Abstract
Summary
Vitamin K2 may preserve bone strength and reduce fracture risk. In this randomised double-blind placebo-controlled trial among healthy postmenopausal Norwegian women, 1 year supplementation of vitamin K2 in the form of Natto capsules had no effect on bone loss rates.
Introduction
Japanese studies indicate that vitamin K2 (menaquinone-7 (MK-7)) intake may preserve bone strength, but this has not been documented in Europeans. The aim of this study was to assess the effect of MK-7 on bone mineral density (BMD) changes in postmenopausal Norwegian women.
Methods
Three hundred thirty-four healthy women between 50 and 60 years, 1–5 years after menopause, were recruited to a randomised double-blind placebo-controlled trial. The participants were randomly assigned into two groups, one receiving 360 µg MK-7 in the form of Natto capsules and the other the same amount of identical-looking placebo capsules containing olive oil. BMD was measured at total hip, femoral neck, lumbar spine and total body at baseline and 12 months together with serum levels of bone-specific alkaline phosphatase, Crosslaps, total osteocalcin (N-mid OC), carboxylated (cOC) and under-carboxylated osteocalcin (ucOC).
Results
After 12 months, there were no statistical differences in bone loss rates between the groups at the total hip or any other measurement site. Serum levels of cOC increased and ucOC decreased in the treatment versus the placebo group (p < 0.001).
Conclusion
MK-7 taken as Natto over 1 year reduced serum levels of ucOC but did not influence bone loss rates in early menopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fracture incidence is varying worldwide. The USA and the Scandinavian countries stand out as having the highest incidences of osteoporotic fractures [1]. Fracture risk is multifactorial and complex [2], but the diagnosis of osteoporosis is based on an assessment of skeletal mass per unit area, defined as bone mineral density (BMD) [3]. A strong relationship between BMD and fracture risk has been documented [4]. During the menopausal transition, most women experience a 1–5-year period of rapid bone loss as a result of the changing hormonal environment [5–7]. This loss of bone mass leads to decreased structural strength and increased risk of fracture [8]. An efficient prevention strategy could give individual health benefits as well as socioeconomic savings.
Low intake of vitamin K is associated with low BMD [9] and increased risk of fracture [10]. Vitamin K promotes post-translational conversion of protein glutamate residues in osteocalcin into gamma-carboxy glutamate (Gla) [11, 12], which is secreted by the osteoblasts. Vitamin K deficiency will cause production of under-carboxylated osteocalcin (ucOC) [13], and ucOC levels are therefore considered to be a sensitive measure of vitamin K status [14]. ucOC is furthermore inversely correlated with hip BMD [15] and regarded as a marker of hip fracture risk in elderly women [13, 16]. Both vitamin K1 (phylloquinone or phytonadione) and K2 (menaquinone) contribute to the vitamin K status in humans [12] (Table 1). Vitamin K2 is a spectrum of multiple forms where menaquinone-4 (MK-4) and menaquinone-7 (MK-7) are reportedly related to bone mass and fracture risk [11, 12]. A systematic review from 2006 indicated that supplementation with phytonadione and MK-4 may reduce bone loss and fracture risk [17]. However, most of the included studies were undertaken in Japan, and heterogeneity was high and quality low in several of the trials [17]. In a 3-year study, daily supplementation of phylloquinone significantly decreased serum ucOC in elderly US women and men without any effect on lumbar or total-body BMD compared to the placebo group [18], and in a recently published study, 12 months treatment with phylloquinone or MK-4 also reduced serum ucOC without any effect on lumbar or proximal femur BMD in postmenopausal US women [19].
Although there are studies suggesting a positive effect on bone formation, the effectiveness of MK-7 is far less studied [20, 21]. In the European diet, different fermented cheeses contain large amounts of MK-7 (62 µ/100 g). Even larger amounts (1,100 µ/100 g) are found in the traditional Japanese dish called Natto, which is prepared on soybeans fermented with Bacillus subtilis [12]. Ecological studies report lower occurrence of fractures in Japanese regions with a high population intake of Natto [22]. Cross-sectional studies indicate that Natto may prevent osteoporosis in premenopausal Japanese women [23]. With Natto’s possible but still undocumented effect on bone [24], the aim of this 12-month double-blind placebo-controlled randomised trial was to assess if dietary supplementation of MK-7 taken in the form of Natto capsules would influence bone loss and bone formation markers in early menopausal Norwegian women, with or without adverse effects.
Materials and methods
The study participants
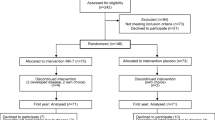
The study, registered with ClinicalTrials.gov (NCT00290212), was conducted in collaboration between the National Research Centre in Complementary and Alternative Medicine (NAFKAM), the University Hospital of North Norway (UNN), Tromsø (Centre 1) and Haukeland University Hospital, Bergen (Centre 2). Four hundred fifty-five healthy women were recruited through newspaper and media advertisement and assessed for eligibility by telephone interview. Eligible participants were included for randomization from February to November 2006, if they had turned 50 but not 61 years by 31 December 2005, were between 1 and 5 years since last menstruation, not using warfarin, hormone replacement therapy (HRT) or other medication influencing bone remodelling. On entry, the participants filled in a questionnaire on lifestyle variables. BMD was measured, and two non-fasting blood samples drawn. Follow-up visits with a new questionnaire, blood sampling and BMD measurements were scheduled at 6 and 12 months. At follow-up, fractures or events, such as being bed ridden, using crutches for more than 2 weeks or possible adverse effects were registered. Adherence was judged either by capsule counts or participants estimating the number of unused capsules. A form to fill in with a prepaid envelope was provided for written reports of any possible adverse effects between visits. After each examination, participants were informed about their BMD status. Participants with T-scores below −2.5 at any site, which is within the osteoporotic range [25], were allowed to participate. However, at the end of the study, all participants with T-scores below −2.0 at the total hip or lumbar spine were offered medical follow-up at the respective centres. Written informed consent was obtained prior to randomization. The Regional Committee of Research Ethics, the Norwegian Data Inspectorate and the Norwegian Directorate of Health and Social Services approved the study and the biobank establishment.
Study medication and randomisation procedure
The treatment and placebo capsules were produced by NATTOKIN Co., Ltd., Kanagawa 220-0061, Japan, to be indistinguishable by colour and size. The treatment capsule extract contained 85 mg powder of Natto’s viscosity extract, 25 mg soy bean isoflavones extract, 56 mg evening prim rose oil, 17 mg bees wax and 17 mg glycerol esters of fatty acids, all per 200 mg. The placebo capsules contained olive oil. The MK-7 content of the capsules were analysed and confirmed by Vita K, Maastricht. They were provided at no cost by NATURAL with a material transfer agreement signed by NATURAL and NAFKAM before the start of the study. A dosage study performed at NAFKAM in 2005 indicated that daily supplement of 360 µg of MK-7 in form of Natto capsules would be sufficient for Norwegian women to achieve plasma levels of MK-7 comparable to levels in similarly aged Japanese women [26]. The treatment group was therefore assigned a daily 360-µg MK-7 dosage in four 90-µg capsules. Recommended daily intake was two capsules twice with meals, but all four capsules could be taken at one preferred time. The study medication was labelled by a research assistant not involved in the study. The central randomization unit at UNN generated the block randomization sequence by computer (random block size). Each centre received blocks of study medication and assigned it to participants in numerical order. All researchers and participants were blinded throughout the study. The randomization code was broken after analyses of BMD changes.
BMD measurements
BMD was measured by dual X-ray absorptiometry (DXA; GE Lunar Prodigy, LUNAR Corporation, Madison, WI, USA) at the following sites: total hip, femoral neck, lumbar spine (L2-L4) and total body. According to the protocol, the total hip measurement was the primary endpoint. Through the national collaboration, Norwegian Epidemiological Osteoporosis Studies (NOREPOS), the densitometers were calibrated in vitro and in vivo before the start of the study, and no differences were detected. The in vivo coefficient of variation (CV%) for the total hip was 1.14% and 0.82% in Centre 1 and 2, respectively [27]. Scanners were calibrated daily against the standard calibration block supplied by the manufacturer (aluminium spine phantom). These phantom measurements showed no drift throughout the study. The measurements were performed according to the same protocol, and all scans were reviewed by one specially trained technician.
Biochemical measurements
The blood samples were centrifuged at 4°C and frozen until analysis at the Hormone Laboratory at Centre 2. The assays used were enzyme-linked immunosorbent assays. Serum osteocalcin (N-mid OC), intact OC and the N-terminal mid-region fragment of OC, and Crosslaps (CL), which measures the degradation product of C-terminal telopeptides of Type-I collagen, were measured by assays from Nordic Bioscience Diagnostics, Herlev, Denmark. The bone-specific alkaline phosphatase (BAP) assay was from Quidel Corporation, San Diego, CA, USA. The carboxylated osteocalcin (cOC) and ucOC assays were obtained from TaKaRa Bio. Inc., Japan. The mean sample pair variation was 4.1%, 12.8%, 5.0%, 4.1%, and 8.4% for N-mid OC, CL, BAP, cOC and ucOC, respectively. Inter-assay CVs were 9.2% (mean value, 17.0 ng/l) and 5.5% (mean value, 43.9 ng/l) for N-mid OC, 23.5% (mean value, 0.38 ng/l) and 8.6% (mean value, 1.11 ng/l) for CL, 7.0% (mean value, 11.5 µ/L) and 2.6% (mean value, 45.3 µ/L) for BAP, and 20% (mean value, 1.33 ng/ml) and 5.67% (mean value, 6.47 ng/ml; manufacturer’s data) for ucOC and 23% (mean value, 2.91 ng/ml) and 1.0% (mean value, 12.1 ng/ml; manufacturer’s data) for cOC.
Study power and sample size
The study was powered for a comparison of mean changes in BMD from baseline to 12-month follow-up. The sample size calculation was based on BMD change data from the Tromsø Study [28]. Women, not using HRT, had the highest bone loss in the time period 1–5 years after menopause, with a mean annual bone loss of 1.1% at the distal forearm. We defined the minimum clinically significant difference between groups to be 0.3 percentage points, representing 1 year bone loss “saved” per 4 years. Assuming 1.1% and 0.8% loss of BMD, with a standard deviation of 0.93%, in the control and treatment groups, respectively, a power of 80% and an alpha of 5%, 152 participants were needed in each group. Assuming a 10% dropout rate, 167 participants were needed in each group, 334 participants in total.
Data preparations and statistics
We categorised smoking status as never, former or present smoking and alcohol intake into low (few times a year), moderate (once a month to once a week) and high (more than once a week). Physical activity was derived from two questions on light and hard activity and combined into a score with three alternatives: low, moderate or high physical activity level. Differences in baseline characteristics between the groups and the centres were assessed by chi-square tests (categorical variables) and independent sample t test (continuous variables). Loss of follow-up, adverse events, self-reported health status and adherence with treatment (yes or no) were compared with chi-square tests. From capsule counts, adherence with treatment was also calculated as mean percentage and tested by independent sample t test.
Change in BMD was calculated as absolute change in g/cm2 (BMD 12 months − BMD baseline) and as percentage change (BMD 12 months − BMD baseline/BMD baseline). BMD levels at baseline and changes in BMD between the groups were compared with independent sample t test. We also compared BMD changes between the groups with participants stratified into two groups on the basis of baseline total hip T-scores: low (T-score < −1.0) and high (T-score > −1.0) BMD groups, respectively. We tested BMD changes between the groups with multiple regression analyses adjusting for age and physical activity level (which differed between the two groups at baseline), using low activity as the reference level. We also added study centre and adherence to treatment to the model, adherence using two options and self-reported or mean percentage adherence. At all sites, the percentage of participants with declining BMD during the study was compared using chi-square tests. Similarly, the least significant change was calculated for each study centre (2.77 × CV%) [29] at the total hip, and the percentage of participants with significant loss in the two groups was compared using chi-square tests.
We tested differences in serum concentration of biochemical measures (BAP, CL, N-mid OC, cOC and ucOC) between the groups at baseline and at 12 months using independent sample t test. Differences in changes in serum concentration were calculated as absolute changes (the measured value at 12 months − baseline value), and comparison of changes between groups was tested by independent sample t test. Two-sided p values below 0.05 were considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
Compliance
The study profile is displayed in Fig. 1. Reasons for discontinuation in 14 participants in each group (8.4%) were various diseases, relocation, lack of motivation or experienced adverse effects (three participants in each group). In the placebo group, four participants started treatment known to affect bone remodelling. Two hundred sixty-four participants (86%) reported to take the study medication as expected, 131 and 133 participants in the treatment and placebo groups, respectively (p = 0.68). Mean adherence rates calculated by capsule counts in 279 participants were 94.4% and 94.9% in the treatment and placebo groups, respectively (p = 0.55).
Adverse events
During the course of the study, five participants in each group sustained a fracture. In the treatment and placebo groups, 40 and 36 participants, respectively, reported events which included forgetting study medication during holidays or reduced weight bearing more than 2 weeks (p = 0.60). In the treatment group, there were two written reports: increased nocturnal hot flushes and abdominal pain. In the placebo group, there were four written reports: on muscular pain, general unwell feeling and two reports on itching. On phone, one participant in the treatment group reported increased palpitations, which ceased at study end. Self-perceived health was reported similarly in the groups at the start of the study (p = 0.69) and by 12 months (p = 0.71).
Effect of intervention on BMD changes
Participants’ baseline characteristics were similar, except for 0.5 years higher age (p = 0.04) and physical activity level (p = 0.04) in the treatment group (Table 2). The mean difference in bone loss rates between the treatment and the placebo group was −0.001 g/cm2 (95% CI, −0.005, 0.003 g/cm2) at the total hip, 0.001 g/cm2 (95% CI, −0.004, 0.005 g/cm2) at the femoral neck, 0.000 g/cm2 (95% CI, −0.008, 0.008 g/cm2) at the lumbar spine and 0.001 g/cm2 (95% CI, −0.003, 0.006 g/cm2) at the total body. There were no statistically significant differences in BMD changes between the groups at any measurement site (Table 3). The p values remained virtually unchanged when the four subjects in the placebo group, who started other treatment, were excluded from the analyses. Adjusting for age and physical activity level did not change the results, nor did adjustment for adherence to treatment. Participants from Centre 1 had more frequent alcohol intake (p = 0.03), but adjusting for study centre in the multiple regression model did not change the results.
Excluding those lost to follow-up or starting other treatment, 123 and 179 (of 141 and 193, respectively) remained in the low and high BMD groups. There were no statistically significant differences in BMD changes between the groups at any measurement site. Dividing the participants according to adherence to treatment did not change the results. Using bone loss rates calculated on the basis of two available measurements at baseline and 12 months at the total hip and femoral neck for participants in Centre 1 did not have any impact on the results. The percentage of women with declining BMD did not differ between the groups at any site, nor did the percentage of women with a significant bone loss differ (data not shown).
Effect of intervention on biochemical measurements
There were no statistically significant differences between the groups in biochemical measurements at baseline. After 12 months, N-mid OC, cOC and ucOC levels differed significantly between the groups (Table 4). At 12 months, BAP was unchanged in the treatment group and had declined in the placebo group, but the changes were not significantly different between the treatment groups. Crosslaps declined in the treatment group and remained unchanged in the placebo group, but the changes were not significantly different between the groups. N-mid OC declined in both groups, −3.1 ng/l (95% CI, −5.1, −1.2) more in the treatment group (p < 001). cOC increased in both groups, 3.8 ng/ml (95% CI, 2.6, 5.1) more in the treatment group (p < 0.001). ucOC declined in both groups, −1.8 ng/ml (95% CI, −2.4, −1.2) more in the treatment group (p < 0.001). Excluding from analyses, four participants on other treatment had only minor influence on the results.
Discussion
There were no statistically significant differences in bone loss at any measurement site in the treatment compared to the placebo group, in early menopausal women between 50 and 60 years in this randomised double-blind placebo-controlled trial. The concentration of cOC increased and ucOC decreased in the treatment group, both significantly more in the treatment group.
The attrition rate was low, and adherence to treatment was high, corresponding to 2.8 and 2.6 weeks without study medication in the treatment and placebo groups respectively, as calculated in 91% of the participants. There are however limitations to the study, the most important being the short follow-up of only 1 year. Although the error of the BMD measurement is low (approximately 1%), the study aims to measure small changes in BMD occurring after only 1 year of follow-up. To minimise the effect of the measurement error, bone loss was also calculated using the mean of two measurements from Centre 1 participants. The results were however unchanged, and there were no significant difference in the percentage of women who experienced a significant bone loss during the follow-up. We calculated that a mean annual loss of 1.1% with a standard deviation of 0.9% [28] would be a conservative estimate for the annual loss in this age group [5, 6]. A mean loss of 0.38% at the total hip in all participants was less than expected [5, 6, 30, 31]. This could be explained by participants being healthy volunteers, with high physical activity level, only 16% were smoking and 82% reported good or very good health, all factors beneficial for bone health [32–34]. An extended follow-up of altogether 24 months would have increased the mean bone loss rates in both groups. The point estimate of bone loss in our study, however, actually suggests a higher bone loss at the primary end point in the Natto compared to the placebo group. It is implausible that additional 12 months study duration suddenly should reverse the trend of higher bone loss compared to the placebo controls.
Another possible limitation is the use of DXA technology. The changes in ucOC levels may induce changes in structural properties of bone not detected by DXA technology [8]. Alternative technology was however not available, and in correspondence with the WHO definition [3], the clinical fracture risk evaluation in most centres include BMD measurements by DXA. The lack of fracture endpoint may be considered as another limitation of the study. However, fracture risk in the age group between 50 and 60 years is still low [35], and BMD is a good surrogate measure of bone strength, predicting 60–70% of its variation [36]. Although recent meta-analyses have questioned the role of calcium [37] and vitamin D [38] intake for fracture prevention in younger adults, the lack of calcium and vitamin D supplementation in each arm is a limitation [39] of the study. A positive interaction between vitamins K and D3 is suggested [40–43], but with the current study design, we were not able to assess this possible effect.
Several studies have indicated an effect of vitamin K2/MK-7 on bone [11, 21, 23, 44], but we have identified only one small clinical trial evaluating the potential effect. In one Japanese study including 73 premenopausal women, BAP increased and ucOC decreased in the group taking vitamin K2 in the form of Natto for lunch 1 year, compared with the no intake group, but there was no statistical difference in stiffness index between the groups, as measured by quantitative ultrasound [20]. Despite differences in design and endpoint measure, these results are comparable to our study.
The association between vitamin K2/MK-4 and BMD has been studied in several trials. Most of the studies indicating a positive effect are on patients with different chronic diseases [45–49], in women with established osteoporosis [40, 50–52], and in studies combining MK-4 and vitamin D3 [40]. In all these studies, the daily dosage is 45 mg of MK-4. Because of differences in side chin length, the effect of MK-7 and MK-4 on bone may not be comparable [12, 53]. With differences in study population and treatment dosage, the MK-4 studies are therefore not easily comparable with ours. However, in a 1-year study on Japanese postmenopausal women [54], BMD decrease was suppressed, but not significantly different from controls. In a 3-year study on Dutch postmenopausal women [55], the authors reported maintenance of calculated bone strength indices. In two 48-week Japanese studies on menopausal women taking 45 mg MK-4 daily, ucOC decreased without any effect on lumbar BMD [56]. Reduced bone loss was only measured in the study group treated both with MK-4 and D3 [57].
Similar results are observed in trials assessing the association between vitamin K1 and bone mass. In a 3-year trial including 474 elderly US men and women [18], 500-µg vitamin K1 taken daily resulted in significantly lower ucOC levels in the treatment group, but no observed effect on BMD [18]. In a recently published study including 381 postmenopausal US women mean age 62.5 years, neither phylloquinone (1 mg) nor MK-4 (45 mg) daily treatment for 12 months had any effect on lumbar spine or proximal femur BMD, although serum ucOC levels declined significantly compared to placebo group [19]. The bone loss rates and lack of observable differences in loss rates between the groups are similar to the results from our study and stand in contrast to the systematic review from 2006 [17]. Possible discrepancies may be explained by the different dosages. Japanese studies may also reflect unique dietary, environmental and/or genetic factors favouring positive association between vitamin K1 and K2 and bone mass in the participants [18]. However, data from a Japanese trial involving 3,000 patients did not indicate any effect of 36 months daily supplementation of 45 mg MK-4 on vertebral fracture risk [58]. These results might have changed the outcome of the systematic review had the data been available for inclusion [59]. Additionally, in a large observational study, there was no association between intake of vitamin K1 and lumbar and femoral neck BMD in peri- and early postmenopausal Danish women [60].
With increasing age, a larger proportion of women use natural health products also targeted for specific diseases such as osteoporosis [61]. The efficacy and safety of many of these products are poorly documented. The results from our study support the assertion that MK-7 taken in the form of Natto capsules has few adverse side effects [61]. Despite limited evidence concerning its effect, MK-7 or vitamin K2 taken as Natto is widely promoted in Europe as an effective dietary supplementation for prevention of osteoporosis in daily dosages between 90 and 180 µg. In this double-blind placebo-controlled randomised trial, 1 year intake of MK-7, taken in the form of 360 µg Natto capsules, did not produce any significant adverse effects in early menopausal women aged between 50 and 60 years. Serum levels of ucOC decreased and cOC increased significantly in the treatment group, indicating that the participants took the medication, and that the medication was absorbed and active, but as in other recent trials [18, 19, 43], BMD was not maintained despite improvement of OC carboxylation. The results indicate that that changes in serum cOC and ucOC levels do not influence determinants of bone strength measured as BMD over a period of 1 year. The possible effects other health measures still needs to be established.
References
Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17:1237–1244
Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N (2005) Assessment of fracture risk. Osteoporos Int 16:581–589
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ III, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
Chapurlat RD, Gamero P, Sornay-Rendu E, Arlot ME, Claustrat B, Delmas PD (2000) Longitudinal study of bone loss in pre- and perimenopausal women: evidence for bone loss in perimenopausal women. Osteoporos Int 11:493–498
Guthrie JR, Ebeling PR, Hopper JL, Barrett-Connor E, Dennerstein L, Dudley EC, Burger HG, Wark JD (1998) A prospective study of bone loss in menopausal Australian-born women. Osteoporos Int 8:282–290
Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, Jannausch M (1998) Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res 13:1134–1140
Seeman E (2003) The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am 32:25–38
Booth SL, Broe KE, Gagnon DR, Tucker KL, Hannan MT, McLean RR, wson-Hughes B, Wilson PW, Cupples LA, Kiel DP (2003) Vitamin K intake and bone mineral density in women and men. Am J Clin Nutr 77:512–516
Feskanich D, Weber P, Willett WC, Rockett H, Booth SL, Colditz GA (1999) Vitamin K intake and hip fractures in women: a prospective study. Am J Clin Nutr 69:74–79
Katsuyama H, Otsuki T, Tomita M, Fukunaga M, Fukunaga T, Suzuki N, Saijoh K, Fushimi S, Sunami S (2005) Menaquinone-7 regulates the expressions of osteocalcin, OPG, RANKL and RANK in osteoblastic MC3T3E1 cells. Int J Mol Med 15:231–236
Vermeer C, Braam L (2001) Role of K vitamins in the regulation of tissue calcification. J Bone Miner Metab 19:201–206
Vergnaud P, Garnero P, Meunier PJ, Breart G, Kamihagi K, Delmas PD (1997) Undercarboxylated osteocalcin measured with a specific immunoassay predicts hip fracture in elderly women: the EPIDOS Study. J Clin Endocrinol Metab 82:719–724
Gundberg CM, Nieman SD, Abrams S, Rosen H (1998) Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 83:3258–3266
Szulc P, Arlot M, Chapuy MC, Duboeuf F, Meunier PJ, Delmas PD (1994) Serum undercarboxylated osteocalcin correlates with hip bone mineral density in elderly women. J Bone Miner Res 9:1591–1595
Szulc P, Chapuy MC, Meunier PJ, Delmas PD (1993) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 91:1769–1774
Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ (2006) Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 166:1256–1261
Booth SL, Dallal G, Shea MK, Gundberg C, Peterson JW, wson-Hughes B (2008) Effect of vitamin K supplementation on bone loss in elderly men and women. J Clin Endocrinol Metab 93:1217–1223
Binkley N, Harke J, Krueger D, Engelke J, Vallarta-Ast N, Gemar D, Checovich M, Chappell R, Suttie J (2009) Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density or geometry in healthy postmenopausal North American women. J Bone Miner Res 24:983–991
Katsuyama H, Ideguchi S, Fukunaga M, Fukunaga T, Saijoh K, Sunami S (2004) Promotion of bone formation by fermented soybean (Natto) intake in premenopausal women. J Nutr Sci Vitaminol (Tokyo) 50:114–120
Yamaguchi M, Sugimoto E, Hachiya S (2001) Stimulatory effect of menaquinone-7 (vitamin K2) on osteoblastic bone formation in vitro. Mol Cell Biochem 223:131–137
Kaneki M, Hedges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Y, Mizuno Y, Hoshino S, Miyao M, Inoue S, Horiki K, Shiraki M, Ouchi Y, Orimo H (2001) Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition 17:315–321
Katsuyama H, Ideguchi S, Fukunaga M, Saijoh K, Sunami S (2002) Usual dietary intake of fermented soybeans (Natto) is associated with bone mineral density in premenopausal women. J Nutr Sci Vitaminol (Tokyo) 48:207–215
Bonjour JP, Gueguen L, Palacios C, Shearer MJ, Weaver CM (2009) Minerals and vitamins in bone health: the potential value of dietary enhancement. Br J Nutr 101:1581–1596
Kanis JA, Gluer CC (2000) An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int 11:192–202
Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K, Okano T (2006) Vitamin K status of healthy Japanese women: age-related vitamin K requirement for gamma-carboxylation of osteocalcin. Am J Clin Nutr 83:380–386
Omsland TK, Emaus N, Gjesdal CG, Falch JA, Tell GS, Forsen L, Berntsen GK, Meyer HE (2008) In vivo and in vitro comparison of densitometers in the NOREPOS study. J Clin Densitom 11:276–282
Emaus N, Berntsen GK, Joakimsen R, Fonnebo V (2006) Longitudinal changes in forearm bone mineral density in women and men aged 45–84 years: the Tromso Study, a population-based study. Am J Epidemiol 163:441–449
Shepherd JA, Lu Y (2007) A generalized least significant change for individuals measured on different DXA systems. J Clin Densitom 10:249–258
Hansen MA, Overgaard K, Christiansen C (1995) Spontaneous postmenopausal bone loss in different skeletal areas—followed up for 15 years. J Bone Miner Res 10:205–210
Melton LJ III, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL (2000) Determinants of bone loss from the femoral neck in women of different ages. J Bone Miner Res 15:24–31
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Law MR, Hackshaw AK (1997) A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major effect. BMJ 315:841–846
Shields M, Shooshtari S (2001) Determinants of self-perceived health. Health Rep 13:35–52
Kanis JA, Johnell O, Oden A, De Laet C, Jonsson B, Dawson A (2002) Ten-year risk of osteoporotic fracture and the effect of risk factors on screening strategies. Bone 30:251–258
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14(Suppl 3):S13–S18
Bischoff-Ferrari HA, wson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Specker B, Orav JE, Wong JB, Staehelin HB, O’Reilly E, Kiel DP, Willett WC (2007) Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr 86:1780–1790
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, wson-Hughes B (2005) Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 293:2257–2264
Lanham-New SA (2008) Importance of calcium, vitamin D and vitamin K for osteoporosis prevention and treatment. Proc Nutr Soc 67:163–176
Iwamoto J, Takeda T, Ichimura S (2000) Effect of combined administration of vitamin D3 and vitamin K2 on bone mineral density of the lumbar spine in postmenopausal women with osteoporosis. J Orthop Sci 5:546–551
Miyake N, Hoshi K, Sano Y, Kikuchi K, Tadano K, Koshihara Y (2001) 1, 25-Dihydroxyvitamin D3 promotes vitamin K2 metabolism in human osteoblasts. Osteoporos Int 12:680–687
Braam LA, Knapen MH, Geusens P, Brouns F, Hamulyak K, Gerichhausen MJ, Vermeer C (2003) Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int 73:21–26
Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD, Shearer MJ (2007) Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 22:509–519
Katsuyama H, Saijoh K, Otsuki T, Tomita M, Fukunaga M, Sunami S (2007) Menaquinone-7 regulates gene expression in osteoblastic MC3T3E1 cells. Int J Mol Med 19:279–284
Sato Y, Honda Y, Kuno H, Oizumi K (1998) Menatetrenone ameliorates osteopenia in disuse-affected limbs of vitamin D- and K-deficient stroke patients. Bone 23:291–296
Sato Y, Honda Y, Kaji M, Asoh T, Hosokawa K, Kondo I, Satoh K (2002) Amelioration of osteoporosis by menatetrenone in elderly female Parkinson’s disease patients with vitamin D deficiency. Bone 31:114–118
Sato Y, Kanoko T, Satoh K, Iwamoto J (2005) Menatetrenone and vitamin D2 with calcium supplements prevent nonvertebral fracture in elderly women with Alzheimer’s disease. Bone 36:61–68
Shiomi S, Nishiguchi S, Kubo S, Tamori A, Habu D, Takeda T, Ochi H (2002) Vitamin K2 (menatetrenone) for bone loss in patients with cirrhosis of the liver. Am J Gastroenterol 97:978–981
Yonemura K, Kimura M, Miyaji T, Hishida A (2000) Short-term effect of vitamin K administration on prednisolone-induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcif Tissue Int 66:123–128
Ishida Y, Kawai S (2004) Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: The Yamaguchi Osteoporosis Prevention Study. Am J Med 117:549–555
Orimo H, Shiraki M, Tomita A, Morii H, Fujita T, Ohata M (1998) Effects of menatetrenone on the bone and calcium metabolism in osteporosis: a double blind placebo controlled study. J Bone Miner Metab 16:106–112
Shiraki M, Shiraki Y, Aoki C, Miura M (2000) Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 15:515–521
Booth SL, Al RA (2008) Determinants of vitamin K status in humans. Vitam Horm 78:1–22
Iwamoto I, Kosha S, Noguchi S, Murakami M, Fujino T, Douchi T, Nagata Y (1999) A longitudinal study of the effect of vitamin K2 on bone mineral density in postmenopausal women a comparative study with vitamin D3 and estrogen-progestin therapy. Maturitas 31:161–164
Knapen MH, Schurgers LJ, Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18:963–972
Ozuru R, Sugimoto T, Yamaguchi T, Chihara K (2002) Time-dependent effects of vitamin K2 (menatetrenone) on bone metabolism in postmenopausal women. Endocr J 49:363–370
Yasui T, Miyatani Y, Tomita J, Yamada M, Uemura H, Miura M, Irahara M (2006) Effect of vitamin K2 treatment on carboxylation of osteocalcin in early postmenopausal women. Gynecol Endocrinol 22:455–459
Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Nakamura T, Sato T, Yamazaki K (2009) Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab 27:66–75
Tamura T, Morgan SL, Takimoto H (2007) Vitamin K and the prevention of fractures. Arch Intern Med 167:94–95
Rejnmark L, Vestergaard P, Charles P, Hermann AP, Brot C, Eiken P, Mosekilde L (2006) No effect of vitamin K1 intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos Int 17:1122–1132
Whelan AM, Jurgens TM, Bowles SK (2006) Natural health products in the prevention and treatment of osteoporosis: systematic review of randomized controlled trials. Ann Pharmacother 40:836–849
Acknowledgements
We are greatly thankful for the contributions from Margrete Garvik and Eva Mette Leknes at the Bone Laboratory, Marit Hjelmeland and professor Ernst Lien at the Hormone Laboratory at Haukeland University Hospital in Bergen, the chief study nurse Aslaug Jacobsen and her colleagues at the research unit at the University Hospital of North Norway, Tromsø, and to professor John Eisman at Garvan Institute of Medical Research, Sydney, for reading and critically commenting the manuscript.
Financial support
The study was financially supported by grants from the Norwegian Osteoporosis Association and Northern Norway Regional Health Authorities. NATURAL provided the study medication and Eckboe’s legacy provided support for blood analyses.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Emaus, N., Gjesdal, C.G., Almås, B. et al. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int 21, 1731–1740 (2010). https://doi.org/10.1007/s00198-009-1126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1126-4