Abstract
Nowadays, honey bees are stressed by a number of biotic and abiotic factors which may compromise to some extent the pollination service and the hive productivity. The EU ban of antibiotics as therapeutic agents against bee pathogens has stimulated the search for natural alternatives. The increasing knowledge on the composition and functions of the bee gut microbiota and the link between a balanced gut microbiota and health status have encouraged the research on the use of gut microorganisms to improve bee health. Somehow, we are assisting to the transfer of the “probiotic concept” into the bee science. In this review, we examine the role of the honey bee gut microbiota in bee health and critically describe the available applications of beneficial microorganisms as pest control agents and health support. Most of the strains, mainly belonging to the genera Lactobacillus, Bifidobacterium and Bacillus, are isolated from honey bee crop or gut, but some applications involve environmental strains or formulation for animal and human consumption. Overall, the obtained results show the favourable effect of applied microbial strains on bee health and productivity, in particular if strains of bee origin are used. However, it is actually not yet possible to conclude whether this strategy will ever work. In particular, many aspects regarding the overall setup of the experiments, the dose, the timing and the duration of the treatment need to be optimized, also considering the microbiological safety of the hive products (i.e. pollen and honey). In addition, a deep investigation about the effect on host immunity and physiology is envisaged. Lastly, the final users of the formulations, i.e. beekeepers, should be taken into account for the achievement of high-quality, cost-effective and easy-to-use products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollination is one of the most important services provided by insects, with a strong ecological, economic and cultural impact. The European honey bee Apis mellifera is regarded as the most relevant pollinating agent, even if a significant contribution comes also from less known Apoidea species, such as bumble bees (Bombus spp.) and wild bees (Macropis spp., Osmia spp. and Xilocopa spp.). The maintenance of genetic diversity in plant population, the productivity of crops and orchards for human nutrition and the floral variety in the environment are unequivocally assured and satisfied by this “free” ecosystem service, whose preservation is also dependent on human actions (Gill et al. 2016). Nowadays, bees are stressed by a number of biotic and abiotic factors which affect honey bee health and productivity. In addition to pathogens, pesticides and lack of flowers, whose implications in insect health have been deeply studied (Goulson et al. 2015; Porrini et al. 2016), climate change, habitat loss and invasive species are becoming equally crucial for beehive integrity (Potts et al. 2010; Bond et al. 2014; Nieto et al. 2014). The parasite Varroa destructor and the microsporidium Nosema ceranae moved, in the last decades, from their natural Asiatic host (Apis cerana) to the European one, finding fertile ground for their development (Higes et al. 2010 and Rosenkranz et al. 2010). Moreover, the presence of V. destructor in every colony seems to exert an important pressure on bee health since the mite found in A. mellifera a less resistant host (Le Conte et al. 2010). The use of veterinary medicines in the beekeeping sector has a strong limitation due to the big concern about antibiotic resistance acquisition/transmission, antibiotic residues in beehive products and, to a lesser extent, the risk of unbalancing the bee gut microbiota. Consequently, antibiotics were banned in EU countries, whereas some acaricides are still permitted (European Commission 2010). Natural substances, such as oxalic acid and thymol, are highly efficient in controlling mite populations if they are correctly applied. The proper handling is important to avoid bee intoxication and, most importantly, to achieve efficacy. Honey bee management needs a deep knowledge of bee behaviour and seasonal cycles and appropriate skills to recognize problems and threats at a given time, in order to successfully employ the colonies for crop pollination or for the hive products. What is often underestimated is that a compromised health status, due to different stressors, can negatively affect the activities of a balanced and healthy gut microbiota both in humans and in animals (Gaggìa et al. 2010). The honey bee gut microbiota displays high affinity with that of mammals (Kwong and Moran 2016); the huge number of bacterial symbionts, inhabiting selected niches in the gut (from honey crop to the rectum), are represented by host-adapted species contributing to host defence, nutrition and physiology (Hamdi et al. 2011). Recent advances on metagenomics have brought new insights in the knowledge of honey bee gut microbiota and its genes (Moran 2015). The host-microbe interaction derives from a long co-evolution process strictly associated with insect labour division, developmental stage and social transmission (Hughes et al. 2008). It is quite surprising to observe that most members of this gut microbiota are maintained by horizontal social transmission (with the exception of the queen) and interaction with the hive environment (Tarpy et al. 2015), providing unique functions related to food storage and transformation. Moreover, the finding that the honey bee genome has significantly fewer immune genes than expected allowed to speculate a contribution of the gut endosymbiont genes in supporting honey bee immunity (Evans et al. 2006) in association with the social immune response described in eusocial insects (Wilson-Rich et al. 2009). Recent works on Drosophila melanogaster have given a picture of the molecular dialog between the microbiota and the insect gut. Many authors described the role of gut microorganisms in supporting the immune system, influencing the epithelial homeostasis, promoting lifespan, larval growth in food shortage and driving the host mating preference (Brummel et al. 2004; Ryu et al. 2008; Buchon et al. 2009; Sharon et al. 2010; Storelli et al. 2011). For these reasons, as in vertebrates, the prosperous gut symbiont community should be considered pivotal for insect life and should be preserved. Beneficial microorganisms have been widely exploited in humans and animals both as food/feed supplements and as pharmaceutical formulations, representing a valid tool to support gut health and alleviate several disorders (Gaggìa et al. 2010; Di Gioia et al. 2014). The use of commensal gut microorganisms and their related secondary metabolites are more and more taken into account to re-establish a disbiotic insect gut community and control disease spread (Crotti et al. 2012; Berasategui et al. 2016). Insects are probably a simpler system to investigate, but such applications, in social bees, could result more difficult to monitor since many variables should be considered (environment, genetic diversity, high complexity at hive level). Researchers are focusing on honey bee microbial gut inhabitants to better understand the host-microbiota interaction and transfer the acquired knowledge from human and animal to bees.
In this review, we discuss the role of the honey bee gut microbiota, focusing on its main activities and we give an overview of the available applications of beneficial microorganism on bee larvae and adults, looking at their potential as pest-control agents and health support.
A look inside the honey bee gut microbiota
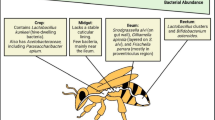
In the last decade, the new available techniques led scientists to investigate the microbial gut symbionts with a particular focus on the functional aspect of host-symbiont interaction. Next-generation sequencing (NGS) has allowed the identification of a distinctive gut bacterial community, which consists of eight dominant groups, comprising over 95 % of the whole community, as described by Moran (2015) and Kwong and Moran (2016). The Gram-negative Gilliamella apicola and Frischella perrara, belonging to the Gammaproteobacteria class, and the Betaproteobacterium Snodgrassella alvi are predominant in the midgut. The rectum is preferentially colonized by the clades Firm-4 and Firm-5, including different Lactobacillus species (e.g. Lactobacillus mellis, Lactobacillus mellifer, Lactobacillus helsingborgensis, Lactobacillus kullabergensis, Lactobacillus melliventris and Lactobacillus kimbladii) and two species belonging to the genus Bifidobacterium (Bifidobacterium asteroides and Bifidobacterium coryneforme). Alphaproteobacteria (related to the genera Bartonella/Brucella and the Acetobacteraceae family) have been described but they are less abundant (Moran 2015; Kwong and Moran 2016). The microbial gut community, evolving in the days following pupae hatching, reaches its definition in 3–5 days (Anderson et al. 2016). The same authors hypothesised that many strains of Lactobacillus Firm-5 are pioneer species, being particularly abundant within the hive, and that cell cleaning and other early behaviours are pivotal in newly emerging bees for promoting the composition of the adult gut microbial community. However, further behavioural mechanisms, such as the grooming, the oral trophallaxis and the oral-faecal route, are reported as well (Martinson et al. 2012; Powell et al. 2014). As in humans and animals, this bacterial core group is composed of facultative anaerobic and microaerophilic bacteria (Kwong and Moran 2016), which are strictly associated with the gut epithelial cells and are involved in several host functions. It is interesting to point out that several species have been only recently isolated and identified (Engel et al. 2013; Kwong and Moran 2013; Olofsson et al. 2014), and studies on their role and interaction with the host are still at the beginning. Besides this core microbiota, some caste-related differences may be found in relation to the social function that honey bees cover during their life (Kapheim et al. 2015). Moreover, a recent study (Rokop et al. 2015) has suggested the presence of a “non-core” microbial group associated with the hive environment, including the food prepared by the bees, which may trigger the development of the gut core microbiota.
The role of gut microorganisms in honey bees
Nutritional support
Social insects create a partnership with the microbial gut symbionts as they possess genes encoding for enzymatic activities (i.e. cellulases, hemicellulases and lignase) essential for the energy uptake from a plant-based diet (Newton et al. 2013). Moreover, the microbial consortium produces fatty acids, amino acids and other necessary nutrients and metabolites (Gündüz and Douglas 2009). Honey bees also require vitamins, including the vitamin B complex, and gut bacteria could represent a relevant source (Brodschneider and Crailsheim 2010). A summary, indicating the main activities of gut symbionts, is reported in Table 1. Fructobacillus species, isolated from bee bread, brood cells and larval gut, were found to utilize the plant complex molecule lignin, which is a component of pollen, thus beginning the breakdown of this important high-protein plant-derived food (Rokop et al. 2015). In a recent metagenomic study, involving 150 pooled guts of A. mellifera worker bees, Engel and Moran (2013) evidenced the presence of different sugar uptake systems in Gammaproteobacteria, Firmicutes and Bifidobacteriaceae (phosphotransferase system families and the arabinose efflux permease family). This is in agreement with Lee et al. (2015) who identified, through metatranscriptome sequencing, the aforementioned bacterial groups as the major contributors (91 %) of the protein-coding transcripts, participating in the breakdown of plant-derived macromolecules and in the fermentation of the monomeric subunits. Interestingly, the energy uptake of the Betaproteobacterium S. alvi exclusively relies on the aerobic oxidation of the products of the fermentation process (citrate, malate, acetate and lactic acid), thus avoiding any competition for nutrients with neighbouring species (Kwong et al. 2014). This represents a simple example of co-evolution within the same niche. A further interesting finding (Engel and Moran 2013) is the pectin degradation activity of G. apicola that is strain specific and leads to pollen cell wall degradation, thus leaving the protein content available for the host. It is clear from these studies that a high degree of genetic diversity can be found within the microbial symbionts, thus suggesting a high adaptability of microorganisms to host metabolic requirements within the same niche (Engel and Moran 2013). The catabolic pathways in lactobacilli (commonly defined lactic acid bacteria, LAB) and bifidobacteria are well known since these two microbial groups are involved in numerous fermentation processes and have a long history of safe use as probiotic and protective microorganisms (Gaggìa et al. 2011). Lee et al. (2015) described a wide range of glycoside hydrolase (GH) activities in the bee gut, such as GH13 and GH16 families, acting on plant cell wall components and highly transcribed within the lactobacilli group. Other GH families were described for their activities on the soluble disaccharides maltose, cellobiose and sucrose. The importance of LAB is also emphasized by their ecological distribution, which is not limited to adult bee gut. They have been isolated from larval guts (Gaggìa et al. 2015) and from the honey stomach of adult bees (Olofsson and Vásquez, 2008), which is a further relevant microbial niche associated with food storage and liquid transfer (water, nectar and royal jelly), adjacent to the midgut. Moreover, LAB are also dominant in the hive environment (bee bread, honey, wax and comb) (Anderson et al. 2013). Among bifidobacteria, some isolates from social insects are known to possess a complete trehalose degradation IV pathway, which is absent in the majority of the other bifidobacterial taxa. Trehalose is indeed used as carbohydrate storage and hemolymph sugar by many insects including honey bee (Milani et al. 2015). Moreover, Milani et al. (2015) confirmed the significant differences in the glycobiome composition of bifidobacterial taxa isolated from social insects compared with human and animal taxa, highlighting a discrete set of GH43 (for the breakdown of complex plant glycans, xylan and arabinoxylans) and GH3 family members. Bottacini et al. (2012) showed that B. asteroides was able to metabolize a range of simple carbohydrates broader than any other tested bifidobacterial species (72 carbohydrate-active proteins). This is consistent with Lee et al. (2015), who detected a class of β-glucosidases within the Actinobacteriaceae family, whose activity is addressed towards oligosaccharides with diverse sizes and compositions, and it has been associated with pollen cell wall degradation. The genome sequencing of B. asteroides also confirmed the presence of a complete biosynthetic pathway for folate (vitamin B9), but not for other B vitamins (Bottacini et al. 2012). Overall, the above studies showed again that species isolated from different hosts possess specific gene sets, suggesting host-specific adaptation. Bifidobacteria are recognized as strictly anaerobic microorganisms, but B. asteroides, inhabiting the honey bee hind gut, possesses genes associated with a respiratory metabolism that help the bacterium to adapt to the oxygen-rich bee gut environment (Bottacini et al. 2012; Sun et al. 2015).
Immunity support
Host protection is another important aspect that is frequently associated with a balanced gut microbiota. It is a fact that different stress factors, such as parasites/pathogens, deficient nutrition and pesticides, can cause immunosuppression (Antúnez et al. 2009; Alaux et al. 2010b; Anbutsu and Fukatsu 2010; Fang et al. 2010; Di Prisco et al. 2013). As already mentioned, honey bee has a simpler immune system compared to other model insects (Evans et al. 2006 ; Barribeau et al. 2015), in favour of more convenient and less expensive social defence strategies which combine prophylactic and activated responses as well as behavioural, physiological and spatial mechanisms (Cremer et al. 2007). However, a significant contribution to host protection is provided by the antagonistic activity of the gut microbiota and its interaction with the humoral and systemic immunity (Dillon et al. 2005; Hedges et al. 2008; Jaenike et al. 2010). In three species of wild bumble bees, a low presence of S. alvi and G. apicola strains was associated with a higher incidence of the pathogen Crithidia spp. (Cariveau et al. 2014). Dillon and Charnley (2002) reported in the desert locust Schistocerca gregaria a real contribution of the gut microbiota to host defence against pathogens by producing antimicrobial phenolic compounds and synthesizing key components of the locust cohesion pheromone. Alterations of this microbiota could consequently compromise honey bee defence mechanisms. In particular, this paragraph will focus on how microorganisms could play a role in host protection, (i) by directly stimulating the bee’s immune system and (ii) by directly inhibiting pathogens through antimicrobial compound production (Table 1).
Given that individual and social defence mechanisms are diverse and complex, one of the main effectors of the innate immunity in honey bee, and more in general in insects, is represented by antimicrobial peptides (AMPs), whose synthesis is under the control of the Toll and Imd signalling pathways (Lemaitre and Hoffmann 2007). Honey bees possess six AMPs, mainly activated at epithelial surfaces, following the exposure to the major cell wall component of Gram-positive bacteria, the Lys-type peptidoglycan (PG): abaecin, hymenoptaecin, apidaecin, defensin-1, defensin-2 and apisimin (Casteels et al. 1989; Casteels et al. 1990; Casteels et al. 1993; Bíliková et al. 2002; Klaudiny et al. 2005). Antimicrobial activity is mainly achieved through alteration of the microbial membrane properties (Imler and Bulet 2005) and intracellular metabolic processes (Brogden 2005). A selective AMP synthesis is induced following exposure to various honey bee larvae/adult pathogens with variable responses (Evans and Lopez 2004; Jefferson et al. 2013; Yoshiyama et al. 2013). Evans and Pettis (2005) showed a higher abaecin expression in colonies with a lower incidence of Paenibacillus larvae (the ethiological agent of the American Foulbrood, AFB). However, some studies also evidenced an increased level of AMPs in response to non-pathogenic bacteria. Higher RNA levels for the abaecin gene have been reported in bee larvae fed with probiotic bacteria of human origin and fermented foods (Evans and Lopez 2004; Yoshiyama et al. 2013). Janashia and Alaux (2016) fed larvae with five different LAB species previously isolated from worker honey bee guts and bee bread, and among them, two strains (B. asteroides 26p and Fructobacillus pseudoficulneus 57) significantly upregulated the expression of apidaecin, while no effect was observed on abaecin, hymenoptaecin and defensin-1 levels. These results, taken together, showed that the honey bee immune response through AMP synthesis is fairly non-specific and the increase of the transcription levels of the different AMPs genes is strain specific and is not related to either the species or the source of the strains. Jefferson et al. (2013) also found a strong positive correlation between the amount of total honey bee gut bacteria and transcript levels of two AMPs, defensin-1 and apidaecin. The hypothesis that the resident gut microorganisms may determine a basal immune response to control its proliferation and consequently harmful microorganisms through AMP synthesis has not yet be investigated in honey bee; however, studies on D. melanogaster and Anopheles mosquitoes go in that direction. An interesting observation in D. melanogaster has revealed that appropriate AMP levels could guarantee the preservation of a balanced gut microbial community structure, with the species Commensalibacter intestini dominant within the Acetobacteraceae family. An induced upregulation of AMP gene expression led to a drastic change in the microbial composition, exerting the growth promotion of the pathogenic commensal Gluconobacter morbifer (Ryu et al. 2008). Acetobacteraceae is indeed a relevant symbiont group of insect gut (adult and larvae) and crop and has significant implications related to both host nutrition and protection (as reviewed by Crotti et al. 2010). Dong et al. (2009) showed that microbe-free aseptic Anopheles mosquitoes displayed an increased susceptibility to Plasmodium infection with a reduced expression of the anti-Plasmodium factors FBNs 6, 9 and 36.
Concerning the production of antimicrobial compounds for host protection, Saraiva et al. (2015) found a relative high presence of genes involved in the biosynthesis of streptomycin and secondary metabolites in the gut microbiota of honey bee, which could play a role in shaping the microbiome. A considerable amount of information also derives from the LAB community and bifidobacteria, which are well-known antimicrobial compound producers. The finding that an important component of the honey bee gut microbiota was represented by lactobacilli and bifidobacteria have increased the interest of scientists in looking for similarity and analogy with the probiotic bacteria widely investigated in humans and animals. Once lactobacilli and bifidobacteria started to be isolated (from honey bee stomach, gut and hive products), numerous in vitro trials confirmed their ability to inhibit honey bee pathogens; in particular P. larvae, Melissococcus plutonius and Ascosphaera apis, the agents of the American and European foulbrood (AFB and EFB) and Chalkbrood disease respectively (Sabaté et al. 2009; Yoshiyama and Kimura 2009; Audisio et al. 2011; Vásquez et al. 2012; Wu et al. 2013; Killer et al. 2014). Although in vitro activity does not necessarily correspond to action in in vivo systems, these assays could provide useful information on the antimicrobial equipment possessed by each strain. Organic acids, strain-specific metabolites and/or bacteriocin production have been described as powerful antimicrobial molecules (Servin 2004; Kleerebezem et al. 2010) and are widely exploited in human and animal food/feed additives, in the food industry to preserve food and in bio-control strategy against phyto-pathogens (Gaggìa et al. 2011; Tontou et al. 2015). Nevertheless, the interactions between microorganisms in the gut of larvae and adult bees are very complex and pathogens are not at all defenceless exposed to the weapons of the gut microbial symbionts. As an example, P. larvae with its secreted non-ribosomal peptides (NRP) and NRP/polyketide hybrids (Müller et al. 2015) is able to eliminate all microbial competitors, despite their antimicrobials, resulting in a pure P. larvae culture in the degraded larval cadavers (Holst 1945).
A recent genomic analysis of 13 LAB strains, isolated from the honey crop, put in evidence that most of them produced extracellular proteins of known/unknown function related with antimicrobial action, host interaction, or biofilm formation. In particular, a putative novel bacteriolysin with 51 % homology with Helveticin J was detected in L. helsingborgensis Bma5N (Butler et al. 2013). At the same time, some strains did not evidence any “antimicrobial function”, thus confirming the high variability among the gut microorganisms inhabiting the same niches. Vásquez et al. (2012) analysed the interaction of some LAB symbionts with the honey crop by SEM and fluorescence microscopy. The resulting images evidenced biofilm formation and structures resembling extracellular polymeric substances (EPS), which are known to be involved in host protection/colonization and cellular recognition (Flemming and Wingender 2010). A further support comes from the work of Ellegaard et al. (2015), which evidenced at genome level the presence of gene clusters associated with the biosynthesis of cell wall exopolysaccharides in both “Firm4” and “Bifido” groups. Martinson et al. (2012) reported, in honey bee workers, the presence of genes in G. apicola and S. alvi encoding a relevant number of functions related to biofilm formation and host interaction (Type IV pili, outer membrane proteins and secretion), whose expression could be relevant for the establishment of a micro-niche insensitive to pathogens colonization. Finally, the Bacillaceae family includes several spore-forming bacteria, isolated from the bee gut and from the hive environment, showing in vitro a strong antibacterial activity against bee pathogens. In this case, it is known from decades that inhibition activity is mainly due to the production of antibiotic molecules (lipopeptides and iturin-like lipopeptides) (Alippi and Reynaldi 2006; Lee et al. 2009; Sabaté et al. 2009; Yoshiyama and Kimura 2009). However, as mentioned above, it must be again emphasized that P. larvae itself, as spore-forming bacteria, produces antibiotics molecules which help the pathogen during infection to defend its niche and dominate the larval gut environment towards resident microorganisms.
The “probiotic concept” in honey bee
It is clear that a balanced gut microbiota offers a wide range of metabolic, trophic and protective functions, which confer health benefit to honey bees. In this perspective, the FAO/WHO probiotic definition (FAO/WHO 2002), which encompasses strain specificity (Sanders et al. 2014), is more than appropriate. However, the transfer of the probiotic concept from vertebrates to invertebrates still requires further considerations, and several questions still need to be investigated and debated. In particular, beyond the health aspect, probiotic microorganisms fulfil a list of biological requirements and safety criteria, e.g. to be non-toxic and non-pathogenic, to have an accurate taxonomic identification, to be normal inhabitants of the targeted host-species, to adhere to the gut epithelium (Hooper and Gordon 2001; Gaggìa et al. 2010). For these reasons, in the present review, authors will refer to “beneficial microorganisms” rather than to probiotic microorganisms, since honey bee gut symbiont characterization is far to be completed. From our and general experience in humans and animals, biotic and abiotic stresses could negatively affect the composition of the gut microbiota and therefore induce specific changes in the microorganism activities at gut level (Gaggìa et al. 2010). The analysis of the honey bee microbial gut community in colonies suffering from Colony Collapse Disorders (CCD) evidenced a variation of some microbial phyla in healthy colonies compared to diseased ones (Cox-Foster et al. 2007); in affected colonies, a decrease of Firmicutes and Alphaproteobacteria was observed. We can deduce that this alteration could reflect physiological changes due to the incoming infection or support the hypothesis that the low presence of beneficial species could weaken host defence. Anyway, we have to ask ourselves if any kind of microbiota modulation, by the administration of selected strains, could restore this perturbation, reduce bee mortality and/or improve honey bee health. In other studies, by introducing a given stress, no perturbation was observed (Babendreier et al. 2007; Hui-Ru et al. 2015). In particular, Hui-Ru et al. (2015) did not evidence significant difference in the microbial gut community of honey bees, under laboratory conditions, following exposure to sub-lethal dose of the neonicotinoid Imidacloprid, whose adverse effects on honey bees have been already documented (Medrzycki et al. 2003; Dively et al. 2015). Nevertheless, it has been also verified how exposure to sub-lethal concentration of pesticides could significantly enhance bee susceptibility towards pathogens (Alaux et al. 2010a; Vidau et al. 2011; Doublet et al. 2015), thus weakening honey bee health and compromising the gut microbiota. Attempts of gut microbiota modulation have been already performed in some insect species (Wittebolle et al. 2009; Ben Ami et al. 2010; Robinson et al. 2010), showing the importance of the endogenous gut microbial community. In the next section, a description of the main application of beneficial microorganisms in honey bees will be reported and commented, including assays in larvae and adults both under laboratory and field conditions.
Application of beneficial microorganisms: state of the art
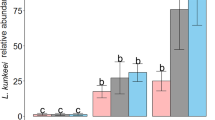
Beneficial microorganisms in honey bee are mainly applied to fight the most widespread pathogens affecting both larvae and adults (Table 2). Most of the bacterial strains used in these studies are isolated from honey bee crop or gut, whose selection derives from in vitro tests based on direct antagonism towards target pathogens. However, some other applications rely on the use of bacterial strains isolated from the environment or on formulation for animal and human consumption. With respect to the AFB, Forsgren et al. (2010) used a mixture of 12 isolates from honey crop—Lactobacillus kunkeei, L. mellis, L. kimbladii, L. kullabergensis, L. helsingborgensis, L. melliventris, L. apis, L. mellifer, B. asteroides and B. coryneforme—with a final concentration of 107 bacteria/ml. The exposure assay was performed by rearing 1st instar honey bee larvae, infected with two different spores concentration of P. larvae. The LAB mixture was supplemented with sugar syrup, both in combination with P. larvae at the time of spore inoculum and 48 h post infection. Results showed the positive effect of LAB supplementation only in the group challenged with the highest dose of P. larvae with a significant reduction of larvae mortality. However, these results are of little biological relevance because the reduced larvae mortality, from 70 to 55 %, is not enough to combat a notifiable epizootic and the colony will probably succumb to the disease, although it might take 1 week longer. Recently, a probiotic mixture, based on two spore-forming bacteria (SFB; Bacillus thuringiensis HD110 and Brevibacillus laterosporus BMG65) in association with Saccharibacter spp., has been developed for the protection of bee larvae against the AFB (Hamdi and Daffonchio, 2011). The efficacy of the invention was tested on P. larvae-infected larvae and the experiments showed that the addition of the bacterial mix to the diet decreased the mortality level from 70 % in the control to 22 % in larvae fed with the microorganism mix. Although the mortality reduction is encouraging, the invention should be investigated in infected apiaries in open field to assess the biological relevance of the microorganism-based product. Concerning EFB, a single laboratory assay has been performed in A. mellifera (Vásquez et al. 2012). The same LAB strains isolated from honey crop and used by Forsgren et al. (2010) were orally administered to honey bee larvae challenged with M. plutonius at three concentrations (107, 106 and 105 bacteria/ml). Irrespective of the infectious dose, mortality was significantly reduced in groups treated with the LAB mixture. However, as outlined in Forsgren et al. (2010), these data does not prove the efficacy of these microorganisms since the reduced mortality between 10 and 20 %, although significant, is biologically irrelevant. Based on these results, it could be interesting to investigate the efficacy of the LAB mixture in infected larvae with a lower dose of the pathogen and perform the treatments as preventive measure before the infection step. The native microbial community inhabiting the honey crop is mainly involved in the production of the bee bread nourishing the brood and constitute the first defence line against potential brood pathogens acquired from the floral environment (Vásquez et al. 2012). Therefore, an application of beneficial microorganisms prior to infection to boost the gut microbiota composition could be more successful in contrasting brood pathogens. However, no data are actually available.
An interesting observation from this study is the antibiotic susceptibility of the LAB strains towards oxytetracycline and tylosin, two antibiotics used in apiculture to fight P. larvae and M. plutonius. All LAB strains were highly sensitive to tylosin, while L. kunkeei Fhon2, L. apis Hma11, L. melliventris Hma8 and L. mellis Hon2 showed resistance to oxytetracycline. Antibiotic resistance is an important concern for insects and human health, if we look at the risk of an increased antibiotic resistance among bee pathogens and accumulation in the hive products. These are some of the reasons leading to the ban of antibiotics in apiculture in EU. Unfortunately, this regulation has not yet been adopted in non-EU countries.
With respect to adult honey bees, beneficial microorganisms are targeted against the emergent pathogen Nosema spp., in particular Nosema ceranae, which multiplies within gut cells and no relevant symptoms can be detected during infection (see details in Higes et al. 2010) (Table 2). The microsporidium is prevalent in southern Europe (Fernandez et al. 2012; Porrini et al. 2016), and it has been associated with reduced honey bee life span and colony weakening (Goblirsch et al. 2013)). However, according to the investigation of Fernandez et al. (2012) in Spanish apiaries, N. ceranae does not necessarily kill honey bee colonies and does not influence beehive production. Almost all the reported experiments are performed in plastic cages under laboratory conditions with newly emerging honey bees. Many issues can be argued about the use of cage experiments. Although the laboratory assessment allows the standardization of the variables and the direct observation of the introduced perturbations (e.g. diet change, pathogen inoculation, beneficial microorganisms and pesticides), most of the behavioural and social interactions both inside and outside the hive are lacking. Moreover, this confinement can also introduce stress factors and influence the experiment itself.
The trial performed by Corby-Harris et al. (2016) showed an improve resistance to Nosema spp. in honey bee adults individually challenged with 104 spores and originating from larvae fed with pollen patty mixed with an inoculum of Parasaccharibacter apium C6. P. apium (Corby-Harris et al. 2014), of the Acetobacteraceae family, is particularly abundant in honey crop, hypopharyngeal glands, royal jelly and larval gut through nurse worker bees feeding behaviour. However, spore load reduction was always biological irrelevant since the decrease was less than 40 % compared to the control group. Similarly, Baffoni et al. (2016) observed a significant decrease of N. ceranae in infected honey bees orally fed with Lactobacillus and Bifidobacterium strains. The ∼1 log reduction observed in challenged and treated insects could be considered irrelevant since the spore number remained high and honey bees would surely die. However, Baffoni et al. (2016) also evidenced a significant reduction in spore load from 2.04 ± 0.91 and 0.78 ± 0.81 (mean log spores/bee ± sd) in honey bees exposed to a low natural infection and treated with the microorganisms; in this particular case, a hypothetical protective effect, contrasting the low infection rate, might be considered of biological relevance. Sabaté et al. (2012) and Audisio et al. (2015) observed a decrease in the amount of spores in field conditions in honey bees orally fed for several months with strains isolated from the gut of healthy insects, namely Bacillus subtilis Mori2 and Lactobacillus johnsonii CRL1647. In both cases, the biological relevance of the reduction (less than 1 log) is still questionable since the spore numbers are still high. The decrease in Nosema incidence observed by Sabaté et al. (2012) is only evident in September and October when a slight spore increase can be observed in the control group. When the control group showed a physiological decrease in the spore number, no relevant reduction is observed in the treated groups. From these data, firm evidences on the positive effect of beneficial microorganism administration against Nosema spp. cannot be drawn. Conversely, Andrearczyk et al. (2014) found an increase of Nosema spp. infection, following administration in both winter and summer bees of a probiotic product recommended for animals. Likely, Ptaszyńska et al. (2016) observed an increased mortality rate in Nosema-infected honey bees fed with the probiotic microorganism L. rhamnosus, both as preventive measure and along the infection. The authors argued that the increased infection was associated with a pH reduction of the honey bee midgut because of the metabolic activity of the supplemented microorganism. However, this consideration relies on previous data (Ptaszyńska et al. 2013), where this association is not clearly and statistically demonstrated and further investigations are envisaged to better understand such interactions. Moreover, the honey bee midgut is a multi-niche environment, harbouring a complex microbial community and fermentation products (as lactic and acetic acids) may be taken up and utilized by some components of this community or by the bee host (Kwong and Moran 2016), thus limiting their contribution to the reduction of gut pH. An interesting approach to study N. ceranae-host interactions comes from Gisder and Genersch (2015). The authors developed a cell culture model by using the lepidopteran cell line IPL-LD 65Y, from Lymantria dispar, which was susceptible to N. ceranae infection and could support the entire microsporidium life cycle. By this approach, the authors tested several molecules for cytotoxicity and inhibition of N. ceranae intracellular development and demonstrated the efficacy of the synthetic antibiotics metronidazole and tinidazole, while a surfactin from Sigma-Aldrich did not show any inhibition and at low concentration was also cytotoxic for the cells.
Microbial gut symbionts could be useful to sustain honey bee health and productivity since, as already described, bacteria from honey bee crop and gut are highly specialized in performing thousands of metabolic activities necessary to honey bee for a normal development (Table 3). However, most of the published data are still not very convincing and experiments should have more replicates. An improved wax gland cells development was observed by Pătruică et al. (2012), following the supplementation of organic acids and two probiotics for human consumption. In particular, lactic acid and a probiotic product containing Lactobacillus and Bifidobacterium spp., both individually and in combination, positively influenced the number, the morphology and the diameter of the wax cells. Audisio and Benítez-Ahrendts (2011) performed two different trials to assess colony health and performance on honey bee hives treated with a cell suspension (105 ufc/ml sugar syrup) of L. johnsonii CRL1647 (every 15 days for 3 months and a monthly administration for 1 year). All the parameters analysed (open and operculated brood area, bee number, honey storage), with some fluctuations every month, were significantly higher in the treated groups. Sabaté et al. (2012) obtained comparable results with the supplementation in field conditions of spores of B. subtilis Mori2, isolated from honey, once a month for eight consecutive months. Alberoni et al. (2015) found a significant increase in honey supers production following the administration of a mixture of lactobacilli and bifidobacteria in hives before the linden (Tilia spp.) honey flow. Moreover, authors investigated at the end of the 4-week treatments the composition of the honey bee microbial gut community by NGS; surprisingly, lactobacilli showed a significant decrease, whereas a significant increase was observed for bifidobacteria and Acetobacteraceae compared to non-treated hives. The bifidobacteria increase confirmed the results obtain under laboratory conditions (Baffoni et al. 2016). The increase of the Acetobacteraceae in the treated group could be considered a promising result since many members of the family have recently emerged as important endosymbionts for honey bees (Crotti et al. 2010). However, further investigations are envisaged to better understand if and how these compositional changes can affect the host-gut microbe interaction.
Overall, data are too sparse and weak to support the hypothesis that beneficial microorganisms have a role in improving honey bee health. Moreover, the introduction within the hive of biological agents, even if beneficial, should be carefully treated, in particular for spore-forming bacteria (SFB). Notably, the use of SFB into the hive poses a serious issue regarding the finding of such bacteria in the stored honey. The European Food Safety Authority (EFSA) is requested to verify, through the qualified presumption of safety (QPS) assessment, the safety of a broad range of biological agents in the context of notifications for market authorization (EFSA Journal 2015), including SFB. The chemical composition in natural honey makes the growth of microorganisms difficult (Snowdon and Cliver 1996); however, SFB are able to survive and may become a risk for human health. Actually, no data are available on the microbiological quality of honey, following SFB application into the hive.
The use of pollen substitute and its fortification with probiotic microorganisms have been also investigated in different trials (Kaznowiski et al. 2005; Kazimierczak-Baryczko and Szymaś 2006; Szymaś et al. 2012), although such alternatives required more studies due to the harmful effect of their components on honey bee gut and the few available data. All three studies used a mixed of protein ingredients (fish meal, egg powder, soybean flour, etc.) with two different probiotic products, for animal ((Biogen-N; Biogen Idec Sp. z.o.o, Poland) and human consumption ((Trilac®; Allergon Health Care, Sweden). Overall, the trials showed an improved condition of bees, confirmed by lower mortality, more developed pharyngeal glands, higher dry matter and fat body content. A positive influence was also assessed on the morphological changes in the midgut epithelium. After 14 days, midgut analysis evidenced a high epithelium, cytoplasm slightly vacuolized and the presence of considerable quantities of peritrophic membranes, which are associated with duration of the feeding, presence of beneficial bacteria and protection towards harmful compounds (Szymaś et al. 2012).
Finally, we are still far to conclude that beneficial microorganisms could actually limit pathogen widespread, support honey bee health and the hive productivity, even if a starting point has been set. Research activities are still sparse and further implementations are envisaged.
Conclusions
The preservation of the European honey bee A. mellifera is imperative; the beekeeping sector and the ecosystems depending on pollinators are suffering from missed pollination and lack of productivity with an associated loss of biodiversity in the long run (Aizen and Harder, 2009; Klein et al. 2007). Nowadays, beekeepers too often rely on subspecies hybrids, with the false hope to increase disease resistance, but the resistance mechanisms against bee pathogens/parasites are usually a result of a co-evolution in local ecosystems (Ruottinen et al. 2014). Overall, the described applications offer to some extent a picture of the favourable influence of beneficial microorganisms on bee health, in particular their potential activity against some pathogens. However, information is scarce and limited to specific investigations. It could be useful, as in human and animal applications, to define some guidelines in order to standardize the studies and draw up appropriate protocols. The dose, the timing, the duration of the administration and the number of strains may influence the efficacy of the treatments. The number of experimental replicates and the repetition along the years should be accurately established. Moreover, investigation methods (i.e. N. ceranae spore number detection) ought to be uniformed in order to improve as major as possible the output accuracy and the trial comparison. It is necessary to address the study towards gut symbionts isolated from healthy honey bee gut possessing the QPS status and omit the use of probiotics for human and animal consumption. This is in authors’ opinion a key factor, since the main issue, which stand out from this review and from the literature, is the specificity of each microbial strain within its gut niche. In particular, metagenomic and transcriptomic studies are envisaged to better describe the bacterial strain(s) and their interaction with the host, following the supplementation. A deep investigation about the effect on host immunity, physiology and composition of the honey bee gut microbiota could improve the rationale of such supplementation. This is finalized to build a robust experimental structure, to minimize risk associated with bio-treatments and to analyse results in a comparable way. Finally, this will allow the realization of microorganism-based products with a reliable scientific literature, which will be more appreciated by beekeepers who are constantly looking for high-quality products combined with an excellent ratio quality/price. The beekeeping sector includes operators having a particular feeling towards honey bees, but sometimes a deep knowledge on their biological activities, including the wide world of gut symbionts, is lacking.
References
Aizen MA, Harder LD (2009) The global stock of domesticated honeybees is growing slower than agricultural demand for pollination. Curr Biol 19:915–918. doi:10.1016/j.cub.2009.03.071
Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP, Le Conte Y (2010a) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ Microbiol 12(3):774–782. doi:10.1111/j.1462-2920.2009.02123.x
Alaux C, Ducloz F, Crauser D, Le Conte Y (2010b) Diet effects on honeybee immunocompetence. Biol Lett 23:562–565. doi:10.1098/rsbl.2009.0986
Alberoni D, Baffoni L, Gaggia F, Ryan P, Murphy K, Ross RP, Biavati B, Stanton C, Di Gioia D (2015) Administration of lactobacilli and bifidobacteria on Apis mellifera L. beehives to increase health of the bee super-organism. In: Microbial Diversity 2015, the challenge of complexity. Perugia, pp 107–108
Alippi AM, Reynaldi FJ (2006) Inhibition of the growth of Paenibacillus larvae, the causal agent of American foulbrood of honeybees, by selected strains of aerobic spore-forming bacteria isolated from apiarian sources. J Invertebr Pathol 91:141–146. doi:10.1016/j.jip.2005.12.002
Anbutsu H, Fukatsu T (2010) Evasion, suppression and tolerance of Drosophila innate immunity by a male-killing Spiroplasma endosymbiont. Insect Mol Biol 19:481–488. doi:10.1111/j.1365-2583.2010.01008.x
Anderson KE, Sheehan TH, Mott BM, Maes P, Snyder L, Schwan MR, Walton A, Jones BM, Corby-Harris V (2013) Microbial ecology of the hive and pollination landscape: bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS One 17;8(12):e83125. doi: 10.1371/journal.pone.0083125
Anderson KE, Rodrigues PAP, Mott BM, Maes P, Corby-Harris V (2016) Ecological succession in the honey bee gut: shift in Lactobacillus strain dominance during early adult development. Microb Ecol 71:1008–1019. doi:10.1007/s00248-015-0716-2
Andrearczyk S, Kadhim MJ, Knaga S (2014) Influence of a probiotic on the mortality, sugar syrup ingestion and infection of honeybees with Nosema spp. under laboratory assessment. Med Weter 70:762–765
Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M (2009) Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ Microbiol 11:2284–2290. doi:10.1111/j.1462-2920.2009.01953.x
Audisio MC, Benítez-Ahrendts MR (2011) Lactobacillus johnsonii CRL1647, isolated from Apis mellifera L. bee-gut, exhibited a beneficial effect on honeybee colonies. Benef Microbes 2:29–34. doi:10.3920/BM2010.0024
Audisio MC, Torres MJ, Sabaté DC, Ibarguren C, Apella MC (2011) Properties of different lactic acid bacteria isolated from Apis mellifera L. Bee-gut. Microbiol Res 166:1–13. doi:10.1016/j.micres.2010.01.003
Audisio MC, Sabaté DC, Benítez-Ahrendts MR (2015) Effect of Lactobacillus johnsonii CRL1647 on different parameters of honeybee colonies and bacterial populations of the bee gut. Benef Microbs 25:1–10. doi:10.3920/BM2014.0155
Babendreier D, Joller D, Romeis J, Bigler F, Widmer F (2007) Bacterial community structures in honeybee intestines and their response to two insecticidal proteins. FEMS Microbiol Ecol 87:87–97. doi:10.1111/j.1574-6941.2006.00249.x
Baffoni L, Gaggìa F, Alberoni D, Cabbri R, Nanetti A, Biavati B, Di Gioia D (2016) Effect of dietary supplementation of Bifidobacterium and Lactobacillus strains in Apis mellifera L. against Nosema ceranae. Benef Microbs 7:45–51. doi:10.3920/BM2015.0085
Barribeau SM, Sadd BM, du Plessis L, Brown MJ, Buechel SD, Cappelle K, Carolan JC, Christiaens O, Colgan TJ, Erler S, Evans J, Helbing S, Karaus E, Lattorff HM, Marxer M, Meeus I, Näpflin K, Niu J, Schmid-Hempel R, Smagghe G, Waterhouse RM, Yu N, Zdobnov EM, Schmid-Hempel P (2015) A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biol 16:1–21. doi:10.1186/s13059-015-0628-y
Ben Ami E, Yuval B, Jurkevitch E (2010) Manipulation of the microbiota of mass-reared Mediterranean fruit flies Ceratitis capitata (Diptera: Tephritidae) improves sterile male sexual performance. ISME J 4:28–37. doi:10.1038/ismej.2009.82
Berasategui A, Shukla S, Salem H, Kaltenpoth M (2016) Potential applications of insect symbionts in biotechnology. Appl Microbiol Biotechnol 100:1567–1577. doi:10.1007/s00253-015-7186-9
Bíliková K, Hanes J, Nordhoff E, Saenger W, Klaudiny J, Šimúth J (2002) Apisimin, a new serine–valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: purification and molecular characterization. FEBS Lett 528:125–129. doi:10.1016/S0014-5793(02)03272-6
Bond J, Plattner K, Hunt K (2014) Fruit and Tree Nuts Outlook: Economic Insight. US Pollination-Services Market. USDA Economic Research Service Situation and Outlook FTS-357SA.
Bottacini F, Milani C, Turroni F, Sánchez B, Foroni E, Duranti S, Serafini F, Viappiani A, Strati F, Ferrarini A, Delledonne M, Henrissat B, Coutinho P, Fitzgerald GF, Margolles A, van Sinderen D, Ventura M (2012) Bifidobacterium asteroides PRL2011 genome analysis reveals clues for colonization of the insect gut. PLoS One 7(9):e44229. doi:10.1371/journal.pone.0044229
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidol 41:278–294. doi:10.1051/apido/2010012
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. doi:10.1038/nrmicro1098
Brummel T, Ching A, Seroude L, Simon AF, Benzer S (2004) Drosophila lifespan enhancement by exogenous bacteria. Proc Natl Acad Sci U S A 101:12974–12979. doi:10.1073/pnas.0405207101
Buchon N, Broderick NA, Chakrabarti S, Lemaitre B (2009) Invasive and indigenous microbiota impact intestinal stem cell activity through multiple pathways in Drosophila. Genes Dev 23:2333–2344. doi:10.1101/gad.1827009
Butler È, Alsterfjord M, Olofsson TC, Karlsson C, Malmström J, Vásquez A (2013) Proteins of novel lactic acid bacteria from Apis mellifera mellifera: an insight into the production of known extra-cellular proteins during microbial stress. BMC Microbiol 13:235. doi:10.1186/1471-2180-13-235
Cariveau DP, Powell JE, Koch H, Winfree R, Moran NA (2014) Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus spp.). ISME J 8:2369–2379. doi:10.1038/ismej.2014.68
Casteels P, Ampe C, Jacobs F, Vaek M, Tempst P (1989) Apidaecins: antimicrobial peptides from honeybees. EMBO J 8:2387–2391
Casteels P, Ampe C, Riviere L, Damme JV, Elicone C, Fleming M, Jacobs F, Tempst P (1990) Isolation and characterization of abaecin, a major antimicrobial peptide in the honeybee (Apis mellifera). Eur J Biochem 187:381–386. doi:10.1111/j.1432-1033.1990.tb15315.x
Casteels P, Ampe C, Jacobs F, Tempst P (1993) Functional and chemical characterization of hymenoptaecin, an antimicrobial peptide that is infection-inducible in the honeybee (Apis mellifera). J Biol Chem 268:7044–7054
Corby-Harris V, Snyder LA, Schwan MR, Maes P, McFrederick QS, Anderson KE (2014) Origin and effect of Alpha 2.2 Acetobacteraceae in honey bee larvae and description of Parasaccharibacter apium gen. nov., sp. nov. Appl Environ Microbiol 80:7460–7472. doi:10.1128/AEM.02043-14
Corby-Harris V, Snyder L, Meador CA, Naldo R, Mott B, Anderson KE (2016) Parasaccharibacter apium, gen. nov., sp. nov., improves honey bee (Hymenoptera: Apidae) resistance to Nosema. J Econ Entomol 109:537–543. doi:10.1093/jee/tow012
Cox-Foster DL, Conlan S, Holmes EC, Palacios G, Evans JD, Moran NA, Quan P-L, Briese T, Hornig M, Geiser DM, Martinson V, vanEngelsdorp D, Kalkstein AL, Drysdale A, Hui J, Zhai J, Cui L, Hutchison SK, Simons JF, Egholm M, Pettis JS, Lipkin WI (2007) A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318:283–287. doi:10.1126/science.1146498
Cremer S, Armitage SA, Schmid-Hempel P (2007) Social immunity. Curr Bio 17:693–702. doi:10.1016/j.cub.2007.06.008
Crotti E, Rizzi A, Chouaia B, Ricci I, Favia G, Alma A, Sacchi L, Bourtzis K, Mandrioli M, Cherif A, Bandi C, Daffonchio D (2010) Acetic acid bacteria, newly emerging symbionts of insects. Appl Environ Microbiol 76:6963–6970. doi:10.1128/AEM.01336-10
Crotti E, Balloi A, Hamdi C, Sansonno L, Marzorati M, Gonella E, Favia G, Cherif A, Bandi C, Alma A, Daffonchio D (2012) Microbial symbionts: a resource for the management of insect-related problems. Microb Biotechnol 5:307–317. doi:10.1111/j.1751-7915.2011.00312.x
Di Gioia D, Aloisio I, Mazzola G, Biavati B (2014) Bifidobacteria: their impact on gut microbiota composition and their applications as probiotics in infants. Appl Microbiol Biotechnol 98:563–577. doi:10.1007/s00253-013-5405-9
Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F (2013) Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci U S A 110:18466–18471. doi:10.1073/pnas.1314923110
Dillon R, Charnley K (2002) Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res Microbiol 153:503–509. doi:10.1016/S0923-2508(02)01361-X
Dillon RJ, Vennard CT, Buckling A, Charnley AK (2005) Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8:1291–1298. doi:10.1111/j.1461-0248.2005.00828.x
Dively GP, Embrey MS, Kamel A, Hawthorne DJ, Pettis JS (2015) Assessment of chronic sublethal effects of imidacloprid on honey bee colony health. PLoS One 10(3):e0118748. doi:10.1371/journal.pone.0118748
Dong Y, Manfredini F, Dimopoulos G (2009) Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5(5):e1000423. doi:10.1371/journal.ppat.1000423
Doublet V, Labarussias M, Miranda JR, Moritz RF, Paxton RJ (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ Microbiol 17:969–983. doi:10.1111/1462-2920.12426
EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) (2015) Statement on the update of the list of QPS - recommended biological agents intentionally added to food or feed as notified to EFSA 3: suitability of taxonomic units notified to EFSA until September 2015. EFSA J 13(12):4331. doi:10.2903/j.efsa.2015.433
Ellegaard KM, Tamarit D, Javelind E, Olofsson TC, Andersson SG, Vásquez A (2015) Extensive intra-phylotype diversity in lactobacilli and bifidobacteria from the honeybee gut. BMC Genom 16:284. doi:10.1186/s12864-015-1476-6
Engel P, Moran NA (2013) Functional and evolutionary insights into the simple yet specific gut microbiota of the honey bee from metagenomic analysis. Gut Microbes 4:60–65. doi:10.4161/gmic.22517
Engel P, Kwong WK, Moran NA (2013) Frischella perrara gen. nov., sp. nov., a Gammaproteobacterium isolated from the gut of the honeybee, Apis mellifera. Int J Syst Evol Microbiol 63:3646–3651. doi:10.1099/ijs.0.049569-0
European Commission (2010) Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. EU 15:1–70. http://ec.europa.eu/health/files/eudralex/vol-5/reg_2010_37/reg_2010_37_en.pdf. Accessed 31 may 2016
Evans JD, Lopez DL (2004) Bacterial probiotics induce an immune response in the honey bee (Hymenoptera: Apidae). J Econ Entomol 97:752–756. doi:10.1093/jee/97.3.752
Evans JD, Pettis JS (2005) Colony-level impacts of immune responsiveness in honey bees, Apis mellifera. Evolution 59:2270–2274. doi:10.1111/j.0014-3820.2005.tb00935.x
Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, Kanost M, Thompson GJ, Zou Z, Hultmark D (2006) Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Bio 15:645–656. doi:10.1111/j.1365-2583.2006.00682.x
Fang Q, Wang L, Zhu J, Li Y, Song Q, Stanley DW, Akhtar Z, Ye G (2010) Expression of immune-response genes in lepidopteran host is suppressed by venom from an endoparasitoid, Pteromalus puparum. BMC Genom 11:484
FAO/WHO (2002) Joint FAO/WHO (Food and Agriculture Organization/World Health Organization) working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada. Guidelines for the evaluation of probiotics in food. Joint working group report on drafting. London, Ontario, 2002:1–11. http://who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf
Fernandez JM, Puerta F, Cousinou M, Dios-Palomares R, Campano F, Redondo L (2012) Asymptomatic presence of Nosema spp. in Spanish commercial apiaries. J Inverte Pathol 111:106–110. doi:10.1016/j.jip.2012.06.008
Flemming HC, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633. doi:10.1038/nrmicro2415
Forsgren E, Olofsson TC, Vásquez A, Fries I (2010) Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidol 41:99–108. doi:10.1051/apido/2009065
Gaggìa F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141:15–28. doi:10.1016/j.ijfoodmicro.2010.02.031
Gaggìa F, Di Gioia D, Baffoni L, Biavati B (2011) The role of protective and probiotic cultures in food and feed and their impact in food safety. Trends Food Sci Tech 22:58–66. doi:10.1016/j.tifs.2011.03.003
Gaggìa F, Baffoni L, Stenico V, Alberoni D, Buglione E, Lilli A, Di Gioia D, Porrini C (2015) Microbial investigation on honey bee larvae showing atypical symptoms of European foulbrood. Bulletin Insect 68:321–327
Gill RJ, Baldock KC, Brown MJ, Cresswell JE, Dicks LV, Fountain MT, Garratt MPD, Gough LA, Heard MS, Holland JM, Ollerton J, Stone GN, Tang CQ, Vanbergen AJ, Vogler AP, Woodward G, Arce AN, Boatman ND, Brand-Hardy R, Breeze TD, Green M, Hartfield CM, O’Connor RS, Osborne JL, Phillips J, Sutton PB (2016) Protecting an ecosystem service: approaches to understanding and mitigating threats to wild insect pollinators. Adv Eco Res 54:135–206. doi:10.1016/bs.aecr.2015.10.007
Gisder S, Genersch E (2015) Identification of candidate agents active against N. ceranae infection in honey bees: establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS One 10(2):e0117200. doi: 10.1371/journal.pone.0117200
Goblirsch M, Huang ZY, Spivak M (2013) Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS One 8(3):e58165. doi:10.1371/journal.pone.005816
Goulson D, Nicholls E, Botias C, Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347:1255957. doi:10.1126/science.1255957
Gündüz EA, Douglas AE (2009) Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proce Roy Soc London Biol Sci 276:987–991. doi:10.1098/rspb.2008.1476
Hamdi C, Daffonchio D (2011) Methods for the prevention and control of pathogenic infections in bees and relative composition. Patent Application WO/2011/138310.
Hamdi C, Balloi A, Essanaa J, Crotti E, Gonella E, Raddadi N, Ricci I, Boudabous A, Borin S, Manino A, Bandi C, Alma A, Daffonchio D, Cherif A (2011) Gut microbiome dysbiosis and honeybee health. J Appl Entomol 135:524–533. doi:10.1111/j.1439-0418.2010.01609.x
Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702–702. doi:10.1126/science.1162418
Higes M, Martin-Hernandez R, Meana A (2010) Nosema ceranae in Europe: an emergent type C nosemosis. Apidol 41:375–392. doi:10.1051/apido/2010019
Holst EC (1945) An antibiotic from a bee pathogen. Science 102:593–594
Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 292:1115–1118. doi:10.1126/science.1058709
Hughes DP, Pierce NE, Boomsma JJ (2008) Social insect symbionts: evolution in homeostatic fortresses. Trends Ecol Evol 23:672–677. doi:10.1016/j.tree.2008.07.011
Hui-Ru J, Yan-Yan W, Ping-Li D, Qiang W, Ting Z (2015) Effects of the sublethal doses of imidacloprid on the bacterial diversity in the midgut of Apis mellifera Ligustica (Hymenoptera: Apidae). Acta Entomol Sin 58:139–146. doi:10.16380/j.kcxb.2015.02.005
Imler J, Bulet P (2005) Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy 86:1–21. doi:10.1159/000086648
Jaenike J, Unckless R, Cockburn SN, Boelio LM, Perlman SJ (2010) Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329:212–215. doi:10.1126/science.1188235
Janashia I, Alaux C (2016) Specific immune stimulation by endogenous bacteria in honey bees (Hymenoptera: Apidae). J Econ Entomol. doi:10.1093/jee/tow065
Jefferson JM, Dolstad HA, Sivalingam MD, Snow JW (2013) Barrier immune effectors are maintained during transition from nurse to forager in the honey bee. PLoS One 8(1):e54097. doi:10.1371/journal.pone.0054097
Kapheim KM, Rao VD, Yeoman CJ, Wilson BA, White BA, Goldenfeld N, Robinson GE (2015) Caste-specific differences in hindgut microbial communities of honey bees (Apis mellifera). PLoS One 10(4):e0123911. doi:10.1371/journal.pone.0123911
Kazimierczak-Baryczko M, Szymaś B (2006) Improvement of the composition of pollen substitute for honey bee (Apis mellifera L.), through implementation of probiotic preparation. J Apic Sci 50:15–22
Kaznowiski A, Szymas B, Jazdzinska E, Kazimierczak M, Paetz H, Mokracka J (2005) The effect of probiotic supplementation on the content of intestinal microflora and chemical composition of worker honey bees (Apis mellifera). J Apic Res 44:10–14. doi:10.1080/00218839.2005.11101139
Killer J, Dubná S, Sedláček I, Švec P (2014) Lactobacillus apis sp. nov., from the stomach of honeybees (Apis mellifera), having an in vitro inhibitory effect on the causative agents of American and European foulbrood. Int J Syst Evol Microbiol 64:152–157. doi:10.1099/ijs.0.053033-0
Klaudiny J, Albert Š, Bachanová K, Kopernický J, Šimúth J (2005) Two structurally different defensin genes, one of them encoding a novel defensin isoform, are expressed in honeybee Apis mellifera. Insect Biochem Mol Biol 35:11–22. doi:10.1016/j.ibmb.2004.09.007
Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, Bron PA (2010) The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34:199–230. doi:10.1111/j.1574-6976.2009.00208.x
Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc Biol Sci 274:303–313. doi:10.1098/rspb.2006.3721
Kwong WK, Moran NA (2013) Cultivation and characterization of the gut symbionts of honey bees and bumble bees: description of Snodgrassella alvi gen. nov., sp. nov., a member of the family Neisseriaceae of the Betaproteobacteria, and Gilliamella apicola gen. nov., sp. nov., a member of Orbaceae fam. nov., Orbales ord. nov., a sister taxon to the order ‘Enterobacteriales’ of the Gammaproteobacteria. Int J Syst Evol Microbiol 63:2008–2018. doi:10.1099/ijs.0.044875-0
Kwong WK, Moran NA (2016) Gut microbial communities of social bees. Nat Rev Microbiol 14:374–384. doi:10.1038/nrmicro.2016.43
Kwong WK, Engel P, Koch H, Moran NA (2014) Genomics and host specialization of honey bee and bumble bee gut symbionts. Proc Natl Acad Sci U S A 111:11509–11514. doi:10.1073/pnas.1405838111
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidol 41:353–363. doi:10.1051/apido/2010017
Lee H, Churey JJ, Worobo RW (2009) Isolation and characterization of a protective bacterial culture isolated from honey active against American foulbrood disease. FEMS Microbiol Lett 296:39–44. doi:10.1111/j.1574-6968.2009.01615.x
Lee FJ, Rusch DB, Stewart FJ, Mattila HR, Newton IL (2015) Saccharide breakdown and fermentation by the honey bee gut microbiome. Environ Microbiol 17:796–815. doi:10.1111/1462-2920.12526
Lemaitre B, Hoffmann J (2007) The host defense of Drosophila melanogaster. Annu Rev Immunol 25:697–743. doi:10.1146/annurev.immunol.25.022106.141615
Martinson VG, Moy J, Moran NA (2012) Establishment of characteristic gut bacteria during development of the honeybee worker. Appl Environ Microbiol 78:2830–2840. doi:10.1128/AEM.07810-11
Medrzycki P, Montanari R, Bortolotti L, Sabatini AG, Maini S, Porrini C (2003) Effects of imidacloprid administered in sub-lethal doses on honey bee behaviour. Laboratory tests. Bull Insect 56:59–62
Milani C, Turroni F, Duranti S, Lugli GA, Mancabelli L, Ferrario C, van Sinderen D, Ventura M (2015) Genomics of the genus Bifidobacterium reveals species-specific adaptation to the glycan-rich gut environment. Appl Environ Microbiol 82:980–991. doi:10.1128/AEM.03500-15
Moran NA (2015) Genomics of the honey bee microbiome. Curr Opin Insect Sci 10:22–28. doi:10.1016/j.cois.2015.04.003
Müller S, Garcia-Gonzalez E, Genersch E, Süssmuth RD (2015) Involvement of secondary metabolites in the pathogenesis of the American foulbrood of honey bees caused by Paenibacillus larvae. Nat Prod Rep 32:765–778. doi:10.1039/C4NP00158C
Newton IL, Sheehan KB, Lee FJ, Horton MA, Hicks RD (2013) Invertebrate systems for hypothesis-driven microbiome research. Microbiome Sci Med 1(1). doi:10.2478/micsm-2013-0001
Nieto A, Roberts SPM, Kemp J, Rasmont P, Kuhlmann M, Criado GM, Biesmeijer JC, Bogusch P, Dathe HH, De la Rúa P, De Meulemeester T, Dehon M, Dewulf A, Ortiz-Sánchez FJ, Lhomme P, Pauly A, Potts SG, Praz C, Quaranta M, Radchenko VG, Scheuchl E, Smit J, Straka J, Terzo M, Tomozii B, Window J, Michez D (2014) European red list of bees. Publication Office of the European Union. http://ec.europa.eu/environment/nature/conservation/species/redlist/downloads/European_bees.pdf.
Olofsson TC, Vásquez A (2008) Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr Microbiol 57:356–363. doi:10.1007/s00284-008-9202-0
Olofsson TC, Alsterfjord M, Nilson B, Butler È, Vásquez A (2014) Lactobacillus apinorum sp. nov., Lactobacillus mellifer sp. nov., Lactobacillus mellis sp. nov., Lactobacillus melliventris sp. nov., Lactobacillus kimbladii sp. nov., Lactobacillus helsingborgensis sp. nov. and Lactobacillus kullabergensis sp. nov., isolated from the honey stomach of the honeybee Apis mellifera. Int J Syst Evol Microbiol 64:3109–3119. doi:10.1099/ijs.0.059600-0
Pătruică S, Dumitrescu G, Stancu A, Bura M, Bănătean Dunea I (2012) The effect of prebiotic and probiotic feed supplementation on the wax glands of worker bees (Apis mellifera). J Anim Sci Biotech 45:268–271
Porrini C, Mutinelli F, Bortolotti L, Granato A, Laurenson L, Roberts K, Gallina A, Silvester N, Medrzycki P, Renzi T, Sgolastra F, Lodesani M (2016) The status of honey bee health in Italy: results from the nationwide bee monitoring network. PLoS One 11(5):e0155411. doi:10.1371/journal.pone.0155411
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353. doi:10.1016/j.tree.2010.01.007
Powell JE, Martinson VG, Urban-Mead K, Moran NA (2014) Routes of acquisition of the gut microbiota of the honey bee Apis mellifera. Appl Environ Microbiol 80:7378–7387. doi:10.1128/AEM.01861-14
Ptaszyńska AA, Borsuk G, Mułenko W, Olszewski K (2013) Impact of ethanol on Nosema spp. infected bees. Med Weter 69:736–741
Ptaszyńska AA, Borsuk G, Zdybicka-Barabas A, Cytryńska M, Małek W (2016) Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis C? Parasitol Res 115:397–406. doi:10.1007/s00436-015-4761-z
Robinson CJ, Schloss P, Ramos Y, Raffa K, Handelsman J (2010) Robustness of the bacterial community in the cabbage white butterfly larval midgut. Microbiol Ecol 59:199–211. doi:10.1007/s00248-009-9595-8
Rokop ZP, Horton MA, Newton ILG (2015) Interactions between co-occurring lactic acid bacteria in honey bee hives. Appl Environ Microbiol 81:7261–7270. doi:10.1128/AEM.01259-15
Rosenkranz P, Aumeier P, Ziegelmann B (2010) Biology and control of Varroa destructor. J Invertebr Pathol 103:96–119. doi:10.1016/j.jip.2009.07.016
Ruottinen L, Berg P, Kantanen J, Kristensen TN, Praebel A, Groeneveld L (2014) Status and conservation of the nordic brown bee: final report. Nordic Genetic Resource Center (NordGen) http://vbn.aau.dk/ws/files/207960984/Ruottinen_et_al_2014.pdf
Ryu JH, Kim SH, Lee HY, Bai JY, Nam YD, Bae JW, Lee DG, Shin SC, Ha EM, Lee WJ (2008) Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319:777–782. doi:10.1126/science.1149357
Sabaté DC, Carrillo L, Audisio MC (2009) Inhibition of Paenibacillus larvae and Ascosphaera apis by Bacillus subtilis isolated from honeybee gut and honey samples. Res Microbiol 160:193–199. doi:10.1016/j.resmic.2009.03.002
Sabaté DC, Cruz MS, Benítez-Ahrendts MR, Audisio MC (2012) Beneficial effects of Bacillus subtilis subsp. subtilis Mori2, a honey-associated strain, on honeybee colony performance. Probiotics Antimicrob Proteins 4:39–46. doi:10.1007/s12602-011-9089-0011-9089-0
Sanders ME, Lenoir-Wijnkoop I, Salminen S, Merenstein DJ, Gibson GR, Petschow BW, Nieuwdorp M, Tancredi DJ, Cifelli CJ, Jacques P, Pot B (2014) Probiotics and prebiotics: prospects for public health and nutritional recommendations. Ann N Y Acad Sci 1309:19–29. doi:10.1111/nyas.12377
Saraiva MA, Zemolin APP, Franco JL, Boldo JT, Stefenon VM, Triplett EW, De Oliveira FA, Roesch LFW (2015) Relationship between honeybee nutrition and their microbial communities. Antonie Van Leeuwenhoek 107:921–933. doi:10.1007/s10482-015-0384-8
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440. doi:10.1016/j.femsre.2004.01.003
Sharon G, Segal D, Ringo JM, Hefetz A, Zilber-Rosenberg I, Rosenberg E (2010) Commensal bacteria play a role in mating preference of Drosophila melanogaster. Proc Natl Acad Sci U S A 107:20051–20056. doi:10.1073/pnas.1009906107
Snowdon JA, Cliver DO (1996) Microorganisms in honey. Int J Food Microbiol 31:1–26. doi:10.1016/0168-1605(96)00970-1
Storelli G, Defaye A, Erkosar B, Hols P, Royet J, Leulier F (2011) Lactobacillus plantarum promotes drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metab 4:403–414. doi:10.1016/j.cmet.2011.07.012
Sun Z, Zhang W, Guo C, Yang X, Liu W, Wu Y, Song Y, Kwok LY, Cui Y, Menghe B, Yang R, Hu L, Zhang H (2015) Comparative genomic analysis of 45 type strains of the genus Bifidobacterium: a snapshot of its genetic diversity and evolution. PLoS One 10(2):e0117912. doi:10.1371/journal.pone.011791
Szymaś B, Łangowska A, Kazimierczak-Baryczko M (2012) Histological structure of the midgut of honey bees (Apis mellifera L.) fed pollen substitutes fortified with probiotics. J Apic Sci 56:5–12. doi:10.2478/v10289-012-0001-2
Tarpy DR, Mattila HR, Newtond ILG (2015) Development of the honey bee gut microbiome throughout the queen-rearing process. Appl Env Microbiol 81:3182–3191. doi:10.1128/AEM.00307-15
Tontou R, Gaggìa F, Baffoni L, Devescovi G, Venturi V, Giovanardi G, Stefani E (2015) Molecular characterisation of an endophyte showing a strong antagonistic activity against Pseudomonas syringae pv. actinidiae. Plant Soil. doi:10.1007/s11104-015-2624-0
Vásquez A, Forsgren E, Fries I, Paxton RJ, Flaberg E, Szekely L, Olofsson TC (2012) Symbionts as major modulators of insect health: lactic acid bacteria and honeybees. PLoS One 7(3):e33188. doi:10.1371/journal.pone.0033188
Vidau C, Diogon M, Aufauvre J, Fontbonne R, Viguès B, Brunet JL, Texier C, Biron DG, Blot N, El Alaoui H, Belzunces LP, Delbac F (2011) Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS One 6:e21550. doi:10.1371/journal.pone.0021550
Wilson-Rich N, Spivak M, Fefferman NH, Starks PT (2009) Genetic, individual, and group facilitation of disease resistance in insect societies. Annu Rev Entomol 54:405–423. doi:10.1146/annurev.ento.53.103106.093301
Wittebolle L, Marzorati M, Clement L, Balloi A, Daffonchio D, Heylen K, De Vos P, Verstraete W, Boon N (2009) Initial community evenness favours functionality under selective stress. Nature 458:623–626. doi:10.1038/nature07840
Wu M, Sugimura Y, Takaya N, Takamatsu D, Kobayashi M, Taylor D, Yoshiyama M (2013) Characterization of bifidobacteria in the digestive tract of the Japanese honeybee, Apis cerana japonica. J Invertebr Pathol 112:88–93. doi:10.1016/j.jip.2012.09.005
Yoshiyama M, Kimura K (2009) Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American foulbrood. J Invertebr Pathol 102:91–96. doi:10.1016/j.jip.2009.07.005
Yoshiyama M, Wu M, Sugimura Y, Takaya N, Kimoto-Nira H, Suzuki C (2013) Inhibition of Paenibacillus larvae by lactic acid bacteria isolated from fermented materials. J Invertebr Pathol 112:62–67. doi:10.1016/j.jip.2012.09.002
Acknowledgments
This work was performed within the VII FP TRAFOON (traditional food network to improve the transfer of knowledge for innovation), grant agreement no. 613912.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animals rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Alberoni, D., Gaggìa, F., Baffoni, L. et al. Beneficial microorganisms for honey bees: problems and progresses. Appl Microbiol Biotechnol 100, 9469–9482 (2016). https://doi.org/10.1007/s00253-016-7870-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7870-4