Abstract
Introduction
Since the soluble receptor activator of the NF-κB ligand (sRANKL) as well as the endogenous anti-resorptive cytokine osteoprotegerin (OPG) are produced by osteoblasts and given that these cells undergo significant changes during antiresorptive treatment, we hypothesized that treatment with bisphosphonates (BP) would be accompanied by changes in serum OPG and sRANKL levels.
Methods
In a prospective, randomized controlled trial of previously untreated postmenopausal women with osteoporosis, oral BP therapy (daily doses of either 10 mg alendronate or 5 mg risedronate) in combination with calcium/vitamin D was compared to calcium/vitamin D treatment alone (control group). Follow-up at 2, 6 and 12 months was completed for 56 patients. Standardized spinal X-rays were performed at baseline, and DEXA measurements at the femoral neck and trochanter were made at baseline and after 1 year. Serum OPG and sRANKL levels were measured with a polyclonal antibody-based ELISA system.
Results
After 1 year, there was a non-significant loss in neck and trochanteric bone mineral density (BMD) in the CTR group and a mean increase of 3.3% and 4.6% in the combined BP group (both p<0.0001), respectively. Serum levels of C-terminal telopeptides of type I collagen (sCTX) and osteocalcin decreased by 12% and 10% at 12 months in the CTR group and by 43% and 23% in the combined BP group, respectively (all significant). OPG serum levels in the CTR group decreased significantly by 9% at 2 months (p<0.005) and remained below pre-treatment levels at later time points. Both the alendronate- and risedronate-treated patient groups showed unaltered OPG levels after 2 months, but they had significantly increased serum levels at 6 and 12 months. Levels of sRANKL were unchanged throughout the treatment period. Univariate regression analysis demonstrated that changes in serum OPG levels after 12 months of BP treatment were positively and better correlated to BMD changes (trochanter: r= 0.59, p<0.0001; neck: r= 0.50, p<0.001) than those of sCTX, which showed the expected negative correlation to BMD change (trochanter: r= –0.35, p=0.03; neck: r= –0.23, p=0.16). With multiple regression analyses at 12 months, R2 values for 1-year changes in trochanteric BMD of 0.33 (OPG alone) and 0.23 (sCTX alone) were significantly improved to the 0.57 when OPG and sCTX changes were combined (p<0.001). Results for the femoral neck were also statistically significant R2=0.35, p<0.001). BMD and OPG changes in the CTR group were not correlated with each other.
Conclusions
We conclude that with BP treatment, changes in serum OPG levels, unlike changes in sCTX levels, are positively correlated to changes in BMD response. The BP-related changes in serum OPG levels during treatment could result from effects on osteoclastogenesis and osteoclast apoptosis as well as from a direct stimulatory effect on osteoblastic OPG production. These changes in OPG levels may be used to predict the individual response of patients to BP treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphosphonates (BP) are effective and widely used drugs for the treatment of osteoporosis. The newer BP – after being internalized by osteoclasts – inhibit the mevalonate pathway [1] and increase the production of Apppi, an ATP analogue [2]. These processes prevent the osteoclast from forming a ruffled border, and the cells subsequently undergo apoptosis. On the cellular level, BP reduce activation frequency and remodeling space, decrease the probability of trabecular perforations and failure and increase bone density [3]. A prolongation of the formation period further allows for an extended secondary mineralization phase [3, 4].
There is a growing body of evidence that BP may also act on osteoblastic bone lineage cells. It has been suggested that BP require osteoblasts for the mediation of bone resorption [5, 6] and the possible modulation of the secretion of osteoblast-derived soluble factor(s) [7]. It was subsequently shown that BP may also inhibit osteoclastic activity by suppressing osteoblastic interleukin-(IL)-6 secretion [8, 9], which is a well-characterized bone-resorbing cytokine. Recent reports propose that BP are capable of stimulating differentiation and inhibiting proliferation of the human fetal osteoblastic lineage cell line hFOB [10] and of decreasing osteoblastic apoptosis in murine vertebral cancellous bone cells [11]. The finding that BP stimulate alkaline phosphatase and osteocalcin expression [10, 12–14] as well as the number of colony-forming units of osteoblasts [15] further supports the idea that these drugs may potentially influence cells of the osteoblastic lineage, independent of their inhibitory action on osteoclasts.
One candidate key factor likely to be involved in the modulation of an osteoblast-derived anti-resorptive factor during BP treatment is osteoprotegerin (OPG), a soluble receptor produced by osteoblastic cells that inhibits osteoclast maturation by binding to nuclear factor κB ligand (RANKL). There are in vitro data showing that OPG is markedly up-regulated in primary human trabecular osteoblasts by pamidronate and zoledronate [13]. This finding has very recently been confirmed by Pan et al. [16] who found a time- and dose-dependent increase in the level of secreted OPG protein and zoledronate at doses at which osteoblasts are thought to be exposed in vivo [17].
In various cross-sectional studies, measurements of serum OPG levels have been found to be either correlated [18, 19] or not correlated [20, 21] to bone density, whereas the local expression of OPG mRNA levels in iliac bone biopsies has been correlated with increased fracture susceptibility [22]. Similarly, fractures have [18, 23] or have not been [24] associated with prevalent or incident fractures.
There are, however, a number of studies demonstrating correlations of OPG with age [19–21, 23] and bone-turnover [20, 21, 23] and associations of OPG with various diseases affecting bone [25–28]. This has led to the notion that circulating OPG relates – at least in part – to metabolic changes within the bone compartment. There is very little information on longitudinal OPG measurements and possible correlations with bone mineral density (BMD) or marker changes in osteoporotic patients undergoing pharmacologic treatment [29].
Since treatment with BP is accompanied by significant changes in bone turnover and is likely to influence osteoblastic lineage cells and OPG secretion, we tested this hypothesis by treating patients with established osteoporosis with two different BP in order to analyse the relationship between changes in serum OPG levels and markers of bone turnover or BMD.
Patients and methods
Study subjects and treatment
At our outpatient endocrinology clinic we recruited 61 postmenopausal women older than 60 who fulfilled criteria for osteoporosis according to WHO guidelines [30] and had a bone mineral density T-score of <−2.5 SD at the femoral neck. Women with a history of renal or gastrointestinal disease, active malignancy, hyperthyroidism or hyperparathyroidism were excluded from the study. Patients using BP, hormone replacement therapy, calcitonin, fluorides, anabolic drugs or calcitriol within the year prior to study enrolment or on medications known to affect bone metabolism (e.g. anticonvulsants, statins, glucocorticoids) were excluded.
All study subjects were randomized (using the permuted block method with a block size of four patients per block) to receive daily oral BP treatment with either risedronate 5 mg (n=21) or alendronate 10 mg (n=20) in combination with 1,000–1,200 mg calcium and 400–800 IU of vitamin D for 12 months. A control (CTR) group (n=20) was treated with calcium and vitamin D only. Follow-up visits were scheduled at 2, 6 and 12 months. One woman (risedronate) was lost to follow-up, one patient died of a heart attack (CTR) and three women stopped oral BP treatment (one on risedronate, two on alendronate) because of adverse gastrointestinal events. Fifty-six patients completed the protocol. Subjects were advised of the nature of the study and gave written informed consent. The study was approved by the local ethics committee.
Bone density measurements
All patients underwent BMD measurements of the femoral neck (Hologic QDR 4500 acclaim) at baseline and after 1 year of treatment. The in vivo coefficient of variation for femoral neck measurements at our institution is 1.6%. Due to a high prevalence of vertebral fractures and degenerative changes, lumbar spine bone density was not considered to be a reliable measurement in these patients and was, therefore, not performed. Bone density measurements are expressed as T- and Z- score values that compare individual results to the normative database (NHANES II).
Spinal radiographs
Before enrolment, all patients underwent standardized X-rays of the spine, and the films were analyzed by an experienced radiologist. Based on the method described by Genant et al. [31], a prevalent vertebral fracture was diagnosed when there was a ≥20% reduction in any of the anterior, middle or posterior height ratios of the vertebral body compared to undeformed adjacent vertebrae.
Laboratory analysis
Blood samples were drawn in the morning between 08.00 and 10.00 a.m. after an overnight fast and the sera aliquoted. Routine serum parameters were measured by an autoanalyzer (Hitachi 747).
Serum OPG was measured using a commercially available, highly sensitive polyclonal antibody-based sandwich enzyme immunoassay (Biomedica GmbH, Vienna, Austria) with an intra- and inter-assay variability of 9% and 10%, respectively, as previously reported [21]. Measurements were performed in undiluted samples according to the manufacturer’s instructions. A normal range of 6–138 pg/ml (mean ± SD: 77±48) was established in our own laboratory using sera from 177 healthy, subjects. The method of recruitment of control subjects as well as the detailed clinical information has been published elsewhere [23]. The lower limit of detection was 1.2 pg/ml. All samples were measured in duplicate and averaged. If the coefficient of variation was >20%, the sample was re-assayed in duplicate. In general, serum levels of OPG showed a high intra-individual reproducibility and were not normally distributed. In a different cohort of 195 healthy female and male individuals above 70 years, we found a correlation of r=0.81 (p<0.0001) between two OPG measurements that were 1 year apart (unpublished data).
Free serum RANKL was measured using a highly sensitive polyclonal antibody-based sandwich enzyme immunoassay (Biomedica GmbH, Vienna, Austria) with an intra- and inter-assay variability of 4% and 7.5%, respectively. The normal range established in 177 of our own healthy controls was 1.9–8.5 pg/ml.
Bone resorption was assessed by measuring serum C-terminal telopeptides of type I collagen (sCTX) concentrations (ELISA, Nichols Diagnostics, San Juan Capistrano, Calif.), and bone formation was assessed by determining osteocalcin serum levels (chemoluminescence immunoassay, Nichols Diagnostics, San Juan Capistrano, Calif.). Intact parathyroid hormone (iPTH) was analysed using a chemoluminescence immunometric assay (Nichols Diagnostics, San Juan Capistrano, Calif.), and 25-hydroxyvitamin D was measured by radioimmunoassay following extraction (Immunodiagnostic Systems, Boldon, UK). For this study, all serum specimens of an individual patient were run in one assay.
Statistical analysis
Based on an initial cross-sectional observation of a 12.5% difference in OPG levels between BP-treated and untreated patients and the calculated high degree of intra-individual reproducibility of serum OPG levels (see above), we hypothesized an average difference of 10% and more to be clinically relevant. In order to demonstrate a similar difference of OPG levels between two groups, we calculated that the inclusion of 20 control and 40 BP-treated subjects would enable a calculated power of 86% to be achieved.
Unless otherwise noted, all data are expressed as mean values ± SD. Differences between baseline demographic, densitometric or biochemical data among CTR- and BP-treated groups were calculated using either the Mann-Whitney U-Test or, for categorical variables, the chi-square test. Within-group percentage differences of BMD or biochemical variables were calculated using the Wilcoxon matched pairs test, and between-group differences (CTR vs. BP) were calculated by the Mann-Whitney U-Test. Changes between OPG serum levels and either sCTX or BMD changes were analysed with Spearman´s R correlation analysis. Changes in femoral neck and trochanteric BMD and their correlation to changes in OPG, sCTX or osteocalcin levels were calculated by univariate and multiple regression analyses. A p value of <0.05 was considered to be significant. All statistical analyses were performed using the statistical software packages Stat View (Abacus Concepts, Berkeley, Calif.) and Statistica (StatSoft, Tulsa, Okla.).
Results
Patient groups and baseline parameters
Baseline data are given in Table 1. Vertebral fractures were present in 26% (CTR), 28% (alendronate) and 47% (risedronate) of the patients. The mean number of vertebral fractures per affected patient was 1.9 (CTR), 1.8 (alendronate) and 1.8 (risedronate) with a range between 1 and 4. Femoral trochanteric Z-scores were significantly higher in the CTR group than in the BP-treated patients due to the drop-out of one patient in the CTR group who had the lowest Z-score. Baseline measurements of trochanteric Z-scores of all 61 initial patients included did not differ significantly between the two groups (p=0.11). Since neither demographic data nor baseline- or follow-up parameters between the alendronate- and risedronate-treated groups revealed any statistically significant difference (data not shown), patients were treated as a single group for further statistical analyses. For selected analyses, however, additional data are also given separately for both BP groups in order to demonstrate the similarity of the biological response.
Baseline sCTX and osteocalcin levels were highly correlated to each other (r=0.77, p<0.0001). Baseline serum OPG levels showed a non-parametric distribution (significant Lilliefors and Kolmogorov-Smirnov test), and a comparable correlation with sCTX and osteocalcin levels (r=0.58 and r=0.55, p<0.0001). Serum OPG was also correlated to PTH (r=0.39, p<0.002) and 25-hydroxyvitamin D (r=–0.30, p<0.001) concentrations. Although PTH and 25-hydroxyvitamin D levels were correlated to each other (r=–0.40, p<0.005), a multiple regression analysis revealed that both parameters were also independently correlated to serum OPG levels (β-coefficients of 0.27 and –0.30, both p<0.05).
Patients with (n=18) and without (n=38) prevalent vertebral fractures differed in their mean serum OPG level (71.0±3.5 vs. 89.7±2.9 pg/ml), which was 21% lower in patients exhibiting fractures at the baseline visit (p<0.0005).
Results regarding changes in BMD, bone markers and serum OPG measurements
Patients in the CTR group showed a non-significant decrease in both femoral BMD measurements (Table 2), whereas in patients receiving BP treatment, similar results were achieved for both alendronate and risedronate (femoral neck: 3.29±0.54 vs. 3.38±0.73%, p=0.85; trochanteric region: 4.54±0.85 vs. 4.66±1.14%, p=0.96).
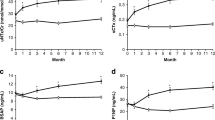
Serum CTX and osteocalcin levels showed the expected magnitude and time course of declining mean levels in both the CTR- and BP-treated groups. Individual serum profiles for sCTX and OPG levels for both treatment groups are given in Fig. 1.
Individual patient profiles of serum C-terminal telopeptides of type I collagen (sCTX) and osteoprotegerin (OPG) concentrations of 19 patients with osteoporosis who were treated with calcium/vitamin D (control group) and 37 patients who were treated with additional oral bisphosphonates (BP) for 1 year
The effects of calcium/vitamin D supplementation in the CTR group were reflected by an increase in vitamin D and a decrease in PTH and sCTX levels (Table 2, Fig. 2). Serum OPG levels were at significantly lower levels 2 months after the initiation of calcium/vitamin D treatment, and they stayed below pretreatment levels during the remainder of the study period (Fig. 2). The early decrease in serum OPG was correlated with the decrease in sCTX levels (Fig. 3).
Changes in the levels of serum C-terminal telopeptides of sCTX and OPG in calcium/vitamin D-treated controls and patients given additional alendronate or risedronate treatment over the course of 1 year [mean (columns) ± SE (narrow bars)]. Levels of significance: a, p<0.05; b, p<0.01; c, p<0.005 compared to baseline measurements. The changes between the control patients and the BP-treated patients were different at all three time points (see Table 2)
Correlation analysis between changes in serum OPG and serum C-terminal telopeptides of sCTX for controls and BP-treated patients after 2 months of treatment demonstrating a significant relationship between changes in serum OPG and bone turnover induced by calcium/vitamin D (control group) and calcium/vitamin D/BP treatment (bisphosphonate group)
Relative to the CTR group, there was a significantly greater decrease in serum sCTX and osteocalcin levels at all time points in the BP-treated group. Serum OPG levels were unchanged at the month 2 time point following initiation of the study, but they increased significantly above baseline thereafter (Fig. 2). Figure 2 also shows that similar changes in sCTX and OPG occurred for both BP. Similarly to the CTR-treated patients, changes in serum OPG and sCTX levels at 2 months were correlated to each other (Fig. 3). Figure 4 presents the correlations between changes in serum OPG and trochanteric as well as neck BMD measurements after 1 year of treatment.
Uni- and multivariate correlation analyses for changes in serum OPG, bone turnover markers and BMD measurements
For the calcium/vitamin D-treated patients, BMD changes were not related to either changes in bone turnover markers or serum OPG levels. After 1 year of BP treatment, 12% and 35% of the variance in trochanteric BMD change could be explained by univariate changes in serum sCTX and OPG levels, respectively (Table 3). Changes in OPG levels for both BP were associated with femoral neck BMD outcome: R2 value was 0.22 for alendronate (p<0.05) and 0.42 for risedronate (p<0.005). Changes in femoral neck BMD at 6 and 12 months were reflected only by changes in serum OPG levels. In additional analyses, the inclusion of age and baseline femoral BMD results had no influence on the significance levels of the regression analysis.
When multiple regression analyses were applied (Table 4), changes in serum sCTX, osteocalcin and OPG independently reflected femoral neck- and trochanteric BMD changes by varying degrees. At 2 months, no marker, either alone or in combination, could predict 1-year BMD changes during BP treatment. At 6 months, R2 values for 1-year changes in trochanteric BMD (sCTX and OPG alone) improved to 0.35 (p<0.001) when both parameters were combined in the analysis. The addition of osteocalcin provided no further improvement. At 12 months, R2 values for 1-year changes in trochanteric BMD also improved to 0.57 (p<0.001) when both OPG and sCTX changes were combined. The addition of osteocalcin also had no influence on this result. Multivariate analysis, including changes in femoral neck BMD measurements and OPG as well as bone turnover markers, yielded highly significant, although lower R2 values. At the femoral neck, as in the trochanteric region, OPG was the best independent parameter reflecting BMD changes.
When multiple regression analysis for the two BP was performed separately, both alendronate- R2=0.43, p<0.01) and risedronate- R2=0.60, p<0.005) treated patients behaved similarly in terms of BMD outcome and changes in OPG and sCTX at the femoral trochanter after 12 months.
There was no significant correlation between OPG and sCTX changes at 6 or 12 months. The three-dimensional surface plot shown in Fig. 5 illustrates how changes in trochanteric BMD related to changes in sCTX or OPG levels or both.
Three-dimensional surface plot illustrating changes in trochanteric bone mineral density in relationship to changes in sCTX and serum OPG levels after 1 year of BP treatment. The decrease in sCTX as well as the increase in OPG is correlated to the increase in trochanteric BMD. The largest increase in BMD occurred when decreases in sCTX were accompanied by increases in serum OPG. For a better orientation, the figure legend provides information on different percentage changes in trochanteric BMD
Discussion
The major finding of this study is that BP treatment results in distinct changes in serum OPG concentrations that, in contrast to markers considered to reflect bone resorption or turnover, are positively correlated to the gain in BMD.
Whenever correlations between treatment-related changes in bone turnover markers and BMD were reported for antiresorptive agents in the past – and found to be significant – they were invariably negative, indicating that increases in BMD are paralleled by decreases in bone turnover [32–35]. The changes in serum OPG levels in the present study indicated, however, that increases in BMD were accompanied by a positive change. Here, BMD changes were better reflected by changes in OPG than by sCTX levels. Whereas sCTX change in a univariate analysis accounted for 12% of the trochanteric BMD variance, OPG achieved 35%. Combining these two markers improved this value to 57%, indicating that sCTX and OPG reflected BMD changes independent of each other.
Early reductions in serum OPG levels in both the control and BP-treated patients were related to decreases in sCTX levels, thereby providing still more evidence that serum OPG concentrations are at least partly reflective of bone turnover [36]. This is also supported in the present study by the significant relationship between baseline bone turnover markers and OPG levels. After 2 months of treatment, however, bone turnover continued to decrease in the BP group, while at the same time serum OPG levels began to rise above pre-treatment levels in both the alendronate- and risedronate-treated patients. Using a similar study design, Sankaralingam et al. [29] found that in 18 osteoporotic women treated with daily risedronate for 1 year the serum OPG levels remained unchanged during the first 6 months followed by a decrease at 1 year. A limitation of their study was the lack of a control group as well as a significant change in BMD with the risedronate-treated group. Similarly to the present study, however, they reported significantly better BMD responses in patients with positive changes in serum OPG levels. Patients with reductions in OPG had a less favourable BMD outcome.
Conceptually, there are at least three mechanisms whereby osteoblastic OPG production may potentially be influenced during treatment with BP: (1) a mechanism in which osteoblastic activity is changed because of reduced osteoclastic activity; (2) a mechanism that involves a direct and specific effect of BP on OPG production; (3) a mechanism that is related to alterations in osteoblast differentiation. Most likely, all of these effects play a role in such a setting.
BP have a direct antiresorptive effect on osteoclasts [37] that feeds back on osteoblast activity by decreasing the proliferation and differentiation of osteoblasts via a coupling mechanism that is still poorly defined. This phenomenon is also reflected by the finding of an 80–90% decrease in the mineralizing surface of transiliac bone specimens 6 and 12 months after the initiation of BP treatment [38, 39]. Consequently, at least at this site this finding has to translate into a significant reduction in the number of trabecular osteoblasts.
If a significant source of circulating OPG indeed originates from osteoblasts, then the changes seen in serum OPG levels during the first year of BP treatment have to be interpreted against the background of a reduction in osteoblast cell number. Because OPG levels rise even above pre-treatment levels, absolute OPG production per osteoblast has to increase markedly. Further and strong support of this conclusion is offered by Viereck et al. [13] as well as recently by Pan et al. [16], both of whom presented evidence of a direct stimulatory effect of pamidronate and zoledronate on OPG gene expression and protein secretion in human osteoblastic cells. In the present study, BP-induced changes in serum OPG levels were similar in the alendronate- and risedronate treated patients, indicating a similar OPG response pattern shared by these two different BP.
It is plausible that both of these mechanisms – i.e. direct effects on OPG production and a decreasing osteoblastic cell population – are relevant for the interpretation of serum OPG levels. Early direct stimulatory effects on osteoblastic OPG production are likely influenced by the concomitant decrease in bone turnover encountered with BP treatment. At later time points, with less relative changes in bone turnover, stimulated osteoblastic OPG production may become more evident. At 12 months, 92% of our BP patients showed serum OPG levels at or above pre-treatment values, as opposed to 53% in the control group. Thus, subsequent to their early direct effects on osteoclasts, BP may then support the inhibition or apoptosis of osteoclasts through paracrine mechanisms originating from osteoblastic lineage cells to work in concert to inhibit bone resorption.
Finally, an increase in OPG production could also be a consequence of a shift towards more fully differentiated osteoblasts, which are known to secrete more OPG protein than less well differentiated cells [40]. This mechanism is likely to play a minor role, however, because serum osteocalcin, a well-established marker of fully differentiated osteoblasts, would then also be expected to increase, which was not the case.
In the present study, we could not detect any significant changes in sRANKL levels during BP treatment. Since the assay measures only soluble forms of RANKL, it is likely that any changes would immediately be masked by serum OPG that would bind to sRANKL. To our knowledge there are no published data on the in vivo effects of BP on sRANKL serum levels. Recent in vitro data, however, indicate that zoledronic acid may decrease RANKL levels through the cleavage of transmembrane RANKL in osteoblast-like cells by the up-regulation of TACE, which is a known enzyme capable of cleaving RANKL [16]. If there is also a role for RANKL in the process of BP-induced changes in bone metabolism in humans, it still remains to be elucidated.
Calcium and vitamin D treatment led to increases in 25-hydroxyvitamin D levels and consecutive reductions in PTH and bone turnover markers in both groups. The decrease in PTH, which was on the order of 10% with calcium/vitamin D supplementation, suggests the presence of a mild deficiency of vitamin D in our patients at baseline [41]. The likely effect of calcium/vitamin D supplements on raising serum calcium levels in these patients seemed to offset any mild hypocalcaemic and PTH-raising effects that BP may provoke [42, 43]. Despite a lowered bone turnover in the CTR subjects, there was a small, non-significant decrease in femoral BMD, which is a common finding at this measurement site and given the treatment period of 1 year [44].
The interpretation of the results of the present study is limited by the small number of patients and the lack of a control group receiving a BP placebo. However, the most interesting aspects of our study relate to relative changes seen within the BP group and are thus less dependent on comparisons with control subjects.
In summary, the effect of BP treatment on serum OPG is most likely a result of a complex interaction of changes in the osteoblastic cell pool on the one hand and simultaneous stimulatory effects on OPG production on the other. Serum OPG changes reflect BMD changes during BP treatment independent of bone turnover.
We hypothesize that BP may trigger antiresorptive processes by two modes of action: during the early treatment phase, primarily through direct anti-osteoclastic effects, while at later treatment stages the suppression of osteoclastic activity may also be influenced by other indirect effects of BP through OPG.
References
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ (1998) Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res 13:581–589
Mönkkönen H, Lehenkari PP, Kellinsalmi M (2004) A new mechanism of action for bisphosphonates: apppi dedicated cytotoxicity of N-BPs. Bone 34:S66–S67
Eriksen EF, Melsen F, Sod E, Barton I, Chines A (2002) Effects of long-term risedronate on bone quality and bone turnover in women with postmenopausal osteoporosis. Bone 31:620–625
Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ (2000) Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27:687–694
Rodan GA, Martin TJ (1981) Role of osteoblasts in hormonal control of bone resorption- a hypothesis. Calcif Tissue Int 33:349–351
Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ (1993) Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest 91:2004–2011
Vitte C, Fleisch H, Guenther HL (1996) Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology 137:2324–2333
Giuliani N, Pedrazzoni M, Passeri G, Girasole G (1998) Bisphosphonates inhibit IL-6 production by human osteoblast-like cells. Scand J Rheumatol 27:38–41
Olmos JM, De Vega T, Perera L, Riancho JA, Amado JA, Gonzalez Macias J (1999) Etidronate inhibits the production of IL-6 by osteoblast-like cells. Methods Find Exp Clin Pharmacol 21:519–522
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC (2000) Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res 60:6001–6007
Plotkin LI, Weinstein RS, Parfitt AM, Roberson PF, Manolagas SC, Bellido T (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374
Gandolfi MG, Pugnaloni A, Mattioli-Belmonte M, Muzzarelli R, De Benedittis A, Mengucci P, Zucchini C, Tesei M, Caudarella R, Biag G (1999) Osteoblast behaviour in the presence of bisphosphonates: ultrastructural and biochemical in vitro studies. Clin Exp Rheumatol 17:327–333
Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Gründker C, Hofbauer LC (2002) Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 291:680–686
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G (1998) Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 22:455–461
Pan B, Farrugia AN, Bik To L, Findlay DM, Green J, Lynch K, Zannettino CW (2004) The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-α converting enzyme (TACE). J Bone Miner Res 19:147–154
Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A (2002) Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol 42:1228–1236
Mezquita-Raya P, de la Higuera M, Garcia DF, Alonso G, Ruiz-Requena ME, de Dios Luna J, Escobar-Jimenez F, Munoz-Torres M (2005) The contribution of serum osteoprotegerin to bone mass and vertebral fractures in postmenopausal women. Osteoporos Int. 16:1368–1374
Oh KW, Rhee EJ, Lee WY, Kim SW, Baek KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW (2005) Circulating osteoprotegerin and receptor activator of NF-kappaB ligand system are associated with bone metabolism in middle-aged males. Clin Endocrinol 62:92–98
Indridason OS, Franzson L, Sigurdsson G (2005) Serum osteoprotegerin and its relationship with bone mineral density and markers of bone turnover. Osteoporos Int 16:417–423
Szulc P, Hofbauer LC, Heufelder AE, Roth S, Delmas PD (2001) Osteoprotegerin serum levels in men: correlation with age, estrogen, and testosterone status. J Clin Endocrinol Metab 86:3162–3165
Abdallah BM, Stilgren LS, Nissen N, Kassem M, Jorgensen HR, Abrahamsen B (2005) Increased RANKL/OPG mRNA ratio in iliac bone biopsies from women with hip fractures. Calcif Tissue Int 76:90–97
Fahrleitner-Pammer A, Dobnig H, Piswanger-Soelkner C, Bonelli C, Dimai HP, Leb G, Obermayer-Pietsch B (2003) Osteoprotegerin serum levels in women: correlation with age, bone mass, bone turnover and fracture status. Wien Klin Wochenschr 115:291–297
Browner WS, Lui LY, Cummings SR (2001) Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab 86:631–637
Ziolkowska M, Kurowska M, Radzikowska A, Luszczykiewicz G, Wiland P, Dziewczopolski W, Filipowicz-Sosnowska A, Pazdur J, Szechinski J, Kowalczewski J, Rell-Bakalarska M, Maslinski W (2002) High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheum 46:1744–1753
Amato G, Mazziotti G, Sorvillo F, Piscopo M, Lalli E, Biondi B, Iorio S, Molinari A, Giustina A, Carella C (2004) High serum osteoprotegerin levels in patients with hyperthyroidism: effect of medical treatment. Bone 35:785–791
Stilgren LS, Rettmer E, Eriksen EF, Hegedus L, Beck-Nielsen H, Abrahamsen B (2004) Skeletal changes in osteoprotegerin and receptor activator of nuclear factor-kappa β ligand mRNA levels in primary hyperparathyroidism: effect of parathyroidectomy and association with bone metabolism. Bone 35:256–265
Moschen AR, Kaser A, Enrich B, Ludwiczek O, Gabriel M, Obrist P, Wolf AM, Tilg H (2005) The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 54:479–487
Sankaralingam S, Frost M, Fogelman I, Hampson G (2003) Early changes in serum osteoprotegerin (OPG) correlates with changes in bone mineral density following treatment with risedronate in post-menopausal women with osteoporosis (Abstract). J Bone Miner Res 18:S158
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Genant HK, Wu CY, Van Kulik C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Ravn P, Hosking D, Thompson D, Cizza G, Wasnich RD, McClung M, Yates AJ, Bjarnason NH, Christiansen C (1999) Monitoring of alendronate treatment and prediction of effect on bone mass by biochemical markers in the early postmenopausal intervention study. J Clin Endocrinol Metab 84:2363–2368
Christgau S, Rosenquist C, Alexandersen P, Hannover Bjarnason N, Ravn P, Fledelius C, Herling C, Qvist P, Christiansen C (1998) Clinical evaluation of the serum crosslaps one step ELISA, a new assay measuring the serum concentration of bone-derived degradation products of type I collagen C-telopeptides. Clin Chem 44:2290–2300
Greenspan SL, Parker RA, Ferguson L, Rosen HN, Maitland-Ramsey L, Karpf DB (1998) Early changes in biochemical markers of bone turnover predict the long-term response to alendronate therapy in representative elderly women: a randomized clinical trial. J Bone Miner Res 13:1431–1438
Rogers A, Hannon RA, Eastell R (2000) Biochemical markers as predictors of rates of bone loss after menopause J Bone Miner Res 15:1398–1404
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4:268–275
Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF (1995) Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res 10:1478–1487
Chavassieux PM, Arlot ME, Reda C, Wie L, Yates AJ, Meunier PJ (1997) Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest 100:1475–1480
Bone HG, Downs RW, Tucci JR, Harris ST, Weinstein RS, Licata AA, McClung MR, Kimmel DB, Gertz BJ, Hale E Polvino WJ (1997) Dose-response relationships for alendronate treatment in osteoporotic elderly women. J Clin Endocrinol Metab 82:265–274
Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL (2000) The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology 141:4768–4776
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Vasikaran SD (2001) Bisphosphonates: an overview with special reference to alendronate. Ann Clin Biochem 38:608–623
Greenspan SL, Holland S, Maitland-Ramsey L, Poku M, Freeman A, Yuan W, Kher U, Gertz B (1996) Alendronate stimulation of nocturnal parathyroid hormone secretion: a mechanism to explain the continued improvement in bone mineral density accompanying alendronate therapy. Proc Assoc Am Physicians 108:230–238
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Acknowledgements
The authors should like to thank Eugenia Lamont for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dobnig, H., Hofbauer, L.C., Viereck, V. et al. Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos Int 17, 693–703 (2006). https://doi.org/10.1007/s00198-005-0035-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-0035-4