Abstract
Summary
Vitamin D is widely used in osteoporosis treatment, although the optimal dose is not known. This 1-year clinical study among 297 women aged 50–80 years old showed that a vitamin D3 dose of 6,500 IU/day was not better than the standard dose of 800 IU/day in improving bone mineral density (BMD) in the hip and spine.
Introduction
The purpose of this study was to determine whether a high dose of vitamin D3 was better than the standard dose in improving BMD and reducing bone turnover in postmenopausal women with reduced bone mass.
Methods
The study was a 1-year randomized double-blind controlled trial comparing high-dose vitamin D3 with the standard dose. Postmenopausal women (n = 297) with a BMD T-score ≤ −2.0 in either lumbar spine (L2–4) or total hip were included and randomized to 6,500 IU vitamin D3/day (20,000 IU twice per week + 800 IU/day) or 800 IU vitamin D3/day (placebo twice per week + 800 IU/day). Both groups were given 1,000 mg elemental calcium/day. The primary endpoint was a change in BMD in total hip and lumbar spine (L2–4).
Results
After 1 year, serum 25-hydroxyvitamin D (25(OH)D) increased [mean (SD)] from 71 (23) to 185 (34) nmol/l and from 71 (22) to 89 (17) nmol/l in the high- and standard-dose vitamin D groups, respectively. BMD at all measurement sites was unchanged or slightly improved with no significant differences between the groups. Although bone turnover was reduced in both groups, the more pronounced reduction in serum levels of the bone formation marker P1NP in the standard-dose group may indicate that this treatment was more efficient. Adverse events did not differ between the groups.
Conclusions
One year treatment with 6,500 IU vitamin D3/day was not better than 800 IU/day regarding BMD in vitamin D-replete postmenopausal women with reduced bone mass and was less efficient in reducing bone turnover.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin D deficiency, as defined by low serum levels of 25-hydroxyvitamin D (25(OH)D), may, in severe forms, lead to reduced intestinal calcium absorption and reduced mineralization of osteoid and, in less severe forms, to secondary hyperparathyroidism with resulting increased bone resorption [1]. There is also evidence for local autocrine and paracrine regulation of bone turnover by 1,25-dihydroxyvitamin D (1,25(OH)2D), which is independent of calcium absorption. Thus, both the enzyme CYP27B1 (1-alpha-hydroxylase), necessary for the formation of 1,25(OH)2D from 25(OH)D, and the vitamin D receptor are present in bone cells [2]. Recent studies suggest that locally produced 1,25(OH)2D is important both in osteoclast differentiation and concurrent downregulation of bone resorption, as well as in optimizing the communication and coupling mechanisms between osteoblasts and osteoclasts [3]. Locally produced 1,25(OH)2D is also important in osteoblast differentiation of human marrow stromal cells [4], and 1,25(OH)2D seems to affect osteoprotegerin secretion in mature osteoblasts [5]. The optimal serum levels of 25(OH)D for bone health is not established, although at least 75 nmol/l is suggested [6]. Concurrently, in a meta-analyses on vitamin D and fracture prevention, antifracture efficacy increased significantly with both higher received dose and higher achieved 25(OH)D levels [7].
Although supplementation with vitamin D together with calcium is an established part of osteoporosis treatment and prevention, the effect of higher doses of vitamin D has not been evaluated. Hypothetically, higher doses might facilitate high calcium absorption, suppress parathyroid hormone (PTH) secretion, and provide sufficient substrate for the local bone production of 1,25(OH)2D. We hypothesized that the doses used in osteoporosis treatment are suboptimal, and the aim of the present study was, therefore, to compare the effect of 1 year treatment with a high dose of 6,500 IU vitamin D3/day (20,000 IU twice/week + 800 IU/day) with a standard dose of 800 IU vitamin D3/day on the bone mineral density (BMD) and bone turnover markers in postmenopausal women with low BMD. The high dose was chosen to obtain high, although still safe, serum 25(OH)D levels [8]. The study was performed in Norway, where vitamin D fortification is restricted to products like margarine (8 mcg/100 g), butter (10 mcg/100 g), and semiskimmed milk (4 mcg/l).
Methods
Study participants
The study was registered at ClinicalTrials.gov (NCT00491920) and included postmenopausal women aged 50–80 years old with a T-score in total hip or lumbar spine (L2–4) ≤ −2.0. The participants should not have used hormone replacement therapy or other therapy affecting bone remodeling during the last 12 months before enrolment. Further exclusion criteria were use of steroids, renal stone disease, systolic blood pressure >175 mmHg or diastolic blood pressure >105 mmHg, serum creatinine >110 μmol/l, suspected primary hyperparathyroidism (serum calcium >2.55 mmol/l, serum calcium >2.50 mmol/l combined with plasma PTH >5.0 pmol/l, or serum calcium >2.45 mmol/l combined with plasma PTH >7.0 pmol/l), or chronic disease like ischemic heart disease, diabetes, granulomatous disease, and cancer.
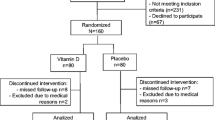
Participants were recruited from January 2007 until March 2009 in three different ways: from our outpatient clinic, through advertisement, and from other completed clinical studies where BMD was measured. These other studies included the 6th Tromsø Study (a population-based study), the NATTO Study (studying the effect of vitamin K on bone loss) [9], and the ACUFLASH Study (studying the effect of acupuncture on menopausal symptoms) [10]. At the least, a 1-month time interval lapsed between the final examination in the NATTO Study or ACUFLASH Study and the inclusion in the present study. No effect of vitamin K on bone loss was found in the NATTO Study [9], and evidence for an effect of acupuncture on bone loss is unconvincing [11]. Hence, we consider the possibility of interference with the results from the intervention in these studies to be negligible. The flow chart shows the recruitment of the participants (Fig. 1).
Protocol
Screening of possible participants included an interview, a dual-energy X-ray absorptiometry (DEXA) scan if not performed during the last 30 days, and a physical examination including blood samples. Subsequently, eligible participants came to a baseline visit at the Clinical Research Unit at the University Hospital of North Norway, where any previous supplements with calcium or vitamin D were discontinued. All participants were then given supplement of 500 mg of calcium and 400 IU of vitamin D3 taken twice daily (Calcigran Forte®, Nycomed, Norway) throughout the 1-year intervention period. Using block randomization with various block sizes and stratifying by smoking status (smoker/nonsmoker) and previous use of bisphosphonates (yes/no), the participants were randomized to either one capsule of vitamin D3 20,000 IU (Dekristol, Mibe, Brehna, Germany) (high-dose vitamin D group in the following) or identical-looking placebo capsules (standard-dose vitamin D group in the following) taken twice per week. In total, this constituted an average daily dose of 6,500 IU vitamin D3 in the high-dose vitamin D group and 800 IU vitamin D3 in the standard-dose vitamin D group. The randomization was performed by the central randomization unit at the University Hospital of North Norway, and the randomization numbers with treatment allocations were given directly to the hospital pharmacy who prepared the medication boxes, which were delivered to the study nurse at baseline for each participant. Thus, the study was a randomized double-blind controlled trial; neither the participants nor the staff performing the examinations nor the researchers knew the randomization status of the participants during the study.
Every third month, adverse events were registered, blood samples drawn, and the participants were supplied with new medication. The unused Calcigran Forte tablets and the capsules were returned and counted. For participants with a long traveling distance (n = 70), the screening and baseline visits were performed the same day. Adverse events were registered through telephone interviews at 3, 6 and 9 months for this group, while the blood samples were taken at the local health center and sent to the University Hospital of North Norway, securing analysis of all samples at the same laboratory. Every third month, the study medication was sent by mail to these participants, who kept all their medication boxes and returned them at the final 12-month visit.
Participants were asked to not use sun beds regularly during the study period, and if going on sunny holidays, they should stop taking their study medication while on vacation, but continue the Calcigran Forte intake. The study was approved by the Regional Committee for Medical Research Ethics, the Norwegian Data Inspectorate, and the Norwegian Medicines Agency. All the participants signed a written consent.
Measurements
Medical history, smoking status, and age at menopause were recorded at enrolment, as were use of sun bed, sunny holidays the previous 12 months, and time spent outdoor during the period of possible ultraviolet B-mediated vitamin D production in the skin at the study location (69° N) [12]. Hence, they were asked about the mean time spent outdoor between 10 AM and 3 PM in March–April and September and between 8 AM and 8 PM in June–August. A food frequency questionnaire [13] and a questionnaire on physical activity (International Physical Activity Questionnaire (IPAQ), short, last 7 days self-administered format) [14] were filled in at baseline, and the intakes of vitamin D and calcium were calculated. The amount of physical activity was calculated based on reported light, moderate, and vigorous activities and reported as units of metabolic equivalents (MET)-minutes per week, in accordance with the IPAQ guidelines. The participants were also classified according to the IPAQ groups inactive, minimally active, or health-enhancing physically active. Body height and weight were measured without shoes and with light clothing at baseline and 12 months, as were BMD of lumbar spine, dual hip, and total body. Body mass index (BMI) was calculated as weight (in kilograms) divided by the square of height (in meters).
A second-void fasting urine sample was obtained, as were nonfasting blood samples. Serum was stored in aliquots at −70°C for later analyses of serum 25(OH)D, 1,25(OH)2D, and bone turnover markers. Serum 25(OH)D was analyzed at the Hormone Laboratory, Haukeland University Hospital, using an in-house developed liquid chromatography double mass spectrometry method [15]. The within-day precision (coefficient of variation [CV]) was ≤3.1%, and the between-day precision (CV) was ≤8.7%.The laboratory takes part in the external quality program DEQAS. Bone turnover markers and 1,25(OH)2D were analyzed at the Hormone Laboratory, Oslo University Hospital. The bone resorption marker C-terminal telopeptide of type 1 collagen (CTX-1) was measured in serum by ELISA technique (IDS, Herlev, Denmark); the reference range for postmenopausal women was <1.35 mcg/l according to the producer. Total CV was ≤9%. The bone formation marker N-terminal propeptide of type 1 procollagen (P1NP) in serum was measured by radioimmunoassay (RIA; Orion Diagnostics, Espoo, Finland); the reference range for postmenopausal women was 16–96 mcg/l according to the producer. Total CV was ≤10%. Serum 1,25(OH)2D was measured by RIA (DiaSorin, Stillwater, MN, USA); the reference range was 42–169 pmol/l and the total CV was ≤17%.
The other analyses were performed continuously at the Department of Clinical Chemistry at the University Hospital of North Norway. Plasma PTH was measured using an automated clinical chemistry analyzer (Immulite 2000, Siemens Healthcare Diagnostics, Los Angeles, CA, USA), reference range 1.1–7.5 pmol/l, and serum calcium was analyzed using an automated analyzer (Hitachi 917) with reagents from Boehringer Mannheim, reference range 2.15–2.55 mmol/l. Serum creatinine was analyzed by an enzymatic colorimetric method (CREA plus, Roche Diagnostics) using an automated clinical chemistry analyzer (Modular P, Roche Diagnostics), reference range 50–90 μmol/l. Ionized calcium was analyzed by ion selectivity (ABL 800 Flex, Radiometer America Inc., Westlake, OH, USA), reference range 1.10–1.34 mmol/l, and phosphate was analyzed by photometric endpoint measurement (Modular P, Roche Diagnostics), reference range was 0.76–1.41 mmol/l when measured in plasma (samples taken at the hospital) and 0.85–1.50 mmol/l when measured in serum (mailed samples).
BMD
BMD was measured by DEXA (GE Lunar Prodigy, Lunar Corporation, Madison, WI, USA) at the following sites: dual hip, lumbar spine, and total body. According to the protocol, the primary endpoints were changes in BMD at the total hip and anterior–posterior L2–4 site, but effects on the femoral neck site and total body were also studied. The CV was <1.0% at both the total hip and the spine. The scanner was calibrated daily against the standard calibration block supplied by the manufacturer (aluminum spine phantom), and these measurements showed no drift throughout the study. We used the mean of the left and right hip measurements for analyses. For participants with metallic implant in one hip, the measurement from the other hip was used in the analyses.
Statistical analyses
Data were checked for normality using visual inspections of histograms and P–P plots. Skewed variables were log-transformed for relevant analyses. For between-group comparisons, Student’s t test or chi-square tests were used, while paired t tests were used to analyze changes from baseline to 12 months within each treatment group. The primary outcome was defined as change in BMD from baseline to 12 months. This was expressed both in absolute values and in percentage. These two were highly correlated (r > 0.99), and only the percentage change is presented here for simplicity. Multiple linear regression analyses, and Pearson (r) or Spearman rho (r s) correlation coefficients for normally and non-normally distributed variables, respectively, were used to assess the association between different variables (baseline values or change in values) and outcomes. In order to compare the results in this study with those from a recent study on vitamin D intake necessary to obtain a serum 25(OH)D concentration of above 75 nmol/l [16], we present the achieved serum 25(OH)D levels for the two treatment groups stratified by baseline serum 25(OH)D below or above 55 nmol/l.
For the primary endpoints, the analyses were performed as intention-to-treat analyses, where the last observation was carried forward for participants that did not complete the study. Adherence to the medication was calculated based on returned Calcigran Forte tablets and study medication. To study effects in subgroups, we predefined the following stratified analyses of interest: below or above the median of baseline serum 25(OH)D and PTH, age below or above 65 years, and with baseline T-score ≤−2.5 or >−2.5 (osteoporosis or normal/osteopenia) at total hip or L2–4. Interactions were tested between subgroups and treatment groups in general linear models. All statistical analyses were performed using the statistical software package SPSS 16.0, and p < 0.05 was considered a significant finding. The results are shown as the mean (SD), unless otherwise stated.
Power calculations
Power calculation prior to the study was based upon a within-group SD of 4% for BMD both at the hip and L2–4 measurement sites. If 400 subjects were included and 300 completed the study, this would give us a power of 90% to detect a difference of 1.5% between the treatment groups at a significance level of 0.05 [17]. During the study, we realized that 400 participants would be difficult to achieve during the available time frame, meanwhile, the dropout rate was lower than expected. Recalculation assuming the same within-group SD showed that a total sample size of 128 individuals in each group would be needed to have a power of 90% to obtain a clinical relevant difference of 1.6% at the total hip at a significance level of 0.05. Assuming a dropout rate of 15%, 300 participants in total would be needed.
Results
Baseline characteristics
Of 905 invited persons, 297 were included in the study (Fig. 1). Their baseline characteristics are shown in Tables 1, 2, and 3. Except for the 1,25(OH)2D levels, which were significantly lower in the standard-dose group, there were no significant differences between the treatment groups at baseline. Vitamin D supplementation (including cod liver oil) was used by 138 participants with a mean dose of 441 (228) IU/day, range 100–1,200 IU/day. Calcium supplementation was used by 86 participants, with a mean dose of 803 (265) mg/day, range 250–1,500 mg. Any kind of vitamin D and/or calcium supplementations were used by 149 participants (50%).
In multiple regression analyses, reported outdoor time, sunny holidays, sun bed use, and vitamin D supplementation were significantly associated with the baseline serum 25(OH)D levels, whereas age, smoking, physical activity, and estimated vitamin D intake were not. There was no correlation between serum 25(OH)D level and BMD at total hip or L2–4 at baseline.
Adherence
For the participants who completed the study, adherence was 97% for the study medication (capsules of vitamin D3 or placebo) and 92% for Calcigran Forte. No differences in adherence were observed between the treatment groups. Sunny holidays during the study were reported by similar numbers in the two treatment groups (61 in the high-dose group and 64 in the standard-dose group (p = 0.69, chi-square test)). Median length of these holidays were 2 weeks with no difference between the treatment groups (p = 0.73, Mann–Whitney U test).
BMD
Intention-to-treat analyses showed that, in both treatment groups and on all measurement sites, BMD was unchanged or slightly increased through the study year (Table 2). There were no statistically significant differences between the treatment groups at any measurement site. Per-protocol analyses in 135 and 140 participants in the high-dose and standard-dose vitamin D group, respectively, revealed similar results, as did analyses in the 148 participants (74 in each group) who did not use calcium and/or vitamin D supplementation at inclusion.
When participants were stratified according to baseline BMD status (baseline T-score ≤−2.5 or >−2.5) for total hip and L2–4, respectively, there were generally slightly better, though not statistically significant, results for the high-dose vitamin D group in the osteoporotic subgroups (Table 4). On the other hand, in the subgroups with baseline T-score > −2.5, there was a trend towards better effect of standard-dose vitamin D, statistically significant at the femoral neck in those with L2–4 T-score > −2.5. Test for interaction between baseline L2–4 BMD status and treatment was significant for the femoral neck (p = 0.03). Similarly, when stratified according to plasma PTH below or above the median (4.8 pmol/l), there were significant better results in the standard-dose vitamin D group at the total hip in the low-plasma PTH group (Table 4). However, interaction tests did not reach significance (p = 0.28). The results did not differ when stratified by baseline serum 25(OH)D below or above the median (69.7 nmol/l; Table 4) or below or above 55 nmol/l or by age (data not shown).
Laboratory measurements
Plasma PTH decreased, and serum ionized calcium, serum 25(OH)D, and urine calcium/creatinine ratio increased in both groups, but significantly more in the high-dose vitamin D group. Serum calcium increased marginally in the high-dose vitamin D group, as did 1,25(OH)2D, while a significant decrease in 1,25(OH)2D was seen in the standard-dose vitamin D group. Serum phosphate decreased in both groups (Table 3).
Of those who completed the study, 33 subjects in the high-dose group and 35 in the standard-dose group had serum levels of 25(OH)D <55 nmol/l at baseline. At 12 months, their serum 25(OH)D levels were 176 (36) and 81 (15) nmol/l, respectively. While all subjects in the high-dose group reached serum 25(OH)D levels above 75 nmol/l, 29% in the standard-dose group still had serum 25(OH)D levels <75 nmol/l after 12 months. Accordingly, for those with baseline serum 25(OH)D levels >55 nmol/l, the serum 25(OH)D levels after 12 months were 188 (33) and 92 (17) nmol/l in the high-dose (n = 101) and standard-dose (n = 104) groups, respectively. Thirteen percent of those in the standard-dose group did not reach serum 25(OH)D levels ≥75 nmol/l.
In both treatment groups, there was a significant decrease in the bone resorption marker CTX-1 and the bone formation marker P1NP. The reduction in P1NP was significantly more pronounced in the standard-dose vitamin D group (Table 3), which was found in per-protocol analyses as well as in intention-to-treat analyses. However, when the analyses were restricted to the 148 participants who did not use any vitamin D or calcium supplementation at baseline, the difference was no longer significant (−11.6 mcg/l in the high-dose group versus −14.2 mcg/l in the standard-dose group, p = 0.33). When all the completing participants were analyzed together, the change in CTX-1 was significantly inversely correlated with the change in BMD at the total hip and the femoral neck (r s = −0.24, p < 0.01 and r s = −0.12, p < 0.05, respectively), and the change in P1NP was significantly inversely correlated with the change in BMD at the total hip, femoral neck, and L2–4 (r s = −0.30, r s = −0.24, and r s = −0.17, respectively; p < 0.01). Subgroup analyses revealed much the same patterns in bone turnover markers as in BMD. Thus, the standard dose reduced serum P1NP more efficiently when baseline plasma PTH was below the median (p < 0.05), when baseline T-score was >−2.5 in hip (p < 0.05) and spine (p < 0.01), and also when age at inclusion was <65 years (p = 0.01). Meanwhile, serum CTX was more reduced in the high-dose group in hip osteoporosis (p < 0.01).
Adverse events
There was no significant difference in the number of adverse events in the two groups (Table 5). Fourteen subjects in the high-dose group did not complete the study (four participants withdrew consent, two had a transient ischemic attack, while the following affected one participant each: skin reactions, planned UV treatment, hypercalcemia (serum calcium 2.77 mmol/l), steroid treatment for reactive arthritis, gastrointestinal symptoms, dizziness with musculoskeletal pains, rheumatoid arthritis, and percutaneous coronary intervention (PCI) with suspected procedure-related urolithiasis). The number of noncompleters in the standard-dose group was eight (three withdrew consent, two had urticaria, while the following affected one participant each: unstable angina pectoris, leg cramps, and cancer). There were no severe hypercalcemias as defined as serum calcium >2.80 mmol/l, and the more modest hypercalcemias (serum calcium 2.60–2.80 mmol/l) occurred at serum 25(OH)D levels across a range of 64–256 nmol/l. According to the protocol, only participants experiencing severe hypercalcemia should be excluded; however, one participant with serum calcium 2.77 mmol/l (control value after 1 week was 2.39 mmol/l) was erroneously excluded. All the other participants continued in the study, and all the hypercalcemias had resolved at retesting without stopping the treatment.
Discussion
The results of this study suggest that, although apparently safe to use, there is no obvious benefit in 1 year treatment with a high dose (6,500 IU/day) of vitamin D supplementation to postmenopausal women with reduced BMD compared to a standard dose of 800 IU/day. Worth noticing was that, in certain subgroups, the standard dose of vitamin D was better than the high dose regarding changes in hip BMD. In addition, standard-dose treatment led to greater reduction in serum P1NP as a measure of bone turnover.
The strengths of this study were that it included an important and relevant population of postmenopausal women with low BMD, adherence was good, and retention in the study high. It was also adequately powered to detect small differences in effects between the treatment groups in the main analyses. The doses used were sufficient to give a substantial increase in serum 25(OH)D, as confirmed in serum measurements.
The study also had several limitations. Firstly, as we considered it ethically problematic, we had no group receiving neither calcium nor vitamin D. The annual reduction in BMD in postmenopausal untreated women is reported to be 0.39–3.2% in the lumbar spine [9, 18–20], 0.50–2.2% in the femoral neck [9, 18–20], and 0.35% in total hip [9]. In comparison, both the treatment regimes in this study led to no change or improvement in BMD in the main analyses. Secondly, the cohort was not treatment naive, as 50% of the participants already used calcium and/or vitamin D supplementation at baseline. As such, the study could be regarded as a maintenance treatment study. The high baseline intake of both vitamin D and calcium could have masked an effect of the intervention. However, analyses in subjects without baseline intake of such supplementation did not change the results.
Thirdly, as the follow-up time was 1 year, we cannot assess long-term effects or safety. Fourthly, although BMD is regarded as a useful surrogate marker of bone health, this has lately been questioned in patients receiving calcium with or without vitamin D supplementation [21], and the ideal endpoint would be fractures. This would need a substantially higher number of participants and also longer follow-up time. Next, although we did predefine the subgroups to be analyzed, the study was not powered for such analyses nor did we adjust for multiple comparisons. The results of the subgroup analyses must, therefore, be interpreted with caution. Finally, the vitamin D status in the included participants was higher than expected from the general Norwegian population [22, 23], as 40.6% had baseline serum 25(OH)D levels above 75 nmol/l, only 15.4% below 50 nmol/l, and 1% below 25 nmol/l. This might be explained by the high prevalence of vitamin D supplementation and sun-seeking activities like sunny holidays, sun bed use, and outdoor activities. It probably also reflected that a substantial proportion of the participants already were aware of their low BMD, and thereby undertaking preventive efforts like vitamin D supplementation [24]. Our study should, therefore, be viewed with caution when it comes to effects of high doses of vitamin D on BMD in vitamin D-deficient subjects with low BMD. Thus, a recent report showed that, in vitamin D-deficient nursing home residents, supplementation with vitamin D 5,000 IU/day and calcium 320 mg/day, which led to a mean serum 25(OH)D at 12 months of 125.6 (38.8) nmol/l, resulted in an impressive 23% increase in BMD at the hip [25]. However, this might reflect mineralization of osteoid due to vitamin D-deficient osteomalacia and not treatment of ordinary osteoporosis.
We did not measure serum 25(OH)D before including subjects in the study. According to a recent work, a vitamin D3 dose similar to the one used by us (5,000 IU/day for 6 months) was suggested to achieve a serum 25(OH)D concentration >75 nmol/l in those with serum 25(OH)D levels below 55 nmol/l and 3,800 IU/day if above that threshold [16]. However, our high dose of vitamin D resulted in much higher serum 25(OH)D levels, both for those below and above a baseline serum 25(OH)D of 55 nmol/l. Our results also demonstrated that 800 IU vitamin D/day is insufficient if a serum 25(OH)D level above 75 nmol/l is to be achieved in all subjects. Clinicians should, therefore, reassess serum 25(OH)D levels in patients supplemented with vitamin D in order to ensure that the target level is achieved.
Ideally, we should have included an intermediate dose of 2,000–4,000 IU vitamin D/day, as also suggested by Bishoff-Ferrari [6]. A daily dose of 4,000 IU vitamin D3 has been reported to reduce bone resorption markers and maintain formation markers in vitamin D-deficient postmenopausal women, while both markers increased in the placebo group [26]; unfortunately, BMD was not measured. In the present study, the bone turnover markers CTX-1 and P1NP were reduced in both treatment groups; however, P1NP was significantly more reduced in the standard-dose group. The reduction of both the formation and the resorption markers was associated with improvement in BMD, as a reflection of reduced bone turnover. This was confirmed in predefined subgroup analyses, adding external validity to the findings.
An increase in serum 1,25(OH)2D was observed in the high-dose vitamin D group. Although toxic doses of 1,25(OH)2D has long been known to induce bone resorption, physiological doses are believed to have the opposite effect by inhibiting PTH-induced bone resorption [27]. A recent report from Anderson et al. showed that serum 1,25(OH)2D levels were negatively correlated with bone CYP27B1 mRNA levels, indicating a reduction in autocrine and paracrine 1,25(OH)2D activity in bone cells when renal 1,25(OH)2D production is high [28]. This might, therefore, at least partly explain less effect of high-dose vitamin D on bone turnover reduction, although we found no correlation between change in serum 1,25(OH)2D and bone turnover markers (data not shown).
Vitamin D toxicity, with hypercalcemia leading to calcification of kidneys and blood vessels, is a frequently discussed problem, and the risk of kidney stones is increased with calcium and vitamin D therapy, as demonstrated in the Women’s Health Initiative [29]. One participant in the high-dose group had an angiography done as part of a cardiac evaluation of bradycardia (apparent before start of study). During a concomitant PCI procedure, she had sudden back pain and also hematuria. No kidney stones were seen in a CT taken thereafter, but the diagnosis cannot be excluded. In order to maintain safety, we measured serum calcium and creatinine every third month during the study. Increases in serum creatinine or serious or sustained hypercalcemias were not observed in any of the treatment groups.
Although several reviews conclude that vitamin D toxicity is not seen before serum 25(OH)D exceeds 500–750 nmol/l [8, 30, 31], the possibility that other adverse health effects may occur below this limit needs careful attention. Illustrating this point was the recently published report from a study where 500,000 IU of vitamin D3 or placebo were given annually for 3–5 years, and an increase in both falls and fractures were seen in the treated group [32]. The mechanisms for this observation is not clear, but might be related to the peaks and falls in serum 25(OH)D levels found with this dosing regime. Although the results from our study on postmenopausal women with reduced BMD cannot be extrapolated to the general population, our results combined with the results of Sanders et al. [32] underscore the need for evaluating possible long-term deleterious effects on skeletal health when using high doses of vitamin D.
In conclusion, combined with calcium 1,000 mg/day, treatment with 6,500 IU vitamin D/day is not better than a standard dose of 800 IU/day regarding BMD in postmenopausal women with reduced bone mass. This high dose may be inferior to the standard dose in subgroups with T-score > −2.5 or low serum PTH and seems less efficient in reducing bone turnover. Further long-term intervention studies with doses in between 800 and 6,500 IU/day are needed to decide the optimal vitamin D treatment of reduced bone mass in postmenopausal women.
References
Need AG, Nordin BE (2008) Misconceptions—vitamin D insufficiency causes malabsorption of calcium. Bone 42:1021–1024
Anderson PH, Atkins GJ (2008) The skeleton as an intracrine organ for vitamin D metabolism. Mol Aspects Med 29:397–406
Kogawa M, Findlay DM, Anderson PH, Ormsby R, Vincent C, Morris HA, Atkins GJ (2010) Osteoclastic metabolism of 25(OH)-vitamin D3: a potential mechanism for optimization of bone resorption. Endocrinology 151:4613–4625
Geng S, Zhou S, Glowacki J (2010) Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1-α-hydroxylase. J Bone Miner Res doi:10.1002/JBMR.298
Baldock PA, Thomas GP, Hodge JM, Baker SU, Dressel U, O’Loughlin PD, Nicholson GC, Briffa KH, Eisman JA, Gardiner EM (2006) Vitamin D action and regulation of bone remodeling: suppression of osteoclastogenesis by the mature osteoblast. J Bone Miner Res 21:1618–1626
Bischoff-Ferrari HA (2007) How to select the doses of vitamin D in the management of osteoporosis. Osteoporos Int 18:401–407
Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav J, Thoma A, Kiel DP, Henschkowski J (2009) Prevention of nonvertebral fractures with oral vitamin D and dose dependency. Arch Intern Med 169:551–561
Vieth R (2006) Critique of the considerations for establishing the tolerable upper intake level for vitamin D: critical need for revision upwards. J Nutr 136:1117–1122
Emaus N, Gjesdal CG, Almås B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fønnebø V (2010) Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteopor Int 21:1731–1740
Borud EK, Alraek T, White A, Fonnebo V, Eggen AE, Hammar M, Åstrand LL, Theodorsson E, Grimsgaard S (2009) The Acupuncture on Hot Flushes Among Menopausal Women (ACUFLASH) study, a randomized controlled trial. Menopause 16:484–493
Borrelli F, Ernst E (2010) Alternative and complementary therapies for the menopause. Maturitas 66:333–343
Engelsen O, Brustad M, Aksnes L, Lund E (2005) Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol 81:1287–1290
Andersen LF, Solvoll K, Johansson LR, Salminen I, Aro A, Drevon CA (1999) Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha-tocopherol in adipose tissue and serum. Am J Epidemiol 150:75–87
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395
Grimnes G, Almaas B, Eggen AE, Emaus N, Figenschau Y, Hopstock LA, Hutchinson MS, Methlie P, Mihailova A, Sneve M, Torjesen P, Wilsgaard T, Jorde R (2010) Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur J Endocrinol 163:339–348
Aloia JF, Patel M, DiMaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK (2008) Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87:1952–1958
Altmann DG (1991) Practical statistics for medical research. Chapman & Hall, Boca Raton
Young R, May H, Murphy S, Grey C, Compston JE (1996) Rates of bone loss in peri- and postmenopausal women: a 4 year, prospective, population-based study. Clin Sci 91:307–312
Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD (1998) A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int 8:282–290
Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D’Anna R, Cannata ML, Corrado F, Adamo EB, Wilson S, Squadrito F (2007) Effects of phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women. Ann Intern Med 146:839–847
Rabenda V, Bruyére O, Reginster JY (2011) Relationship between bone mineral density changes and risk of fractures among patients receiving calcium with or without vitamin D supplementation: a meta-regression. Osteoporos Int 22:893–901
Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G (2010) Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol 171:903–908
Christensen MH, Lien EA, Hustad S, Almås B (2010) Seasonal and age-related differences in serum 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D and parathyroid hormone in patients from Western Norway. Scand J Clin Lab Invest 70:281–286
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221
Mocanu V, Stitt PA, Costan AR, Voroniuc O, Zbranca E, Luca V, Vieth R (2009) Long-term effects of giving nursing home residents bread fortified with 125 mcg (5000 IU) vitamin D3 per daily serving. Am J Clin Nutr 89:1132–1137
Von Hurst PR, Stonehouse W, Kruger MC, Coad J (2010) Vitamin D supplementation suppresses age-induced bone turnover in older women who are vitamin D deficient. J Steroid Biochem Mol Biol 121:293–296
Ueno Y, Shinki T, Nagai Y, Murayama H, Fujii K, Suda T (2003) In vivo administration of 1,25-dihydroxyvitamin D3 suppresses the expression of RANKL mRNA in bone of thyroparathyroidectomized rats constantly infused with PTH. J Cell Biochem 90:267–277
Anderson PA, Iida S, Tyson JH, Turner AG, Morris HA (2010) Bone CYP27B1 gene expression is increased with high dietary calcium and in mineralising osteoblasts. J Steroid Biochem Mol Biol 121:71–75
Jackson RD, LaCroix AZ, Gass M et al (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683
Jones G (2008) Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 88:582S–586S
Heaney RP (2008) Vitamin D—criteria for safety and efficacy. Nutr Rev 66:S178–S181
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010) Annual high-dose oral vitamin D and falls and fractures in older women. JAMA 303:1815–1822
Acknowledgements
The study was funded by a grant from The Norwegians Women Public Health Association in Troms. The superb assistance by the staff at the Research Unit, from Marian Remijn and Line Wilsgaard at the DEXA laboratory, Inger Myrnes and Astrid Lindvall at the Department of Medical Biochemistry at the University Hospital of North Norway, Otto Bårholm at the Hormone Laboratory, Haukeland University Hospital, and Anikken Kristiansen and Nina Norstrand at the Hormone Laboratory, Oslo University Hospital is greatly appreciated. We are grateful for the generous supply of Calcigran Forte from Nycomed Norway.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grimnes, G., Joakimsen, R., Figenschau, Y. et al. The effect of high-dose vitamin D on bone mineral density and bone turnover markers in postmenopausal women with low bone mass—a randomized controlled 1-year trial. Osteoporos Int 23, 201–211 (2012). https://doi.org/10.1007/s00198-011-1752-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1752-5