y, Yoshiyama M, Kimura K, Yang B, Peng G, Kadowaki T (2013) Molecular detection of protozoan parasites infecting Apis mellifera colonies in Japan. Environ Microbiol Rep 5(1):74–77. https://doi.org/10.1111/j.1758-2229.2012.00385.x
Moritz RFA, Southwick EE (1992) Bees as superorganisms: an evolutionary reality. Springer, Berlin
Morse RA (1989) History of subsection; apiculture and social insects. Bull Entomol Soc Am 35:116–118. https://doi.org/10.1093/besa/35.3.115
Mouches C, Bové JM, Albisetti J, Clark TB, Tully JG (1982) A spiroplasma of serogroup IV causes a May-disease-like disorder of honeybees in Southwestern France. Microb Ecol 8:387–399. https://doi.org/10.1007/BF02010677
Neumann P, Carreck NL (2010) Honey bee colony losses. J Apicul Res 49(1):1–6. https://doi.org/10.3896/IBRA.1.49.1.01
Oldroyd BP (2007) What’s killing American honey bees? PLoS Biol 5(6):e168
Olofsson TC, Vásquez A (2008) Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr Microbiol 57:356–363. https://doi.org/10.1007/s00284-008-9202-0
Olofsson TC, Alsterfjord M, Nilson B, Butler È, Vásquez A (2014) Lactobacillus apinorum sp. nov., Lactobacillus mellifer sp. nov., Lactobacillus mellis sp. nov., Lactobacillus melliventris sp. nov., Lactobacillus kimbladii sp. nov., Lactobacillus helsingborgensis sp. nov. and Lactobacillus kullabergensis sp. nov., isolated from the honey stomach of the honeybee Apis mellifera. Int J Syst Evol Microbiol 64(9):3109–3119. https://doi.org/10.1099/ijs.0.059600-0
Page RE Jr, Peng CY (2001) Aging and development in social insects with emphasis on the honey bee, Apis mellifera L. Exp Gerontol 36(4–6):695–711
Pătruică S, Dumitrescu G, Stancu A, Bura M, Bănătean Dunea I (2012) The effect of prebiotic and probiotic feed supplementation on the wax glands of worker bees (Apis mellifera). J Anim Sci Biotech 45:268–271
Paxton RJ, Klee J, Korpela S, Fries I (2007) Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38(6):558–565
Peng YCS, Marston JM (1986) Filtering mechanism of the honey bee proventriculus. Physiol Entomol 11(4):433–439
Plischuk S, Meeus I, Smagghe G, Lange CE (2011) Apicystis bombi (Apicomplexa: Neogregarinorida) parasitizing Apis mellifera and Bombus terrestris (Hymenoptera: Apidae) in Argentina. Env Microbiol Rep 3:565–568. https://doi.org/10.1111/j.1758-2229.2011.00261.x
Porrini MP, Audisio MC, Sabaté DC, Ibarguren C, Medici SK, Sarlo EG, Garrido PM, Eguaras MJ (2010) Effect of bacterial metabolites on microsporidian Nosema ceranae and on its host Apis mellifera. Parasitol Res 107:381–388. https://doi.org/10.1007/s00436-010-1875-1
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25(6):345–353. https://doi.org/10.1016/j.tree.2010.01.007
Prell H (1926) The amoeba-disease of adult bees: a little-noticed spring-time disease. Bee World 8:10–13
Ptaszyńska AA, Borsuk G, Mułenko W, Olszewski K (2013) Impact of ethanol on Nosema spp. infected bees. Med Weter 69:736–741
Ptaszyńska AA, Borsuk G, Zdybicka-Barabas A, Cytryńska M, Małek W (2016) Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol Res 115:397–406. https://doi.org/10.1007/s00436-015-4761-z
Raffiudin R, Crozier RH (2007) Phylogenetic analysis of honey bee behavioral evolution. Mol Phylogenet Evol 43(2):543–552
Ravoet J, Maharramov J, Meeus I, De Smet L, Wenseleers T, Smagghe G, de Graaf DC (2013) Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS One 8(8):e72443. https://doi.org/10.1371/journal.pone.0072443
Regassa LB, Gasparich GE (2006) Spiroplasmas: evolutionary relationships and biodiversity. Front Biosci 11:2983–3002
Roetschi A, Berthoud H, Kuhn R, Imdorf A (2008) Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 39:362–371. https://doi.org/10.1051/apido:200819
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Inv Pathol 103:96–119. https://doi.org/10.1016/j.jip.2009.07.016
Runckel C, Flenniken ML, Engel JC, Ruby JG, Ganem D, Andino R, DeRisi JL (2011) Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 6(6):e20656. https://doi.org/10.1371/journal.pone.0020656
Ruottinen L, Berg P, Kantanen J, Kristensen TN, Praebel A, Groeneveld L (2014) Status and conservation of the nordic brown bee: final report. Nordic Genetic Resource Center (NordGen). http://vbn.aau.dk/ws/files/207960984/Ruottinen_et_al_2014.pdf. Accessed 31 May 2016
Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ (2008) Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319(5864):777–782. https://doi.org/10.1126/science.1149357
Sabaté DC, Carrillo L, Audisio MC (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160(3):193–199. https://doi.org/10.1016/j.resmic.2009.03.002
Sabaté DC, Cruz MS, Benítez-Ahrendts MR, Audisio MC (2012) Beneficial effects of Bacillus subtilis subsp. subtilis Mori2, a honey-associated strain, on honeybee colony performance. Probiotics Antimicrob Proteins 4:39–46. https://doi.org/10.1007/s12602-011-9089-0011-9089-0
Saraiva MA, Zemolin APP, Franco JL, Boldo JT, Stefenon VM, Triplett EW, De Oliveira FA, Roesch LFW (2015) Relationship between honeybee nutrition and their microbial communities. Antonie Van Leeuwenhoek 107:921–933. https://doi.org/10.1007/s10482-015-0384-8
Scardovi V, Trovatelli LD (1969) New species of bifidobacteria from Apis mellifica L. and Apis indica F. A contribution to the taxonomy and biochemistry of the genus Bifidobacterium. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg Abt II 123:64–88
Schwarz RS, Evans JD (2013) Infections with single and mixed-species of trypanosome and microsporidia elicit distinct, ephemeral cellular and humoral immune responses in honey bees. Dev Comp Immunol 40:300–310. https://doi.org/10.1016/j.dci.2013.03.010
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107(46):20051–20056
Shin SC, Kim SH, You H, Kim B, Kim AC, Lee KA, Yoon JH, Ryu JH, Lee WJ (2011) Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674. https://doi.org/10.1126/science.1212782
Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F (2011) Lactobacillus plantarum promotes drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metabol 14(3):403–414
Tontou R, Gaggìa F, Baffoni L, Devescovi G, Venturi V, Giovanardi G, Stefani E (2015) Molecular characterisation of an endophyte showing a strong antagonistic activity against Pseudomonas syringae pv. actinidiae. Plant Soil 405:97. https://doi.org/10.1007/s11104-015-2624-0
Vanengelsdorp D, Meixner MD (2010) A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103:S80–S95. https://doi.org/10.1016/j.jip.2009.06.011
Vásquez A, Olofsson TC (2009) The lactic acid bacteria involved in the production of bee pollen and bee bread. J Apicul Res 48(3):189–195
Vásquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L, Olofsson TC (2012) Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS One 7(3):e33188. https://doi.org/10.1371/journal.pone.0033188
Vidau C, Diogon M, Aufauvre J, Fontbonne R, Viguès B, Brunet JL, Texier C, Biron DG, Blot N, El Alaoui H, Belzunces LP, Delbac F (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS One 6:e21550. https://doi.org/10.1371/journal.pone.0021550
Wallberg A, Han F, Wellhagen G, Dahle B, Kawata M, Haddad N, Simões ZL, Allsopp MH, Kandemir I, De la Rúa P, Pirk CW, Webster MT (2014) A worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis mellifera. Nat Genet 46(10):1081–1088. https://doi.org/10.1038/ng.3077
Wilkins S, Brown M, Andrew A, Cuthbertson GS (2007) The incidence of honey bee pests and diseases in England and Wales. Pest Manag Sci 63:1062–1068. https://doi.org/10.1002/ps.1461
Williams GR, Sampson MA, Shutler D, Rogers R (2008) Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honeybees (Apis mellifera)? J Invertebr Pathol 99:342–344. https://doi.org/10.1016/j.jip.2008.04.005
Winston ML (1987) The biology of the honey bee. Harvard Univ. Press, Cambridge, MA
Wu M, Sugimura Y, Takaya N, Takamatsu D, Kobayashi M, Taylor D, Yoshiyama M (2013) Characterization of bifidobacteria in the digestive tract of the Japanese honeybee, Apis cerana japonica. J Invertebr Pathol 112:88–93. https://doi.org/10.1016/j.jip.2012.09.005
Yoshiyama M, Kimura K (2009) Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American foulbrood. J Invertebr Pathol 102:91–96. https://doi.org/10.1016/j.jip.2009.07.005
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Gaggìa, F., Baffoni, L., Alberoni, D. (2018). Probiotics for Honeybees’ Health. In: Di Gioia, D., Biavati, B. (eds) Probiotics and Prebiotics in Animal Health and Food Safety. Springer, Cham. https://doi.org/10.1007/978-3-319-71950-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-71950-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-71948-1
Online ISBN: 978-3-319-71950-4
eBook Packages: MedicineMedicine (R0)