Abstract
This study reveals the changes in bone mineral density (BMD), the turnover rate, and the balance [multiple of median formation/multiple of median resorption (MoMf/MoMr)] affected by the selection of different bone resorption inhibitors after 24-month daily teriparatide (20 µg/day) administration. The turnover rate was calculated as √(MoMf2 + MoMr2), where MoMf = bone-specific alkaline phosphatase (BAP) value/18.6 and MoMr = tartrate-resistant acid phosphatase 5b (TRACP-5b) value/463. One hundred and twenty-one osteoporotic women (mean age 82.4 years) were randomly administered minodronate (50 mg/28 days), raloxifene (60 mg/day), or eldecalcitol (0.75 µg/day) after teriparatide discontinuation. BMD was measured at 0, 24, and 48 weeks; BAP values and TRACP-5b were measured at 0, 12, 24, 36, and 48 weeks after administration of bone resorption inhibitors. In the minodronate group, BMD increased significantly from week 0 to weeks 24 and 48. The turnover rate was significantly reduced at week 12, and remained so over the entire course in all three groups. The speed of change of turnover rate was greatest in the minodronate group. The balance in the minodronate group shifted significantly toward formation dominance at week 12 (to 0.97 from 0.87) and then again toward resorption dominance (to 0.84) at week 24. However, no further advancement in resorption dominance was observed until week 48. Conversely, the balance in the raloxifene and eldecalcitol groups shifted toward resorption dominance gradually over the entire course. In conclusion, the BMD-increasing effect was greatest with minodronate administration and depends not only on the decrease in turnover rate but also on changes in balance after teriparatide discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Until recently, osteoporosis treatment mainly involved bone resorption inhibitors in Japan. Since the release of daily teriparatide in 2010, bone formation promotors have also played a role in the treatment of osteoporosis [1]. However, based on efficacy and safety considerations, teriparatide is subject to restriction on its duration of use; in Japan, this has been determined to be up to 24 months. Studies have shown that the effect of teriparatide in reducing fractures and ameliorating back pain are sustained even after discontinuation [2, 3]. However, as other studies have demonstrated that bone mineral density (BMD) declines if no medication for osteoporosis is given after cessation of teriparatide, continued treatment with a bone resorption inhibitor is necessary after teriparatide treatment [2, 4–6]. However, no studies have yet addressed which bone resorption inhibitor is an effective choice after completing 24-month teriparatide administration.
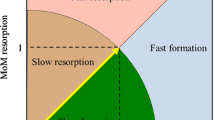
The efficacy of osteoporosis medication is evaluated on the basis of the measurement of BMD and bone turnover markers (BTMs), which are measures of different aspects of bone strength [7]. Therefore, it is important to include both BMD and BTMs in evaluations [8]. In bone metabolism, bone formation and resorption are considered to be mutually dependent through crosstalk between osteoblasts and osteoclasts [9]. Thus, BTM assessment often involves only bone formation markers or bone resorption markers in Japan. However, it is difficult to use this method to evaluate bone turnover from aspects of both formation and resorption and to examine the balance between these. In addition, administration of teriparatide increases variance in multiple of median formation (MoMf) and multiple of median resorption (MoMr) over time [10]. In such cases, erroneous conclusions can be drawn if therapeutic efficacy is evaluated using only bone formation markers or bone resorption markers. It is therefore considered necessary to evaluate both the formation and resorption markers simultaneously [9–12]. Bieglmayer et al. proposed an evaluation using the bone turnover rate (turnover rate) and the bone formation–resorption balance (balance) calculated from bone formation and resorption marker levels [13].
The present study assumed the increase in BMD as an endpoint and hypothesized that the BMD-increasing effect depended on the changes not only in turnover rate but also in balance affected by the selection of different bone resorption inhibitors for continued treatment after teriparatide discontinuation. This study was a randomized controlled trial including patients who had completed 24 months of treatment with teriparatide and was designed to reveal the changes in BMD, turnover rate, and balance for continued treatment after teriparatide discontinuation.
Materials and methods
The subjects were 121 postmenopausal women who had been diagnosed with primary osteoporosis according to the Japanese diagnostic criteria for primary osteoporosis (2012 Version) [14], who had completed 24 months of 20-µg/day teriparatide treatment with ≥90% adherence (Table 1), and who met any of the following three conditions at the start of teriparatide treatment—(1) those with a history of vertebral fracture or hip fracture; (2) those aged <80 years with no history of fragility fracture but with a lumbar spine or hip BMD of <65% of the young adult mean (YAM); and (3) those aged ≥80 years with no history of fragility fracture but with a lumbar spine or hip BMD of <70% of the YAM. Because the risk of falling is increased in patients aged ≥80 years, we set the YAM criteria in <70% of these patients [15]. Patients who were taking any medication known to alter bone metabolism, who had any underlying disease known to affect bone metabolism, or who experienced any fracture during the teriparatide treatment were excluded. Written informed consent for the study and its publication was obtained from all subjects.
The subjects were randomly allocated to one of the following three groups using a block replacement method based on their lumbar spine BMD values before they were administered any bone resorption inhibitor—(1) the minodronate group who received 50 mg minodronate at intervals of 28 days; (2) the raloxifene group who received 60 mg raloxifene daily; and (3) the eldecalcitol group who received 0.75 µg eldecalcitol daily (Table 1). In addition, the minodronate group and the raloxifene group received 1.0 µg of alfacalcidol daily. There was no washout period between the last dose of teriparatide and the first dose of any of the bone resorption inhibitors.
BMD values of the 2nd to 4th lumbar vertebrae and the left proximal femur (total) were measured by dual-energy X-ray absorptiometry (DXA) (Discovery A; Hologic, Inc., Bedford, MA, USA) at the start of administration (baseline, week 0) and 24 and 48 weeks later. Any measurement site with a history of surgery was excluded from measurements. Bone-specific alkaline phosphatase (BAP) and tartrate-resistant acid phosphatase-5b (TRACP-5b) were measured at baseline and at 12, 24, 36, and 48 weeks. These values were divided by the respective median values for untreated postmenopausal women with osteoporosis (BAP 18.6 μg/L; TRACP-5b 463 mU/dL) to obtain values for MoMf (BAP measurement/18.6) and MoMr (TRACP-5b measurement/463) [16]. The turnover rate and balance were calculated as √(MoMf2 + MoMr2) and MoMf/MoMr, respectively.
IBM SPSS Statistics 20J software was used for statistical analyses. Temporal changes were compared using Wilcoxon signed-rank test, and the Kruskal–Wallis test was used for three-group comparisons.
This study was approved by the Ethical Review Board of Asahi General Hospital.
Results
Medication was discontinued for 24 of 121 participants—in the minodronate group, for missing a visit or exhibiting <90% adherence to the regimen (6 patients), a fracture (1 patient), femoral pain (1 patient), and death (1 patient); in the raloxifene group, for missing a visit or exhibiting <90% adherence (4 patients), a fracture (2 patients), and stomach pain (1 patient); and in the eldecalcitol group, for missing a visit or <90% adherence (4 patients), a fracture (1 patient), hypercalcemia (1 patient), and death (2 patients). After excluding these patients, a total of 97 patients were included in the following analyses (32, 35, and 30 in the minodronate, raloxifene, and eldecalcitol groups, respectively).
Changes in BMD
The lumbar spine and femur BMD values at week 24 were higher than their respective baseline values in 23 and 22 subjects in the minodronate group, 19 and 20 in the raloxifene group, and 17 and 12 in the eldecalcitol group, respectively. At week 48, these values were higher than their baseline values in 22 and 20 subjects in the minodronate group, 17 and 18 in the raloxifene group, and 11 and 14 in the eldecalcitol group, respectively. In the minodronate group, the increases in the lumbar spine and femur BMD values from baseline to week 24 and week 48 were of statistical significance (Table 2). The rate of change of BMD differed significantly between the three groups for the lumbar spine at week 24 and week 48 and for the femur at week 24 (Fig. 1).
Changes in bone turnover markers
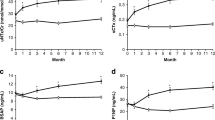
Both BAP and TRACP-5b were significantly reduced at week 12 in all three groups. The difference in the rates of decline in BAP and TRACP-5b was smallest in the eldecalcitol group at week 12, and this difference increased after week 24 in the raloxifene and eldecalcitol groups (Fig. 2). The rate of change in BAP and TRACP-5b did not exceed the minimum significant change in three subjects (two in the raloxifene group and one in the eldecalcitol group) and nine subjects (four in the raloxifene group and five in the eldecalcitol group), respectively, at week 12 [8].
The turnover rate was also reduced significantly at week 12 in all three groups and remained reduced over the entire course of the study. From week 12 onward, the values in all groups were lower than the median value (√2) in untreated postmenopausal women with osteoporosis (Fig. 3). The rate of change was the greatest in the minodronate group throughout the entire course of the study, with no differences observed between the raloxifene and eldecalcitol groups (Fig. 4).
In the minodronate group, compared to baseline, the balance shifted significantly toward formation dominance at week 12 and then again toward resorption dominance at week 24. No further advancement in resorption dominance was observed at weeks 36 and 48. In contrast, the balance in the raloxifene and eldecalcitol groups shifted toward resorption dominance gradually over the entire course. Significant decreases in the bone formation–resorption balance from the baseline level were observed from week 36 onward in the raloxifene group and from week 24 onward in the eldecalcitol group (Fig. 5). In contrast, the changes in the rates of balance at weeks 12, 36, and 48 with reference to the baseline were positive values in the minodronate group, and differed significantly between the three groups (Fig. 6).
Discussion
The effectiveness of teriparatide in increasing the BMD of the lumbar spine is shown to decrease after >12 months of continuous use [4, 12]. It has also been reported that the bone formation marker P1NP decreases after using teriparatide for >12 months [12, 17]. These data suggest that the efficacy of teriparatide attenuates over time after a year of use. Therefore, when switching from teriparatide to another osteoporosis drug, it must also be considered that post-teriparatide therapy is affected differently depending on when teriparatide was discontinued. There have been reports that compared drugs for treatment after discontinuing teriparatide; however, in most of these reports, the duration of teriparatide administration until discontinuation was <24 months (range 9−20 months) [4, 6, 18, 19]. The subjects in the present study had received teriparatide for 24 months with ≥90% adherence. Thus, unlike previous studies, this study focused on changing medication at the time point when the efficacy of teriparatide was most attenuated. The results of the present study should therefore help with the selection of therapeutic agents for patients who have completed 24 months of treatment with teriparatide.
In this study, the rate of BMD increase was highest in the minodronate group, followed by the raloxifene group; in the eldecalcitol group, the only increase observed was for lumbar spine BMD at week 24. A significant difference was found among the three groups in the rate of BMD change 24 weeks after drug switching. The three drugs used in the study were bone resorption inhibitors with different mechanisms of action. Minodronate potently inhibits farnesyl diphosphate synthesis, and its osteoclast inhibition effect is stronger than that of any other oral bisphosphonates [20]. The bone resorption-inhibiting activity of minodronate is >10,000 times stronger than that of etidronate and 10–100 times stronger than that of alendronate [21]. According to data for Japanese patients, minodronate reduces urinary type I collagen N-telopeptide (NTX), a bone resorption marker, by 49.5 and 56.7% at 6 and 24 months after administration, respectively [22]. Raloxifene is a selective estrogen receptor modulator that has been reported to lower NTX by 34.2 and 33.5% at 24 and 52 weeks after administration, respectively, in Japanese patients [23]. Eldecalcitol suppresses the expression of the receptor activator of nuclear factor kappa-b ligand in osteoblast lineage cells and induces bone minimodeling. In Japanese patients, eldecalcitol has been shown to reduce NTX by 20–30% over 6–36 months after administration [24]. The NTX reduction rate suggests that the potency of the three drugs in bone resorption suppression is as follows in decreasing order—minodronate, raloxifene, and then eldecalcitol. In the present study, the decline in TRACP-5b was 49.9% in the minodronate group, 30.0% in the raloxifene group, and 27.9% in the eldecalcitol group at week 12. Despite no vitamin D supplementation in the eldecalcitol group, the potency of the three drugs on bone resorption suppression was of the same order as that in the previous reports. It therefore appears that, in post-teriparatide treatment, the more potent the bone resorption suppressor drug, the greater the increase in BMD that can be expected.
Absolute values of turnover rates decreased to an increasingly greater degree from week 12 onward in the following order—minodronate group, raloxifene group, and eldecalcitol group. The rate of change was greatest in the minodronate group over the entire course from week 12, with the decrease maintained until week 48. This appeared to be attributable to the potent bone resorption suppression effect of minodronate, which would have contributed greatly to the decrease in turnover. There were no differences in the speed of change in turnover rate between the raloxifene and eldecalcitol groups from week 12 onward, suggesting that the potency difference in bone resorption suppression between raloxifene and eldecalcitol made no appreciable contribution to their changes in turnover rate. All three drugs showed no major changes in turnover rate from week 12 onward, suggesting that the effect of teriparatide on turnover was already small at week 12.
Meanwhile, the absolute value of the balance was increased in the minodronate group and decreased in the raloxifene and eldecalcitol groups at week 12. The rate of change of the balance at week 12 was positive in the minodronate and eldecalcitol groups, probably because there was still some remaining influence from teriparatide at week 12. In other words, minodronate greatly reduced TRACP5b through its potent bone resorption suppression effect but did not substantially reduce BAP affected by teriparatide, thus shifting the balance toward bone formation. In the case of eldecalcitol, which has a weak suppressive effect on bone resorption, TRACP5b was only slightly reduced, and BAP reduction was even less, resulting in a relative positive rate of balance change. In the minodronate group, the rate of balance change became negative at week 24 but was positive and gradually increased at weeks 36 and 48. In the raloxifene and eldecalcitol groups, the rate of change for the balance gradually increased to large negative values from week 24 onward, with a greater change observed in the eldecalcitol group. This was presumably because raloxifene, a weak bone resorption suppressor, shifted the balance gradually toward bone resorption dominance, whereas minodronate, with its potent bone resorption suppression effect, shifted the balance toward bone formation dominance over time. Eldecalcitol made an even greater shift toward bone resorption dominance than raloxifene, probably because the former is an even weaker bone resorption suppressor than the latter. These findings indicate that the residual effect of teriparatide must be taken into account when assessing the balance before week 12; however, thereafter, the balance seems to change according to the bone resorption suppression effect of the drug.
It was necessary to consider pre-treatment with teriparatide when the changes in BMD and BTMs were evaluated in this study. The rates of increase in BMD were lower in the three groups than in patients who were treated with each medicine without pre-treatment for osteoporosis (5, 3.3, and 2% in the minodronate, raloxifene, and eldecalcitol groups, respectively, at 6 months, as per the results of previous studies) [23–25]. Therefore, it appears to be a limitation that the BMD-increasing effect was weaker in patients provided pre-treatment with teriparatide than in those not provided pre-treatment. Conversely, the rates of decrease in BTMs were higher in the minodronate and eldecalcitol groups than in patients without pre-treatment for osteoporosis (45.5% in BAP and 49.5% in NTX in the minodronate group and 29% in BAP and 28% in NTX in the eldecalcitol group at 6 months) [22, 24]. This was regarded as a possible reason for the turnover rates being higher at the beginning of this study in the minodronate (2.10) and eldecalcitol (1.98) groups than in the no pre-treatment group and appears to be a benefit of pre-treatment with teriparatide. However, the rate of decrease in BTMs was lower in the raloxifene group than in the no pre-treatment group (41.3% in BAP and 34.2% in NTX at week 24, as per the results of previous studies) [23]. Furthermore, no difference was observed in the rates of decrease in the turnover rate between the raloxifene and eldecalcitol groups throughout this study, whereas the potency of raloxifene on bone resorption suppression was greater than that of eldecalcitol. It appears that the changes in BTMs in the raloxifene group are affected by teriparatide by a mechanism different from that in the minodronate and eldecalcitol groups.
When teriparatide is administered, the activation of osteoclasts occurs in association with the activation of osteoblasts, and bone resorption is also enhanced [6, 10, 26]. Thus, switching to a drug with a more potent bone resorption suppression effect is thought to reopen the ‘anabolic window’ [18]. In the present study, the rate of change was larger in TTRACP-5b than in BAP at week 12 in the minodronate group, in which bone resorption suppression was likely to be the strongest. Therefore, the balance at week 12 had significantly shifted toward formation dominance compared with that at week 0. This suggests that the anabolic window had reopened by week 12 in the minodronate group, resulting in the increment of BMD at week 24. The balance, however, shifted again toward resorption dominance from week 24 onward. Thus, it is likely that the effects of the anabolic window, which had been opened by week 12, became reduced again after week 24 and did not lead to an increased BMD at week 48. However, the balance rate of change remained positive at weeks 36 and 48 in the minodronate group compared to that at the time the drug was changed, which may explain why BMD was not significantly reduced at week 48. In the raloxifene group, both lumbar spine and femur BMD values were increased at weeks 24 and 48 compared to those at the time of drug change, although the differences were not statistically significant. In the eldecalcitol group, however, BMD values decreased compared to those at the time of drug change (except for the lumbar spine BMD at week 24). In both the raloxifene and the eldecalcitol groups, the balance shifted toward resorption dominance throughout the course of the study period. However, the difference between the rate of changes in BAP and TRACP-5b increased gradually in the raloxifene group, although it increased greatly in the eldecalcitol group after week 24. Therefore, the slope of the change in balance was greater for the eldecalcitol group than for the raloxifene group. In other words, the balance shift toward resorption dominance in the eldecalcitol group was greater than that in the raloxifene group, which may explain the differences in how BMD changed. These findings explained why that BMD-increase effect was weaker in the eldecalcitol group than in the raloxifene group despite a similar change in the turnover rates in these groups. Therefore, it appears that not only the reduced turnover rate but also the altered balance makes a major contribution to the increase in BMD.
There were six limitations in this study. First, the subjects were of an advanced age (mean 82.4 years); this was due to one of the conditions for starting teriparatide treatment. However, this age range makes the study more relevant to the actual clinical practice in Japan, where teriparatide is administered to osteoporosis patients with a high risk of fracture. Second, the subjects were randomized using a block replacement method based on their lumbar spine BMD. Therefore, unevenness in the number of participants in three groups was present at the time of study initiation (41, 42, and 38 subjects in the minodronate, raloxifene, and eldecalcitol groups, respectively). Third, there was no control group in which treatment was not provided after teriparatide discontinuation. Fourth, there were reports that lower serum 25(OH)D levels increased the risk of inadequate response to antiresorptives and that the effect of eldecalcitol on BMD was independent of serum 25(OH)D levels [27, 28]. However, serum 25(OH)D levels were not evaluated in this study. Fifth, because the turnover rate and the balance were calculated on the basis of the BTM values of untreated postmenopausal women with osteoporosis as a reference, the effects of treatment could be relatively evaluated with them. However, the depth of the examination did not facilitate evaluation of the absolute values of the turnover rate and of the balance. Therefore, they could only be used to observe the relative changes in bone metabolism. Finally, we did not include denosumab, which is shown to have a potent suppressive effect on bone resorption. These topics are therefore issues for future studies.
The novel points of this study were that the subjects received teriparatide for 24 months with ≥90% adherence and that the BMD-increasing effects of bone resorption inhibitors were evaluated using both turnover rate and balance. In conclusion, as the BMD-increasing potency of minodronate, raloxifene, and eldecalcitol was anticipated to decrease in this order, minodronate was the best choice to be used as medication after 24 months of treatment with teriparatide. All these drugs had significantly reduced the bone turnover rate by week 12; the rate of decrease was greatest with minodronate. When analyzing the bone formation–resorption balance, the residual effects of teriparatide should be taken into account up to week 12. The BMD-increasing effect depends not only on the decrease in turnover rate but also on changes in balance. The consideration of both turnover rate and balance was necessary to evaluate the effects of BTMs in post-teriparatide therapy.
References
Yamamoto T, Taketsuna M, Guo X, Sato M, Sowa H (2014) The safety and effectiveness profile of daily teriparatide in a prospective observational study in Japanese patients with osteoporosis at high risk for fracture: interim report. J Bone Miner Metab 32:699–708
Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 20:1507–1513
Jakob F, Oertel H, Langdahl B, Ljunggren O, Barrett A, Karras D, Walsh JB, Fahrleitner-Pammer A, Rajzbaum G, Barker C, Lems WF, Marin F (2012) Effects of teriparatide in postmenopausal women with osteoporosis pre-treated with bisphosphonates: 36-month results from the European Forsteo Observational Study. Eur J Endocrinol 166:87–97
Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, Audran M, Boonen S, Brixen K, Gomes JM, Obermayer-Pietsch B, Avramidis A, Sigurdsson G, Glüer CC (2009) Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res 24:726–736
Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS (2009) Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab 94:2915–2921
Adami S, San Martin J, Muñoz-Torres M, Econs MJ, Xie L, Dalsky GP, McClung M, Felsenberg D, Brown JP, Brandi ML, Sipos A (2008) Effect of raloxifene after recombinant teriparatide [hPTH(1-34)] treatment in postmenopausal women with osteoporosis. Randomized controlled trial. Osteoporos Int 19:87–94
NIH consensus Development Panel on Osteoporosis Prevention (2001) Diagnosis, and therapy. JAMA 285:785–795
Nishizawa Y, Ohta H, Miura M, Inaba M, Ichimura S, Shiraki M, Takada J, Chaki O, Hagino H, Fujiwara S, Fukunaga M, Miki T, Yoshimura N (2013) Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition). J Bone Miner Metab 31:1–15
Canalis E, Giustina A, Bilezikian JP (2007) Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357:905–916
Nakatoh S (2016) The importance of assessing the rate of bone turnover and the balance between bone formation and bone resorption during daily teriparatide administration for osteoporosis: a pilot study. J Bone Miner Metab 34:216–224
Rubin MR, Bilezikian JP (2003) The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med 19:415–432
Miyauchi A, Matsumoto T, Sugimoto T, Tsujimoto M, Warner MR, Nakamura T (2010) Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 47:493–502
Bieglmayer C, Kudlacek S (2009) The bone marker plot: an innovative method to assess bone turnover in women. Eur J Clin Investig 39:230–238
Soen S, Fukunaga M, Sugimoto T, Sone T, Fujiwara S, Endo N, Gorai I, Shiraki M, Hagino H, Hosoi T, Ohta H, Yoneda T, Tomomitsu T (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Barrett-Connor E, Weiss TW, McHorney CA, Miller PD, Siris ES (2009) Predictors of falls among postmenopausal women: result from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int 20:715–722
Nakatoh S (2014) Utility of calculations bone turnover rates and bone formation/resorption ratios in osteoporosis care (in Japanese). Osteoporos Jpn 22:133–140
Panico A, Lupoli GA, Marciello F, Lupoli R, Cacciapuoti M, Martinelli A, Granieri L, Iacono D, Lupoli G (2011) Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med Sci Monit 17:CR442–CR448
Muschitz C, Kocijan R, Fahrleitner-Pammer A, Pavo I, Haschka J, Schima W, Kapiotis S, Resch H (2014) Overlapping and continued alendronate or raloxifene administration in patients on teriparatide: effects on areal and volumetric bone mineral density—the CONFORS Study. J Bone Miner Res 29:1777–1785
Ebina K, Hashimoto J, Kashii M, Hirao M, Kaneshiro S, Noguchi T, Tsukamoto Y, Yoshikawa H (2017) The effects of switching daily teriparatide to oral bisphosphonates or denosumab in patients with primary osteoporosis. J Bone Miner Metab 35:91–98
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ (2001) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296:235–242
Fleisch H (2000) Bisphosphonates in bone disease. Academic Press, San Diego
Matsumoto T, Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Morii H, Ohashi Y, Nakamura T (2009) Effect of daily oral minodronate on vertebral fractures in Japanese postmenopausal women with established osteoporosis: a randomized placebo-controlled double-blind study. Osteoporos Int 20:1429–1437
Morii H, Ohashi Y, Taketani Y, Fukunaga M, Nakamura T, Itabashi A, Sarkar S, Harper K (2003) Effect of raloxifene on bone mineral density and biochemical markers of bone turnover in Japanese postmenopausal women with osteoporosis: results from a randomized placebo-controlled trial. Osteoporos Int 14:793–800
Matsumoto T, Ito M, Hayashi Y, Hirota T, Tanigawara Y, Sone T, Fukunaga M, Shiraki M, Nakamura T (2011) A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone 49:605–612
Hagino H, Shiraki M, Fukunaga M, Nakano T, Takaoka K, Ohashi Y, Nakamura T, Matsumoto T (2012) Three years of treatment with minodronate in patients with postmenopausal osteoporosis. J Bone Miner Metab 30:439–446
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184
Díez-Pérez A, Olmos JM, Nogués X, Sosa M, Díaz-Curiel M, Pérez-Castrillón JL, Pérez-Cano R, Muñoz-Torres M, Torrijos A, Jodar E, Del Rio L, Caeiro-Rey JR, Farrerons J, Vila J, Arnaud C, González-Macías J (2012) Risk factors for prediction of inadequate response to antiresorptives. J Bone Miner Res 27:817–824
Matsumoto T, Kubodera N (2007) ED-71, a new active vitamin D3, increases bone mineral density regardless of serum 25(OH)D levels in osteoporotic subjects. J Steroid Biochem Mol Biol 103:584–586
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was planned as an investigator-initiated study, and necessary costs were supported by a research grant from Astellas Pharma.
About this article
Cite this article
Nakatoh, S. Effect of osteoporosis medication on changes in bone mineral density and bone turnover markers after 24-month administration of daily teriparatide: comparison among minodronate, raloxifene, and eldecalcitol . J Bone Miner Metab 36, 221–228 (2018). https://doi.org/10.1007/s00774-017-0829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0829-4