Abstract
Summary
The study aims to estimate the direct disease-related costs of osteoporotic vertebral compression fractures (OVCF) in patients with newly diagnosed fracture in the first year after index in Germany. Analyses reveal that OVCFs are associated with significant costs. In light of high and increasing incidence, the results emphasize importance of research in this field.
Introduction
OVCF are among the most common fractures related to osteoporosis. They have been shown to be associated with excess mortality and meaningful healthcare costs. Costs calculations have illustrated the significant financial burden to society and national social security systems. However, this information is not available for Germany. Therefore, aim of the study was to estimate the direct disease-related costs of OVCF in patients with newly diagnosed fracture in the first year after index in Germany.
Methods
Data were obtained from a claims dataset of a large German health insurance fund. Subjects ≥60 years with a new vertebral fracture between 2006 and 2010 were studied retrospectively compared to a matched paired OVCF-free patient group. All-cause and fracture-specific medical costs were calculated in the 1-year baseline and follow-up period. Generalized linear model (GLM) was estimated for total follow-up healthcare cost.
Results
A total of 2,277 pairs of matched OVCF and OVCF-free patients were included in the analysis. Baseline costs were higher in the OVCF group. Mean unadjusted all-cause healthcare cost difference in the four quarters following the index date between OVCF and OVCF-free patients was 8,200 € (p < 0.001). Of the difference, almost two third was attributable to inpatient services and one quarter to prescription drug costs. The GLM procedure revealed that OVCF-related costs in the first year after the index date add up to 6,490 € (p < 0.001; CI 5,809 €–6,731 €).
Conclusions
Despite limitations of this study, our results are consistent with other research and demonstrate that OVCFs are associated with significant costs. The results underline the importance of medical interventions that can help to prevent fractures and treatments, which are cost-effective and can prevent recurrent fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is defined as a “systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture” [1]. In Europe, it has become one of the major widespread diseases. In Germany, more than 8 million people suffer from osteoporosis in the age group 50+, representing 1/4 of that population [2]. Furthermore, the incidence of osteoporosis shows a trend towards further increases which is mainly related to demographic changes and changes in lifestyle [3]. The lifetime risk of a 50-year-old person to experience an osteoporotic fracture has been estimated at 13–22 % for men and at 40–50 % for women [4]. For the year 2000, it has been estimated that 9 million osteoporotic fractures have occurred worldwide [5].
Osteoporotic vertebral compression fractures (OVCF) are among the most common fractures related to osteoporosis [6]. The estimated incidence of OVCF in patients 50 years or older is 307 per 100,000 year in Germany [7]. This value increases nearly eightfold in women aged between 85 and 89 years compared to those aged 60–64 years. OVCFs significantly contribute to the loss of health-related quality of life and life years of middle-aged women, often in the context of several other morbid conditions [6]. Furthermore, they have been shown to be associated with excess mortality, and meaningful health care costs [6, 8]. The overall annual costs of all types of osteoporotic fractures have been estimated to be € 37 billion in the European Union, illustrating the significant financial burden to society and national social security systems [7]. In 2005, osteoporotic fractures accounted for 2.1 % (€ 3.3 billion) of total health care expenditures in Germany [2].
Several studies on the costs of osteoporosis fractures have been conducted in many different countries worldwide. Most studies, however, have focused on the costs of osteoporosis-related hip fractures [9]. Furthermore, results of such studies in different countries cannot be transferred due to differences in healthcare systems, methods of pricing and reimbursement, data availability, as well as methods for estimating costs [10].
Previous research has discovered that the majority of OVCF patients in Germany are treated conservatively (88 %) [11]. Those operated (12 %) incur a 4-year mean overall costs after first diagnosis of 42,510 € and 39,014 € for percutaneous vertebroplasty and balloon kyphoplasty, respectively [11]. The current study uses the same patient sample (conservatively treated and operated patients).
Information on disease-related costs of OVCF is not available for Germany. This information, however, would be important to assess the relevance of a certain disease and support political decision making and model building. Therefore, the aim of the present study was to estimate the direct disease-related costs of OVCF in patients with newly diagnosed fracture in the first year after index fracture in Germany.
Methods
Data source
Data were obtained from a claims dataset of a large German health insurance fund (AOK Niedersachsen). The AOK Niedersachsen covered approximately 2.4 million insurants in 2011. Data were available for the years 2005 to 2010. The database contains basic patient information (e.g., age, gender) as well as detailed information on inpatient claims, outpatient claims, pharmacy claims, rehabilitation claims, sick leave payments, and claims for devices. No clinical information (e.g., disease activity, severity grades of a disease, symptom scores, lab test results, QoL data, smoking status, BMI, etc.) were available. All available information could be merged via an identification number for each patient. However, data had been made anonymous by the AOK before the data transfer to the research group. Given the data source, no ethical approval was required for the study.
Patient selection
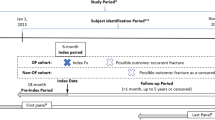
The identification of the study population was based on inpatient and outpatient ICD-10 (international classification of disease, 10th revision) diagnoses codes. All patients who had at least one OVCF diagnosis (ICD-10: M80.-8 “Osteoporosis with pathological fracture”; site subclassification “other (including vertebra)”) in the inpatient sector or two confirmed diagnoses in the outpatient sector (within 1 year) between January 01, 2006 and December 31, 2010 were included in the study. First, OVCF diagnosis in the study period was defined as index date for each patient. Patients had to be at least 60 years old on index date. A 1-year look-back period was incorporated to discriminate between incident and prevalent OVCF patients. Study patients with an OVCF diagnosis in this look-back period or an insufficient look-back period were, therefore, excluded. Patients included in this study had at least 2 years of continuous eligibility in the AOK plan—1 year before and 1 year after the index date. However, we repeated our analysis including patients that had less than 2 years eligibility, as a sensitivity analysis.
To estimate the OVCF-related costs, an OVCF-free comparison group from the AOK Niedersachsen was selected randomly using an exact 1:1 matching, based on age (year of birth) and gender. Individuals in the comparison sample also had a minimum of 1 year continuous eligibility in the AOK plan before and after the index date. The comparison group index date was defined as the same index date of the respective matched OVCF patient.
Economic outcome
The main outcome in this study was the total health care costs. Total all-cause health care costs included inpatient costs, outpatient costs, pharmacy costs, costs for rehabilitation, sick leave payments, and costs for devices for any reason. Except for outpatient costs from doctor visits, all costs components were directly available in the dataset. To calculate the monetary value of outpatient services, the number of points assigned to each medical action was multiplied by the valid point value according to German guidelines [12, 13].
Costs were estimated from the perspective of the German Social Health Insurance during the 1-year period following the index date. However, the exact date of a diagnosis within a quarter in the outpatient sector is not available in German claims data. Therefore, 1-year total health care costs were estimated as the costs in the index quarter plus the three subsequent quarters for each patient. The same approach was used for all cost components to assure a consistent proceeding. To further address meaningful differences between OVCF and OVCF-free patients, total all-cause health care costs in the four quarters prior to the index quarter were assessed for the study sample.
Patient characteristics
Available characteristics of all patients were incorporated in the analysis. They included age (at index year), gender, and comorbidities indicated by the diseases of the Elixhauser Comorbidity Algorithm [14]. This algorithm accounts for 30 different diseases based on distinctive ICD-9-CM codes, which are represented by 30 dichotomous variables. In Germany, however, ICD-10 classification is used. Hence, we used the ICD-10 coding algorithms adapted by Quan et al. [15]. Furthermore, we calculated a single score based on the aforementioned 30 dichotomous variables using the specific weighting factors evaluated by van Walraven et al. [16]. This summarizes the burden of disease. All comorbidity measures were based on the inpatient and outpatient diagnoses in the four quarters before the index quarter. In the present study, the Elixhauser Algorithm was preferred to the Charlson Index [17], another well accepted comorbidity measure, because it accounts for more groups of diseases.
Statistical analysis
Health care cost differences between OVCF patients and OVCF-free patients were calculated using descriptive analysis and multivariate regression. Data were preprocessed using the above mentioned matching in order to make the results more accurate [18]. Health care costs are commonly considered to be skewed because of a substantial fraction of patients with low costs and a few study participants who require much more health care. The assumption of normal distribution of our data was tested and rejected by the Kolmogorov–Smirnov test. Therefore, Wilcoxon-signed rank sum tests were used to determine statistical significance for cost differences between both groups and for all other continuous variables in the descriptive analysis; whereas, differences for categorical variables were determined using McNemar’s test.
The multivariate regression was used to further address differences between both groups after the previously described matching method and to estimate the incremental costs. The regression aims to make both the OVCF group and matched comparison group comparable regarding all included variables, particularly age, gender, and comorbidities. Regression-adjusted cost difference was estimated using a generalized linear model (GLM) with a Poisson distribution and a power link (0.6). To select the family and link of model, a modified Park Test [19] and a combination of Pregibon link test [20] as well the Hosmer–Lemeshow test [21] were used, respectively. In the case of non-normal distributed data, linear regression models could lead to biased estimates [22]. Therefore, GLM’s are applied commonly. They can provide significantly more robust coefficient estimates and avoid the problem of retransformation to the original cost scale (with, e.g., Duan smearing estimation) that undermines log OLS [19, 23]. Nevertheless, because the GLM model is nonlinear, the estimated regression coefficients do not equal the marginal or incremental effect of a one-unit change in the covariate of interest on the conditional mean.
We used total all-cause health care costs during the 1-year period (four quarters) following the index date as the dependent variable for the GLM to predict the incremental costs of care for individuals with OVCF compared with those without OVCF. The GLM adjusted for the following factors: age (at index year), gender, index year, pre-index costs, and 30 comorbidities defined by the Elixhauser algorithm (in the four pre-index quarters). The single index value by Walraven et al. [16] was not used in the GLM. Following the GLM, average marginal effects were calculated for each independent variable. This procedure creates an identical covariate structure for, e.g., OVCF and OVCF-free group by treating everyone as if they were in the OVCF group and predicting cost for each individual and then treating everyone as if they were in the OVCF-free group and predicting cost. The difference between the computation of both predictions is the marginal effect for that case; and the average of all effects is the average marginal effect of having OVCF [24]. Bootstrap resampling methods were used to estimate the p values and 95 % confidence intervals of the health care cost differences. Furthermore, a difference-in-difference (DID) approach was used as a sensitivity analysis of total cost differences [25]. The DID formula is as follows: DID = (Treatment After − Treatment Before ) − (Control After − Control Before ). The idea of the DID approach is that it removes biases in post-index period cost comparisons between both groups that could be due to permanent differences between those groups, as well as biases from comparisons over time in the treatment group that could be the result of trends [25]. All analyses were performed using STATA software, version 13.0.
Results
A total of 4,465 individuals were identified who had a relevant diagnosis in the inpatient or outpatient sector. Of these, 207 patients were excluded from the study because they were younger than 60 years at the time of their first diagnosis. Six hundred fifty-one patients were excluded due to an insufficient look-back period. Furthermore, 1,333 patients with less than 1 year continuous eligibility after the index date were excluded. As a result, a total of 2,277 pairs of matched OVCF patients and OVCF-free comparison members were included in the analysis (Fig. 1).
Table 1 reports the characteristics of the study sample.
The average age of the study cohort was 77 years (standard deviation (SD) = 7.0). The majority of patients were female (91.8 %), reflecting the fact that the OVCF probability is higher among women than men. Because of the previously conducted matching on age and gender, no differences regarding these characteristics between both groups could be found. The mean Elixhauser Score for the OVCF patients was 5.9 (SD = 8.0), which was significantly higher than the score of the matched comparison group (mean = 3.5; SD = 6.2; p < 0.001). The differences between OVCF patients and OVCF-free patients in terms of comorbidities are, furthermore, reflected by the higher prevalence of the diseases defined by the Elixhauser algorithm save for obesity in OVCF patients. Slightly more than half of the indicators show significant differences between the groups. Furthermore, a high prevalence of hypertension is found in both groups.
Table 2 reports the unadjusted all-cause cost differences between the age and gender-matched cohorts in the four quarters both before and after the index date.
The mean unadjusted all-cause health care costs for OVCF patients in the four quarters before and after the index date were 6,182 € and 11,435 € per patient, respectively. Higher mean costs per patient were found for all health care services. However, the clearest increases were found for costs for inpatient services and prescription drug costs. Mean unadjusted costs for the OVCF-free cohort in the same periods were 3,006 € and 3,235 € per patient, respectively. Thus, the mean unadjusted all-cause health care cost difference in the four quarters following the index date between OVCF and OVCF-free patients was 8,200 € (p < 0.001). Of the difference, 60.6 % was attributable to inpatient services, 28.1 % percent to prescription drug costs, 5.0 % to costs for devices, 3.6 % to outpatient services, 2.5 % to rehabilitation, and 0.2 % to sick pay (Fig. 2). All cost differences between both groups were found to be highly significant in the four quarters both before and after the index date, except for the differences regarding sick pay. The all-cause health care cost difference changed by only 1 % if patients with less than 2 years eligibility were also considered.
In addition, we also conducted a multivariate regression analyses (GLM model) that provides insight into the impact of other covariables (e.g., gender, age, comorbidities). The results of the regression are provided in Table 3.
As stated in the method section, the estimated regression coefficients do not equal the marginal effect of a one-unit change in the covariate of interest on the costs. Therefore, marginal effects are also provided in Table 3.
The results reveal that all variables had a significant impact on the all-cause health care costs. The adjusted mean health care costs in OVCF and OVCF-free patients were found to be 10,421 € and 3,930 €, respectively. Hence, the OVCF-related costs in the first year after the index date add up to 6,490 € (p < 0.001; parametric CI 5,809 €–6,731 €), adjusted for age, gender, index year, costs in the pre period, and comorbidities (Table 4). Furthermore, overall costs tend to increase with age, higher costs in the pre period, and the index year; whereas, costs are lower for women than men. The impact of Elixhauser comorbidities is not consistent. However, the marginal effects of the variables reflect the impact of the respective variable in the overall study sample holding all other independent variable values constant; not only for OVCF patients. Therefore, Fig. 3 provides insights into the OVCF-related costs (average marginal effect) with respect to age and gender. The results reveal that the OVCF-related costs are about 457 € higher for the 60-year-old men than for woman at the same age. This difference remains almost the same over the entire age range. Furthermore, costs increase slightly from 6,303 € for 60 year old women to 6,566 € for 90 year old women. Using the DID approach, the OVCF disease-related costs were found to be 5,024 € [(11,435 €–6,182 €)–(3,235 €–3,006 €) = 5,024 € (p < 0.001)].
Discussion
To our knowledge, this is the first study to analyze the disease-related health care costs of OVCF from a payer perspective in Germany. Total health care costs of OVCF patients in the first year following the index were found to be considerably higher than in a disease-free comparison group. Mean health care costs per patient before index were found to be 6,182 € and 3,006 € for the OVCF and matched comparison group, respectively. In the year after the index, the costs increased to 11,435 € and 3,235 €, respectively. Hence, unadjusted differences add up to 8,200 €. Differences in inpatient and prescription drug costs accounted for almost 90 % of the total health care costs difference. Costs for sick pay and rehabilitation were not relevant for the results (costs for rehabilitation were, however, statistically significantly different). After adjusting for differences in age, gender, comorbidities, and other aspects between the OVCF and OVCF-free cohort using multivariate regression methods, cost differences decreased slightly to 6,490 €. This amount can be considered as the disease-related costs of OVCF in newly diagnosed patients in the first year following the index diagnosis. The calculation of marginal effects for other regression variables revealed that age, male sex, and higher pre-period costs are associated with higher disease-related costs.
Our findings are hardly comparable to the results obtained from other studies due to differences in data, method, and setting. Our findings are, however, within the range of costs calculated by Ström et al. [6]. They revealed that the first year disease-related costs of a clinical vertebral fracture range between 5,585 € and 6,845 € for individuals 50 years of age or older in Germany. Furthermore, results for other countries are available [7]. Many studies aimed to analyze the total health care costs of OVCF patients, e.g., Gabriel [26], Ray [27], and De Laet [28]. Shi et al. [29] calculated disease-related health care cost of OVCFs of $ 6,701 in a US-Medicare patient population older than 64 years. The study period, however, was from 2001 until 2004. Rousculp et al. [30] also used a US-Medicare database to analyze the disease-related costs of different osteoporotic fractures. They conclude that osteoporosis accounts for about $ 4,000 of health care costs on average in the first year after the initial fracture and varies markedly for different types of fractures. Vertebral fractures were among the four most expensive fracture types. Häussler et al. [2] analyzed the disease-related health care costs of patients with osteoporotic fractures for the year 2003 for Germany. Costs were found to be 9,962 € per patient per year. Their approach, however, also considered costs from the German long-term care insurance and is, thus, not comparable to our results.
A number of limitations of the present study are related to the characteristics of German health insurance claims data. As stated before, no clinical information is available due to data protection regulations. Thus, results could be biased by those factors. However, we applied state-of-the art methods using regression analysis after matching to make groups comparable in terms of age, gender and comorbidities [31]. This approach has been shown to correct for remaining sample bias [32].
Our analysis takes on a payer perspective including all costs carried by the German statutory health insurance (SHI). Hence, our results do not include patients’ out-of-pocket payments or costs carried indirectly by society, like productivity losses. Fracture-related productivity losses have been estimated to be small and are only applicable to individuals younger than 65 years of age [33]. However, expenses for over-the-counter (OTC) drugs or bone-density measurements are usually not carried by the SHI. Costs are, therefore, likely to be higher from an overall economic perspective. Furthermore, the present analysis focuses on the costs of incident patients. Even though, the consequences related to fractures can be differentiated into an incident (acute phase) and prevalent phase. Therefore, our results cannot be applied to all OVCF patients. However, the costs in the acute phase are higher than in the prevalent phase [34]. Further studies are necessary to determine the costs of prevalent patients.
In addition, the present study was limited to patients aged 60 years and older. It is unclear if the results remain the same if younger patients had been considered. Shi et al. [29] estimated that direct healthcare costs for patients 50–64 were consistently higher than those of a ≥65 cohort. Hence, disease-related costs are likely to increase for a younger cohort. Moreover, data from the AOK Niedersachsen were used for our analysis. The transferability of the results to the overall German SHI population must therefore be examined critically. Furthermore, the present study only analyzed the costs in the first year after the index diagnosis. Robust estimates for these costs are currently not available [6]. However, Ong et al. [35] revealed that the costs in the first and second year after index diagnosis differ substantially for patients who underwent kyphoplasty or vertebroplasty. Therefore, it would be interesting to clarify the long-term disease-related costs of vertebral fractures, which require further studies.
The statistical methods, particularly, the GLM regression, also have certain limitations. These apply in particular to the specification of the model by selecting family and link. As stated in the method section, we selected family and link of the model using different tests to ensure an appropriate model specification. Furthermore, we used the DID approach as a sensitivity analysis. Based on this approach, the OVCF disease-related costs are lower than those obtained from the GLM. The validity of the DID approach is based on the assumption that the underlying trends in the outcome variable (costs here) are the same for both treatment and control group. This assumption cannot be tested with only two observations. However, the contrast between the DID and GLM approach might be related to the differences in comorbidities between the treatment and the control group. The GLM approach accounts for these differences. The DID approach works only if the underlying trend if parallel over time. However, the differences in comorbidities could have influenced the costs of both groups differently, even if the fracture would have not occurred. Assuming that more comorbidities increase the costs disproportionately high over time, the differences in comorbidities can explain higher costs following the GLM analysis compared to the DID analysis. Besides, the question arises whether the exact matching approach used in this study is the best, or other approaches would have been better. It has been proved that exact matching is in many ways the ideal compared, e.g., to propensity score matching if most of the individuals can be matched [36]. Due to the very large dataset from which the comparison group was drawn, there were no problems to this regard.
Further limitations are related to the patient selection procedure. Patients were excluded if they did not have a minimum of 1 year continuous eligibility in the AOK plan before and after the index date. However, there are censored observations in our data and some of these did not meet inclusion requirements. Thus, the results could be biased by this approach. As stated in the results section, only marginal changes have resulted from the inclusion of censored patients. Thus, the cost difference between both groups is not sensitive to this aspect.
Conclusions
The results of this study demonstrate that osteoporotic vertebral fractures are associated with significant costs. Disease-related costs of OVCF in patients with newly diagnosed fracture in the first year after index add up to 6,490 € using German claims data. Against the backdrop of scarce budgets, the results underline the importance of medical interventions that can help to prevent fractures and treatments, which are cost-effective and can prevent recurrent fractures. They also warrant further research in order to identify patients with osteoporosis as early as possible, particularly postmenopausal women, to reduce the economic burden of disease.
References
(1991) Consensus development conference: prophylaxis and treatment of osteoporosis. The American Journal of Medicine 90:107–110. doi: 10.1016/0002-9343(91)90512-V
Häussler B, Gothe H, Göl D, Glaeske G, Pientka L, Felsenberg D (2007) Epidemiology, treatment and costs of osteoporosis in Germany—the BoneEVA Study. Osteoporos Int 18:77–84. doi:10.1007/s00198-006-0206-y
Becker S, Ogon M (2006) Balloon kyphoplasty. Springer, Wien
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA (2011) Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 22:1277–1288. doi:10.1007/s00198-011-1601-6
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jónsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 6:59–155. doi:10.1007/s11657-011-0060-1
Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jönsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. Arch Osteoporos 8. doi: 10.1007/s11657-013-0136-1
Lau E, Ong KL, Kurtz SM, Schmier J, Edidin AA (2008) Mortality following the diagnosis of a vertebral compression fracture in the Medicare population. J Bone Joint Surg Am 90:1479–1486
Dimai H, Redlich K, Peretz M, Borgström F, Siebert U, Mahlich J (2012) Economic burden of osteoporotic fractures in Austria. Health Econ Rev 2:12. doi:10.1186/2191-1991-2-12
Sedrine WB, Radican L, Raginster J (2001) On conducting burden-of-osteoporosis studies: a review of the core concepts and practical issues. A study carried out under the auspices of a WHO Collaborating Center. Rheumatology 40:7–14. doi:10.1093/rheumatology/40.1.7
Lange A, Kasperk C, Alvares L, Sauermann S, Braun S (2014) Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine 39:318–326. doi: 10.1097/BRS.0000000000000135
Kassenärztliche Bundesvereinigung (ed) (2008) Erweiterter Bewertungsausschuss nach § 87 Abs. 4 SGB V—7. Sitzung, 27./28. August 2008; Teil A zur erstmaligen Festlegung des Orientierungswertes nach § 87 Abs. 2e Satz 1 Nr. 1 SGB V für das Jahr 2009. 2008
Prenzler A, Zeidler J, Braun S, Schulenburg J (2010) Bewertung von Ressourcen im Gesundheitswesen aus der Perspektive der deutschen Sozialversicherung. Pharmacoeconomics Ger Res Art 8:47–66. doi:10.1007/BF03320765
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36:8–27
Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10. Med Care 43:1130–1139
van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ (2009) A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 47:626–633. doi:10.1097/MLR.0b013e31819432e5
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
Ho DE, Imai K, King G, Stuart EA (2006) Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal 15:199–236. doi:10.1093/pan/mpl013
Manning WG, Mullahy J (2001) Estimating log models: to transform or not to transform? J Health Econ 20:461–494. doi:10.1016/S0167-6296(01)00086-8
Pregibon D (1980) Goodness of link tests for generalized linear models. J R Stat Soc: Ser C: Appl Stat 29:15–24. doi:10.2307/2346405
Hosmer DW, Lemeshow S (2000) Applied logistic regression, 2nd edn. Wiley-Interscience, New York
Wedderburn RW (1974) Quasi-likelihood functions, generalized linear models, and the Gauss-Newton method. Biometrika 61:439–447. doi:10.2307/2334725
Manning WG (1998) The logged dependent variable, heteroscedasticity, and the retransformation problem. J Health Econ 17:283–295. doi:10.1016/S0167-6296(98)00025-3
Glick HA, Doshi JA, Sonnad SS, Polsky D (2007) Economic evaluation in clinical trials. Oxford Univ. Press, Oxford
Wooldridge JM (2010) Econometric analysis of cross section and panel data, 2nd edn. MIT Press, Cambridge
Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Melton LJ, Hammond CS (2002) Direct medical costs attributable to osteoporotic fractures. Osteoporos Int 13:323–330
Ray NF, Chan JK, Thamer M, Melton LJ (1997) Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res 12:24–35. doi:10.1359/jbmr.1997.12.1.24
de Laet CE, van Hout BA, Burger H, Weel AE, Hofman A, Pols HA (1999) Incremental cost of medical care after hip fracture and first vertebral fracture. The Rotterdam study. Osteoporos Int 10:66–72
Shi N, Foley K, Lenhart G, Badamgarav E (2009) Direct healthcare costs of hip, vertebral, and non-hip, non-vertebral fractures. Bone 45:1084–1090. doi:10.1016/j.bone.2009.07.086
Rousculp MD, Long SR, Wang S, Schoenfeld MJ, Meadows ES (2007) Economic burden of osteoporosis-related fractures in Medicaid. Value Health 10:144–152. doi:10.1111/j.1524-4733.2006.00161.x
Rovithis D (2013) Do health economic evaluations using observational data provide reliable assessment of treatment effects? Health Econ Rev 3:21. doi:10.1186/2191-1991-3-21
Rubin DB (1973) The use of matched sampling and regression adjustment to remove bias in observational studies. Biometrics 29:185–203. doi:10.2307/2529685
Borgström F (2006) Health economics of osteoporosis, Stockholm
Borgström F, Sobocki P, Strom O, Jonsson B (2007) The societal burden of osteoporosis in Sweden. Bone 40:1602–1609. doi:10.1016/j.bone.2007.02.027
Ong KL, Lau E, Kemner JE, Kurtz SM (2013) Two-year cost comparison of vertebroplasty and kyphoplasty for the treatment of vertebral compression fractures: are initial surgical costs misleading? Osteoporos Int 24:1437–1445. doi:10.1007/s00198-012-2100-0
Stuart EA (2010) Matching methods for causal inference: a review and a look forward. Stat Sci 25:1–21. doi:10.1214/09-STS313
Conflicts of interest
None.
The study was funded by an unrestricted grant from Medtronic International. Herescon GmbH has received payment from Medtronic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lange, A., Zeidler, J. & Braun, S. One-year disease-related health care costs of incident vertebral fractures in osteoporotic patients. Osteoporos Int 25, 2435–2443 (2014). https://doi.org/10.1007/s00198-014-2776-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2776-4