Abstract
Summary
This study evaluates the effect of alendronate on osteoclastogenesis, cytokine production, and bone resorption in postmenopausal women. We suggest that it acts on mature bone resorbing osteoclasts after 3 months of treatment, whereas, after 1 year, it diminishes their formation by reducing their precursors and serum RANKL.
Introduction
Osteoclasts are the target cells of bisphosphonates, though the most drug-sensitive steps of their formation and activity have not been determined. The present study evaluates the effect of alendronate on osteoclastogenesis, cytokine production, and bone resorption in postmenopausal women.
Methods
The study was conducted on 35 osteoporotic women; 15 were pretreated with alendronate 70 mg/week, whereas, 20 were treated with calcium 1 g/day and vitamin D 800 IU/day. After 3 months, 30 received alendonate 70/mg, vitamin D 2800 IU/week, and calcium 1 g/day for 12 months (combined therapy), whereas, the other five patients remained on calcium 1 g/day and vitamin D 800 IU/day. The following parameters were assessed before and after therapy: changes in bone resorption markers, circulating osteoclast precursors, formation of osteoclasts in peripheral blood mononuclear cell cultures, their viability, and variations in cytokines production.
Results
After 3 months of alendronate, there was no significant reduction in the number of osteoclast precursors, osteoclast formation and viability, and cytokine levels, whereas, there was a significant reduction of bone resorption markers. One year of the combined therapy, on the other hand, reduced osteoclast precursors, osteoclast formation, and serum RANKL, whereas, calcium plus vitamin D alone had no effect.
Conclusions
We suggest that alendronate mainly acts on mature bone resorbing osteoclasts in the short term, whereas, its long-term administration diminishes their formation by reducing their precursors and serum RANKL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chemical structure of bisphosphonates (BPs) is similar to that of inorganic pyrophosphate. Owing to their high affinity for the bone matrix, they are widely used to treat diseases with increased bone resorption. Their mechanism of action depends on the presence of one or more amine groups. Nonnitrogen-containing bisphosphonates, such as clodronate, etidronate, and tiludronate, induce osteoclast (OC) apoptosis through their intracellular accumulation in cytotoxic nonhydrolysable ATP analogs, whereas, the nitrogen-containing BPs pamidronate, alendronate, ibandronate, zoledronate, and risedronate act as isoprenoid diphosphate lipid analogs, and inhibit farnesyl pyrophosphate synthase, an enzyme in the mevalonate pathway [1].
Inhibitions of this pathway prevent the synthesis of the isoprenoid lipids essential for the farnesylation and geranylation of small GTPase signaling proteins and inhibit OC action. BPs have well-known cellular effects that cause OC retraction, condensation, and cellular fragmentation and induce apoptosis, both in vitro [2–4] and in vivo [5]. BPs also inhibit OC recruitment and differentiation [6–8], their attachment to the bone surface [9, 10], and the ruffled border formation [11], essential for resorption. It has been suggested that BPs act both directly on OCs through internalization and through their effect on OC recruitment by nonactive precursors and through the inhibition of pro-osteoclastogenic cytokines [12, 13]. The role of BPs in reducing pro-osteoclastogenic cytokines is still debated. Both an increase in their levels [14–16] and no effect or reduction [12, 17–20] have been described. Some studies suggest that BPs may also affect osteoblasts by inducing the release of a factor that inhibits OC activity or formation [21, 22].
Although it is still unclear which steps of OC formation and activity are most sensitive to BPs, we have previously suggested that 3 months’ oral therapy with a nitrogen-containing BPs (risedronate) considerably reduces OC precursor recruitment, bone resorption, and the production of TNF alpha and RANKL [12].
The present study compares the short and long-term effect of alendronate on osteoclastogenesis, cytokine production, and bone turnover in postmenopausal osteoporosis.
Methods
Patients and markers of bone turnover
The study was approved by the “Clinical Study Review Committee” of the Azienda Sanitaria Ospedaliero Universitaria San Giovanni Battista of Torino, and all the patients signed an informed consent statement prior to recruitment.
Thirty-five women with postmenopausal osteoporosis were enrolled. Subjects taking calcium and vitamin D, thyroid hormones, corticosteroids, estrogen, BPs, strontium ranelate, parathyroid hormone, and raloxifene were excluded. All subjects had been in spontaneous menopause for at least 1 year and were osteoporotic according to the World Health Organization criteria [23]. Bone mineral density was measured by double-emission X-ray absorptiometry with a Hologic QDR 4500 (Hologic Inc.). Secondary osteoporosis was ruled out by means of medical history, physical examination, and blood tests: serum calcium and phosphorus, bone alkaline phosphatase, serum protein electrophoresis, and 25-OH vitamin D. Serum CTX (measured with an α-Cross Laps ® RIA from Osteometer BioteTech A/S) was used as the bone resorption marker.
Treatment
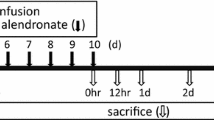
Patients were randomly assigned to receive 70 mg/week alendronate (Fosamax®, kindly provided by Merck Sharp & Dohme SpA Italy) without calcium and vitamin D supplement (15 subjects) or with calcium 1 g/day and vitamin D 800 IU/day (Cacit VitD3®, kindly provided by Procter & Gamble; 20 subjects) per os for 3 months (pretreatment). Thirty patients then received a combined therapy composed of alendronate 70 mg/week plus vitamin D 2,800 IU/week (Fosavance®, kindly provided by Merck Sharp & Dohme SpA Italy) plus calcium 1 g/day (Cacit 1000®, kindly provided by Procter & Gamble); whereas, the other five patients (first five patients randomized to pretreatment with calcium and vitamin D) continued to receive calcium plus vitamin D only until the end of the study period (Fig. 1).
Blood was drawn from an antecubital vein after an overnight fast of ten or more hours before and after pretreatment, and then after 3, 9, and 12 months. All measurements were taken from a single sample at a single time point: spine and femoral neck BMD were measured at the end of the study.
Cell isolation and cultures
Peripheral blood mononuclear cells (PBMC) were obtained with the Ficoll–Paque method from 40 ml peripheral blood in lithium heparin, as previously described [24]. PBMC cultures were performed in triplicate for each subject in 16-well plates (BD Biocoat™ Osteologic™ Bone Cell Culture System, Becton Dickinson & Co. 4 × 105 cell per well) using alpha minimal essential medium (α-MEM: Gibco) supplemented with 10% fetal bovine serum (FBS), benzyl penicillin (100 IU/ml), and streptomycin (100 μg/ml; unstimulated condition), or in the same medium plus M-CSF (25 ng/ml) and RANKL (30 ng/ml; stimulated condition). RPMI (Gibco, UK) was used for cell isolation. All cultures were maintained at 37°C in a humidified 5% CO2 atmosphere.
Osteoclast formation
Cells were fed every 3 days; the medium was recovered at each change, pooled and frozen at −80°C. On the 21st day, the cultures were fixed and stained for Tartrate Resistant Acid Phosphatase (TRAP; Acid Phosphatase, Leukocyte staining kit, Sigma Diagnostics) and with an immune technique to express the vitronectin receptor (VNR; Becton Dickinson & Co). The formation of TRAP + and VNR + multinucleated (>3 nuclei) cells was quantified by counting the stained cells in each well. The count was blind to subject treatment.
OCs were always identified by the same operator as previously described [24], and the mean of three wells for each subject in stimulated or unstimulated condition was calculated. The PBMC cultures were performed at basal level, after pretreatment and after 12 months of combined therapy.
The ability of OCs to resorb bone in vitro was evaluated at the end of the culture period by removing the cells with 14% sodium hypochlorite after counting; a Nikon Coolpix digital camera attached to an inverted microscope was used to photograph the surface of each well [12].
Flow cytometry
Three-color flow cytometry was performed on a FACSCalibur flow cytometer (Becton Dickinson & Co). The effect of therapy on OC precursors was evaluated by staining PBMC with fluorescein (FITC)-conjugated anti-VNR, phycoerythrin (PE)-conjugated anti-CD14, and allophycocyanin (APC)-conjugated anti-CD11b mAb or with the corresponding isotype control, followed by incubation at 4°C for 30 min. CD14+/CD11b+/VNR- cells were regarded as early OC precursors [25, 26] and triple-positive (CD14+/CD11b+/VNR+) cells as OC precursors according to the literature [27–32].
To determine whether alendronate also acts on PBMCs other than OC precursors, we evaluated the PBMC subset at baseline and after therapy in the first five patients randomized to alendronate. Briefly, PBMCs were stained with FITC-conjugated anti-CD19, peridinin chlorophyll protein (PerCP)-conjugated anti-CD3, and APC-conjugated anti-CD14 mAb or with the corresponding isotype control, followed by incubation at 4°C for 30 min. Cells were classified as B (CD19+), T (CD3+) lymphocytes, and monocytes (CD 14+) according to their forward scatter and side scatter.
Membrane antigen expression was analyzed with the CellQuest (Becton Dickinson & Co) software and displayed as bivariate dot plots or histograms. Each plot depicts the results from 10,000 events representing viable cells gated by cell size and granularity.
All the antibodies were purchased from Becton Dickinson & Co. Flow cytometry was performed at basal level after pretreatment and after 3, 9, and 12 months of combined therapy or calcium plus vitamin D.
Cytokine measurement
ELISA kits were used to measure TNFα (Quantikine; R&D System), OPG (Biomedica; Biomedica Medizinprodukte GmbH & Co KGA), and free s-RANKL (Biomedica; Biomedica Medizinprodukte GmbH & Co KGA) levels in the pooled unstimulated culture supernatants to see whether they were altered by the treatment. Total s-RANKL (Apotech; Apotech Corporation & Immunodiagnostik) levels were measured in the serum. Each measurement was performed in duplicate for each patient. Serum cytokines were measured at basal level, after pretreatment and after 3 and 12 months of combined therapy; supernatant cytokines were measured at basal level after pretreatment and after 12 months of combined therapy.
Cell viability
Differences in cell viability were assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5,diphenyltetrazolium bromide (MTT) assay, before and after 12 months of combined therapy in four patients randomly chosen from each group. PBMC were cultured in 96 well per plates in the presence or absence of M-CSF (25 ng/ml) and RANKL (30 ng/ml). On days 10, 21, and 30, 10 µl of MTT/PBS solution (5 mg/ml) were added to each well (containing 100 µl of cells), followed by 4 h incubation at 37°C in humidified 5% CO2 atmosphere. The reaction was stopped by the addition of 100 µl of 0.04 N HCl in absolute isopropanol. The plates were read in a microtiter plate reader (Automatic Microtiter Reader, Biorad) using a 570-nm filter. To automatically subtract background noise, we used dual wavelength settings of 570 and 630 nm according to the manufacturer's instructions. Only OC precursors and serum cytokines were evaluated in the five patients who received calcium plus vitamin D for 15 months (Fig. 1).
Statistics
The normal distribution of each parameter was determined with curtosi test: baseline patient characteristics, BMD, OC in cultures, PBMC subsets, and cell viability were normally distributed, whereas, the others were not.
To rule out selection bias, the baseline characteristics of the three groups of treatment were compared by means of one-way analysis of variance. Student's paired t test was used to compare OC number, PBMC subsets, and cell viability at baseline and after the therapy, at different time points. BMD was compared between baseline and end of the study by Student’s paired t test. A Wilcoxon’s test was used to compare CTX, number of circulating precursors, and cytokine levels at baseline and after therapy at different time points. The SPSS 15.0 software package was used to process the data with p < 0.05 as the significance cutoff.
Results
The baseline characteristics of the three groups of subjects were not significantly different (Table 1).
Combined therapy reduces osteoclast formation
In order to evaluate the effect of alendronate in vivo on in vitro OC formation, PBMC cultures were performed with and without the addition of M-CSF and RANKL at different time points. After 3 months, neither alendronate nor calcium plus vitamin D significantly reduces OC formation, whether with or without the addition of M-CSF and RANKL; whereas, after 12 months of combined therapy, there was a significant reduction in OC formation and activity (Fig. 2). There was no significant increase of OC formation after the addition of M-CSF and RANKL.
a Effect of therapy on OC formation and activity: OCs (white arrows) in a light micrograph (×10 after TRAP staining before (upper left panel) and after in vivo treatment with alendronate (3 months) or with combined therapy (12 months upper right panel), the lower panels shows the pit formation assays performed on hydroxyapatite-coated wells after cell removal. The micrographs refer to OCs generated in PBMC cultures without stimulus after 21 days of culture. b Graphs showing the number of OC in unstimulated (without M-CSF and RANKL, left) and stimulated (with M-CSF and RANKL added, right) conditions in PBMC cultures from osteoporotic women pretreated with alendronate (continuous line) or calcium and vitamin D (hatched line) for 3 months and with combined therapy for 12 months. The symbols show the mean and SE for all patients, p significant values calculated with paired Student’s t test are indicated (the hatched lines refer to the group pretreated with calcium and vitamin D). The gray boxes show the t3 months pretreatment period
By contrast, the bone resorption marker CTX was decreased after 3 months of pretreatment with alendronate but not after calcium and vitamin D alone (Fig. 3).
Effect of therapy on in vivo bone resorption: graph showing the level of serum CTX in osteoporotic women pretreated with alendronate (continuous line) or calcium and vitamin D (hatched line) for 3 months and with combined therapy for 12 months. The symbols show the mean and SE for all patients, p significant values calculated with Wilcoxon’s test are indicated (the hatched lines refer to the group pretreated with calcium and vitamin D). The gray box shows the 3 months pretreatment period
Combined therapy reduces circulating OC precursors
The effect of alendronate on bone marrow output of circulating OC precursors was assessed by comparing their presence in PBMC at baseline and after therapy. There was no significant decrease in either of the pretreatment groups, whereas, a significant reduction was observed after 9 and 12 months of the combined therapy only. The percentage of CD14+/CD11b+/VNR- cells was also less after 12 months of combined therapy.These cells are regarded as early OC precursors [25, 26] (Fig. 4).
These data suggest that the short-term effect of alendronate is exerted on mature bone resorbing OCs rather than on OC precursors.
Effects of therapy on circulating OC precursors: FACS analysis of circulating OC precursors from PBMC of osteoporotic women labeled with FITC-conjugated anti-VNR, PE-conjugated anti-CD14, and APC-conjugated anti-CD11b mAbs before and after alendronate and calcium plus vitamin D in vivo treatment. a Dot plots represent VNR + and CD11b + cells gated on CD14+ cells (OC precursors). Percentages of positive cells are indicated. b Graphs represent the percentage of CD14+ CD11b + VNR + cells (OC precursors) before and after therapy. The symbols show the mean and SE for all patients, p significant values calculated with Wilcoxon’s test are indicated. c Graphs represent the percentage of CD14+/CD11b+/VNR- cells (early OC precursors) before and after therapy. The symbols show the mean and SE for all patients, p significant values calculated with Wilcoxon’s test are indicated. The gray boxes show the 3 months pretreatment period
The effect of alendronate on other PBMCs was determined by evaluation of the percentage of B (CD19+), T (CD3+) lymphocytes, and monocytes (CD14+) at basal level, after pretreatment with alendronate and after 12 months of combined therapy. The drug had no significant influence on these cells (Supplemental Figure).
The high specificity of alendronate for OC precursors is illustrated by the fact that their number fell by more than 90% (and that of early OC precursors by more than 10%) after 12 months of combined therapy, whereas, there was no change in the total amount of CD14+ cells (general monocyte population). This finding suggests that alendronate acts selectively against OC precursors alone.
Combined therapy reduces serum RANKL
To determine whether the combined therapy reduced osteoclastogenesis by influencing cytokine production in vitro and in vivo, the levels of TNF alpha, RANKL, and OPG were measured in culture supernatants and in serum. An increase in TNF alpha level only was found in supernatants after 3 months of alendronate (Fig. 5a). Serum total RANKL and serum OPG were significantly reduced after 3 and 12 months of combined therapy, whereas, the serum level of TNF alpha was not affected (Fig. 5b). These data point to an early but short-lived proinflammatory effect of alendronate and that combined therapy reduces in vivo RANKL production and hence, OC formation and activity. The positive correlation between serum RANKL and CTX (R = 0.46, p = 0.011) is in line with this view. The reduction of serum OPG may reflect the reduction of bone turnover.
Effects of therapy on cytokine production. a In vitro cytokine production measured in the supernatants of PBMC cultures in patients pretreated with calcium and vitamin D (hatched line) or with alendronate (continuous line). b In vivo cytokine production measured in the serum of patients pretreated with calcium and vitamin D (hatched line), with alendronate (continuous line), or with calcium and vitamin D during the entire study period (dotted line). The symbols show the mean and SE for all patients, p significant values calculated with Wilcoxon’s test are indicated. The gray boxes show the 3 months pretreatment period
Combined therapy does not reduce PBMC viability
To assess whether the reduction in OC formation after 1 year was due to a reduction in PBMC viability, an MTT test was performed on days 10, 21, and 30 of culture in the presence or absence of growth factors. PBMC viability was never significantly reduced after alendronate nor after calcium and vitamin D or combined therapy, both in stimulated and in unstimulated conditions (data not shown).
Clinical outcome
We observed a significant increase in BMD at the lumbar spine (0.72 ± 0.017 g/cm2 at baseline versus 0.75 ± 0.017 g/cm2 end of the study, p = 0.004) and a nonsignificant increase at the femoral neck (0.43 ± 0.17 g/cm2 at baseline versus 0.60 ± 0.014 g/cm2 after 15 months of therapy, p = NS); in patients treated with calcium and vitamin D alone, there was a nonsignificant reduction of BMD at both sites (data not shown).
Discussion
BPs are widely used to treat osteoporosis, Paget’s disease, and myeloma: their target cells, however, have not been clearly identified. Some studies have suggested that mature OCs are their only target [7, 8], whereas, others would include OC precursors [12, 33, 34] and osteoblasts [22]. Coxon et al. [35] have recently demonstrated that BP uptake by nonresorbing cells is increased when they are cultured in the presence of resorbing OCs. Our group and other authors have previously shown that OC precursors in peripheral blood are increased in diseases characterized by increased bone resorption [32, 36–39] and decreased after antiresorptive therapy [12]. It has also been shown that OC formation depends mainly on the amount of precursors recruited and on the balance between pro- and antiosteoclastogenic cytokines of which RANKL, TNF alpha, and OPG [36, 40, 41] have received the most attention.
The aim of the present study was to evaluate the effect of short-term oral alendronate and 1-year combined therapy on OC recruitment, formation, and activity. The presence of circulating precursors, the formation, and viability of cells produced in PBMC cultures and cytokine levels in culture medium and serum were evaluated.
Our data confirmed the early effect of oral alendronate on bone resorption in vivo, as demonstrated by the reduction of CTX after 3 months of pretreatment, whereas, OC formation in PBMC cultures was unaffected. After 12 months of combined therapy, OC formation was significantly reduced in both the presence and the absence of M-CSF and of RANKL, which directly stimulates osteoclastogenesis. The absence of increased OC formation after the addition of M-CSF and RANKL is in line with previous studies on osteoclastogenesis from PBMCs in various bone lytic diseases (see [42] for review). These observations suggest that the composition of PBMCs from patients with increased bone resorption is sufficient to increase osteoclast formation and activity in vitro. In order to evaluate the mechanism by which therapy reduces OC formation, we examined the OC precursors in peripheral blood: a significant decrease of OC precursors (CD14+/CD11b+/VNR + cells) in patients treated for at least 9 months with combined therapy was found; whereas, early OC precursors (CD14+/CD11b+/VNR- cells) decreases only after 12 months of combined therapy. In the pretreatment period with alendronate, we do not observe a reduction of OC precursors even though there was a significant decrease in bone resorption; these data could suggest that short-term therapy with alendronate acts mainly on the mature bone OC and does not influence precursor recruitment, whereas, OC formation in the long term is also reduced through the decrease of OC precursors. As alendronate, like risedronate [12], acts on circulating OC precursors, we evaluated its effects on other types of PBMCs, namely monocytes, B, and T-cells: our data demonstrated that alendronate acts specifically on OC precursors without affecting other cell types. Alendronate acts specifically on monocytes committed towards the OC lineage (CD14+/CD11b+/VNR- and CD14+/CD11b+/VNR+) without affecting the general monocyte population: CD14+.
Increased levels of TNF alpha were observed after 3 months alendronate, whereas, after 1 year of combined therapy, TNF alpha levels returned to baseline. These data could indicate an early proinflammatory effect of alendronate and confirm previous data from an in vivo animal study [43] and in vitro studies on human cells [15, 44, 45] suggesting that alendronate can increase TNF production from T-cells. However, it is important to point out that a systemic increase in the TNF alpha levels or acute phase reactions were not observed in these patients; moreover, the observed increase in TNF alpha was transient since TNF returned to baseline after 1 year of therapy.
Alendronate had no effect on the production OPG and RANKL by PBMC, whereas, serum levels of RANKL were significantly reduced by combined therapy after 3 and 12 months. This is in keeping with in vitro indications that zoledronic acid, another BP, decreases RANKL levels through cleavage of trans-membrane RANKL in osteoblast-like cells by upregulating TACE, an enzyme known to cleave RANKL [46].
Serum OPG levels were significantly reduced by treatment; this datum may provide further evidence that serum OPG concentrations are at least partly reflective of bone turnover [13, 40] and confirms our previous work on risedronate [12]. Our observation is in contrast with a previous work by Dobnig et al. [13] that demonstrated an increase in OPG levels after long-term treatment with alendronate or risedronate. The present study suggests striking differences in the effect of alendronate with respect to risedronate. In our previous work, we showed that risedronate reduces OC formation from PBMC after only 3 months by reducing OC precursors, RANKL, and TNF production. Alendronate seems to have a different onset of action. It reduces bone resorption by targeting mature OC (CTX reduction) rather than OC precursor and cytokine production in the short term; only after at least 9 months of combined therapy was a reduction in OC precursors observed. Moreover, alendronate, which is different from risedronate, does not influence PBMC viability. These differences in targeting OC precursors may be clinically relevant and may help to explain the supposed quicker onset of action of risedronate [47]. In the authors’ opinion, the differences between these two oral BPs can be related to their different affinity for hydroxyapatite [48]; the higher affinity of alendronate could explain its lower availability for nonresorbing cells, as bone marrow precursors and its different time-dependent action in bone tissue.
Further studies are required to elucidate the mechanism underlying the different action of alendronate and risedronate.
The major limitation of this study is the small size of its cohort. This, together with the high variability of the biological markers, could explain some negative findings in the study.
In conclusion, we suggest that alendronate reduces the activity of mature OC in the short period and OC formation in the long period by acting on both OC precursor recruitment and RANKL production without affecting OC precursor viability.
References
Rogers MJ (2003) New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9:2643–2658
Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ (2001) Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone 28:465–473
Sudhoff H, Jung JY, Ebmeyer J, Faddis BT, Hildmann H, Chole RA (2003) Zoledronic acid inhibits osteoclastogenesis in vitro and in a mouse model of inflammatory osteolysis. Ann Otol Rhinol Laryngol 112:780–786
Rogers MJ, Chilton KM, Coxon FP, Lawry J, Smith MO, Suri S, Russell RG (1996) Bisphosphonates induce apoptosis in mouse macrophage-like cells in vitro by a nitric oxide-independent mechanism. J Bone Miner Res 11:1482–1491
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487
Cecchini MG, Felix R, Fleisch H, Cooper PH (1987) Effect of bisphosphonates on proliferation and viability of mouse bone marrow-derived macrophages. J Bone Miner Res 2:135–142
Boonekamp PM, van der Wee-Pals LJ, van Wijk-van Lennep MM, Thesing CW, Bijvoet OL (1986) Two modes of action of bisphosphonates on osteoclastic resorption of mineralized matrix. Bone Miner 1:27–39
Rodan GA, Reszka AA (2002) Bisphosphonate mechanism of action. Curr Mol Med 2:571–577
Colucci S, Minielli V, Zambonin G, Cirulli N, Mori G, Serra M, Patella V, Zambonin Zallone A, Grano M (1998) Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated surfaces. Calcif Tissue Int 63:230–235
Carano A, Teitelbaum SL, Konsek JD, Schlesinger PH, Blair HC (1990) Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J Clin Invest 85:456–461
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88:2095–2105
D'Amelio P, Grimaldi A, Di Bella S, Tamone C, Brianza SZ, Ravazzoli MG, Bernabei P, Cristofaro MA, Pescarmona GP, Isaia G (2008) Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J Bone Miner Res 23:373–379
Dobnig H, Hofbauer LC, Viereck V, Obermayer-Pietsch B, Fahrleitner-Pammer A (2006) Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos Int 17:693–703
Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, Ludwig H, Leuenberger P, Burckhardt P, Thiebaud D (1996) Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone 18:133–139
Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK (2005) The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol 139:101–111
Thompson K, Rogers MJ (2004) Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res 19:278–288
Mossetti G, Rendina D, De Filippo G, Viceconti R, Di Domenico G, Cioffi M, Postiglione L, Nunziata V (2005) Interleukin-6 and osteoprotegerin systems in Paget's disease of bone: relationship to risedronate treatment. Bone 36:549–554
Papadaki HA, Tsatsanis C, Christoforidou A, Malliaraki N, Psyllaki M, Pontikoglou C, Miliaki M, Margioris AN, Eliopoulos GD (2004) Alendronate reduces serum TNFalpha and IL-1beta, increases neutrophil counts, and improves bone mineral density and bone metabolism indices in patients with chronic idiopathic neutropenia (CIN)-associated osteopenia/osteoporosis. J Bone Miner Metab 22:577–587
Santini D, Fratto ME, Vincenzi B, La Cesa A, Dianzani C, Tonini G (2004) Bisphosphonate effects in cancer and inflammatory diseases: in vitro and in vivo modulation of cytokine activities. BioDrugs 18:269–278
Gur A, Denli A, Cevik R, Nas K, Karakoc M, Sarac AJ (2003) The effects of alendronate and calcitonin on cytokines in postmenopausal osteoporosis: a 6-month randomized and controlled study. Yonsei Med J 44:99–109
Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ (1993) Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 91:2004–2011
Vitte C, Fleisch H, Guenther HL (1996) Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 137:2324–2333
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381
D'Amelio P, Grimaldi A, Pescarmona GP, Tamone C, Roato I, Isaia G (2005) Spontaneous osteoclast formation from peripheral blood mononuclear cells in postmenopausal osteoporosis. Faseb J 19:410–412
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG, Schwarz EM (2003) Mechanisms of TNF-alpha- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J Clin Invest 111:821–831
Dalbeth N, Smith T, Nicolson B, Clark B, Callon K, Naot D, Haskard DO, McQueen FM, Reid IR, Cornish J (2008) Enhanced osteoclastogenesis in patients with tophaceous gout: urate crystals promote osteoclast development through interactions with stromal cells. Arthritis Rheum 58:1854–1865
Ramnaraine M, Pan W, Clohisy DR (2006) Osteoclasts direct bystander killing of cancer cells in vitro. Bone 38:4–12
Massey HM, Flanagan AM (1999) Human osteoclasts derive from CD14-positive monocytes. Br J Haematol 106:167–170
Shalhoub V, Elliott G, Chiu L, Manoukian R, Kelley M, Hawkins N, Davy E, Shimamoto G, Beck J, Kaufman SA, Van G, Scully S, Qi M, Grisanti M, Dunstan C, Boyle WJ, Lacey DL (2000) Characterization of osteoclast precursors in human blood. Br J Haematol 111:501–512
Faust J, Lacey DL, Hunt P, Burgess TL, Scully S, Van G, Eli A, Qian Y, Shalhoub V (1999) Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem 72:67–80
Matayoshi A, Brown C, DiPersio JF, Haug J, Abu-Amer Y, Liapis H, Kuestner R, Pacifici R (1996) Human blood-mobilized hematopoietic precursors differentiate into osteoclasts in the absence of stromal cells. Proc Natl Acad Sci USA 93:10785–10790
Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O, Ferracini R (2005) Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. Faseb J 19:228–230
van Beek ER, Lowik CW, Papapoulos SE (1997) Effect of alendronate treatment on the osteoclastogenic potential of bone marrow cells in mice. Bone 20:335–340
Van Beek ER, Lowik CW, Papapoulos SE (2002) Bisphosphonates suppress bone resorption by a direct effect on early osteoclast precursors without affecting the osteoclastogenic capacity of osteogenic cells: the role of protein geranylgeranylation in the action of nitrogen-containing bisphosphonates on osteoclast precursors. Bone 30:64–70
Coxon FP, Thompson K, Roelofs AJ, Ebetino FH, Rogers MJ (2008) Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 42:848–860
D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G (2008) Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone 43:92–100
Brunetti G, Colucci S, Pignataro P, Coricciati M, Mori G, Cirulli N, Zallone A, Grassi FR, Grano M (2005) T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J Periodontol 76:1675–1680
Olivier BJ, Schoenmaker T, Mebius RE, Everts V, Mulder CJ, van Nieuwkerk KM, de Vries TJ, van der Merwe SW (2008) Increased osteoclast formation and activity by peripheral blood mononuclear cells in chronic liver disease patients with osteopenia. Hepatology 47:259–267
Tjoa ST, de Vries TJ, Schoenmaker T, Kelder A, Loos BG, Everts V (2008) Formation of osteoclast-like cells from peripheral blood of periodontitis patients occurs without supplementation of macrophage colony-stimulating factor. J Clin Periodontol 35:568–575
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. JMNI 4:268–275
Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L, Buffoni L, Manfredi R, Ruffini E, Ottaviani D, Ciuffreda L, Mussa A, Ferracini R (2006) IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor. PLoS ONE 1:e124
De Vries TJ, Everts V (2009) Osteoclast formation from peripheral blood of patients with bone-lytic diseases
Deng X, Yu Z, Funayama H, Yamaguchi K, Sasano T, Sugawara S, Endo Y (2007) Histidine decarboxylase-stimulating and inflammatory effects of alendronate in mice: involvement of mevalonate pathway, TNFalpha, macrophages, and T-cells. Int Immunopharm 7:152–161
Pietschmann P, Stohlawetz P, Brosch S, Steiner G, Smolen JS, Peterlik M (1998) The effect of alendronate on cytokine production, adhesion molecule expression, and transendothelial migration of human peripheral blood mononuclear cells. Calcif Tissue Int 63:325–330
Toyras A, Ollikainen J, Taskinen M, Monkkonen J (2003) Inhibition of mevalonate pathway is involved in alendronate-induced cell growth inhibition, but not in cytokine secretion from macrophages in vitro. Eur J Pharm Sci 19:223–230
Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K, Zannettino AC (2004) The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE). J Bone Miner Res 19:147–154
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH (2007) Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 1117:209–257
Acknowledgments
This work was supported by an unconditioned grant from the Merck Sharp & Dohme SpA Italy and a grant from the Fondazione Internazionale Ricerche Medicina Sperimentale (FIRMS) Compagnia San Paolo. M.A. Cristofaro was supported by a fellowship from the Ministry for Education, University and Research (MIUR); P. D’Amelio was supported by a fellowship from the Regione Piemonte. Alendronate and alendronate plus vitamin D were kindly provided by Merck Sharp & Dohme SpA Italy; calcium and vitamin D supplements were kindly provided by Procter & Gamble.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
Supplemental Figure 1
Effects of therapy on PBMCs: FACS analysis of PBMCs’ subpopulation labeled with APC-conjugated anti-CD14 (monocytes), FITC-conjugated anti-CD19 (B lymphocytes), and PerCP-conjugated anti-CD3 (T lymphocytes) mAbs before and after alendronate (3 months) or with combined therapy (12 months). a Dot plots represent CD14+ cells gated on monocytes (upper panels), CD19+ cells gated on lympocytes (middle panels), and CD3+ cells gated on lympocytes (lower panels). b Graph represents the percentage of CD14+, CD19+, and CD3+ cells before and after therapy. The bars show the mean and SE for five patients (PPT 369 kb).
Rights and permissions
About this article
Cite this article
D’Amelio, P., Grimaldi, A., Cristofaro, M.A. et al. Alendronate reduces osteoclast precursors in osteoporosis. Osteoporos Int 21, 1741–1750 (2010). https://doi.org/10.1007/s00198-009-1129-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-009-1129-1