Abstract
Factors involved in inflammation are linked with those critical for bone remodeling. We examined the association between serum high sensitivity C-reactive protein (hsCRP) levels and bone mineral density (BMD) in healthy women. Serum concentrations of hsCRP and total alkaline phosphatase (ALP) were measured in premenopausal ( n =3,662) and postmenopausal ( n =1,031) women aged 30 years or older. BMD was measured at the femoral neck and lumbar spine using dual energy X-ray absorptiometry. According to the WHO definition, osteopenia was diagnosed at –2.5< T -score <–1.0 SD, and osteoporosis was diagnosed at T -score ≤–2.5 SD at any sites. Compared with normal subjects, log-transformed serum hsCRP levels were higher in osteopenic and osteoporotic subjects (all, P <0.001) with linearity ( P for trend <0.001), after adjustment for age, BMI and menopausal status. Menopausal status did not have a significant interaction on the association ( P =0.457). In both premenopausal and postmenopausal women, serum total ALP levels were higher in the subjects with higher hsCRP quintiles than those with the lowest quintile (all, P for trend <0.001). Multivariate-adjusted odds ratio (OR) for osteoporosis and osteopenia were 1.35 (95% CI, 1.08 to 1.68) in the highest hsCRP quintile of premenopausal women, and OR for osteoporosis was 1.54 (95% CI, 1.10 to 2.53) in the highest hsCRP quintile of postmenopausal women. These findings suggest that subclinical systemic inflammation may be associated with bone turnover rate and bone mass in healthy women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is considered an imbalance of the remodeling process, in which bone resorption exceeds bone formation. Growing understanding of this process has shown that factors involved in inflammation are linked with those critical for bone physiology and remodeling [1, 2]. For example, pro-inflammatory cytokines, which are critical mediators of inflammatory responses, have also been found to regulate bone metabolism, even in individuals without immunological diseases [3]. One of the most important of these cytokines is interleukin (IL)-6, which is produced by osteoblasts, monocytes and T-cells and has been implicated in the pathogenesis of various metabolic bone diseases, including postmenopausal osteoporosis [4], Paget’s disease [5], polyostotic fibrous dysplasia [6], osteoporosis associated with hematologic malignancy [7] and Gorham-Stout disease [8]. The IL-6 has been shown to promote osteoclast differentiation and activation [9]. IL-1 is another potent stimulator of bone resorption [10] that has been linked to the accelerated bone resorption seen in idiopathic [11] and postmenopausal osteoporosis [12]. Tumor necrosis factor (TNF)-α was shown to be involved in tumor-induced bone resorption [13] and non-tumor-induced osteopenia [14], as well as to stimulate bone resorption and to inhibit bone formation [14]. In osteoblasts and bone marrow stromal cells, these pro-inflammatory cytokines have been observed to up-regulate the receptor activator of nuclear factor-κB ligand (RANKL) [15], leading to increased osteoclastogenesis and bone resorption. Activated T lymphocytes are also an important source of RANKL [16], supporting the concept that an inflammatory state can contribute to pathologic bone loss.
C-reactive protein (CRP), a member of the pentraxin family of innate immune recognition proteins, is regarded as a sensitive marker of systemic inflammation [17]. CRP is predominantly produced in the liver, and IL-1, IL-6 and TNF-α have been identified as regulators of CRP production [18, 19]. Recently, more sensitive immunoassays for CRP (high sensitivity CRP, hsCRP) have become available, making possible the measurement and comparison of low CRP levels in blood. These sensitive assays have revealed the relationship between hsCRP levels and the development and progression of coronary heart disease [20, 21] and osteoarthritis [22]. In this study, we investigated the association between hsCRP levels and bone mineral density (BMD) in healthy pre- and postmenopausal women.
Subjects and methods
Subjects
The study population consisted of 11,732 Korean women aged 30 years or older in whom BMD was measured consecutively at the Health Promotion Center of Asan Medical Center (Seoul, Korea) between January 2002 and July 2003. A specially trained nurse administered a questionnaire to all subjects to obtain information on their smoking and drinking habits, leisure time physical exercise (more/less than two times per week), medication history, history of previous medical or surgical diseases and reproductive history. The height and weight of each subject were measured while the subject was dressed in light clothing and without shoes. Oral temperature was measured, and a physician examined each patient. Routine laboratory and imaging examinations included complete blood counts; liver, renal and thyroid function tests; serum concentrations of glucose, calcium, phosphorus, alkaline phosphatase (ALP), rheumatoid factor, FSH and hsCRP; urinalysis; stool occult blood test; simple chest X-ray; duodenofibroscopy; sigmoidoscopy; abdominal ultrasonography; mammography and Pap smear.
Women were excluded if any information on menopausal history was missing or if the subject had undergone hysterectomy prior to natural menopause. Women who had taken drugs, including vitamin D, which might affect bone metabolism or hsCRP level for more than 6 months or within the previous 12 months were also excluded. Subjects were excluded if they had suffered from any disease that might affect bone metabolism or hsCRP level. Women who had had a stroke or dementia were also excluded because of concerns related to their limited physical activity. Also excluded were women with fever (oral temperature ≥38.0°C) or any abnormal findings on routine laboratory and imaging examinations, including abnormal leukocyte count (<4.0 or >10.0×109/l); elevated serum aspartate aminotransferase (≥40 U/l), alanine aminotransferase (≥40 U/l), or total ALP (>120 U/l) concentration; abnormal serum calcium (<8.3 mg/dl, <2.1 mmol/l, or >10.0 mg/dl, >2.5 mmol/l) or phosphorus (<2.5 mg/dl, <0.8 mmol/l; or >4.5 mg/dl, >1.4 mmol/l) concentration; decreased serum albumin concentration (<3.0 g/dl<30 g/l); elevated serum creatinine (≥1.5 mg/dl, ≥133 μmol/l) or fasting glucose (≥126 mg/dl, ≥7.0 mmol/l) concentration; abnormal serum free T4 (≤0.8 ng/dl, ≤10 pmol/l, or ≥1.9 ng/dl, ≥24 pmol/l) or TSH (≤0.5 or ≥5.0 mU/l) concentration, or serum rheumatoid factor ≥20 IU/ml. Finally, in order to exclude the subjects with infectious or immune diseases, 26 subjects with hsCRP levels >10 mg/l were excluded [21]. Of the initial 11,732 women, 4,693 were deemed eligible for study, of whom 3,662 were premenopausal and 1,031 were postmenopausal. Postmenopausal status was defined as cessation of menses for at least 1 year, which was confirmed by a serum FSH concentration >30 IU/l. This study was approved by the Ethics Committee of Asan Medical Center.
Biochemical measurements
Serum hsCRP concentration was measured by a particle-enhanced immunoturbidometric method using a Cobas Integra 700 (Roche Diagnostic System, Basel, Switzerland). According to the EP5-A proposal of the National Committee for Clinical Laboratory Standards, the intra- and inter-assay coefficients of variations (CVs) were 1.3 and 3.0%, respectively. The median hsCRP concentration in 1,399 healthy persons was 0.64 mg/l, and the 25th, 75th, and 95th percentiles were 0.34, 1.23, and 4.01 mg/l, respectively [23].
Serum ALP concentration was measured by the Bowers and McComb method at 37°C with AMP buffer using a Toshiba 200 FR Autoanalyzer (Toshiba Medical Systom Co., Ltd., Tokyo). The intra- and inter-assay CVs were 0.7 and 1.3%, respectively, and the reference interval was 40–120 U/l.
BMD measurements
Areal BMD (g/cm2) was measured at the non-dominant femoral neck and the anterior-posteriorlumbar spine (L2–L4) using dual energy X-ray absorptiometry (QDR 4500-A, Hologic, Inc., Waltham, Mass.), software version 4.84. The in vivo precision of the machine was 1.2% for the femoral neck and 0.85% for the lumbar spine. Normal T -score was calculated using the installed software of the apparatus, in which mean ± SD BMD was established from those of the northeastern Asian young women [24]. According to the WHO definition, osteopenia was diagnosed at –2.5< T -score <–1.0 SD, and osteoporosis was diagnosed at T -score ≤–2.5 SD at any sites.
Analyses
Bivariate associations between menopausal status and clinical and non-clinical variables were determined using the chi-square test for categorical variables and the t test with a normal distribution for continuous variables. Serum hsCRP concentration, which showed a positively skewed distribution, was compared using the Wilcoxon rank-sum test, and was categorized into five levels at the nearest quintile points in other analyses. Analysis of variance (ANOVA) test and chi-square test were used to compare clinical and behavioral characteristics according to quintiles for serum hsCRP level. The Tukey method was used for multiple comparisons in the ANOVA test. After adjustments for age and body mass index (BMI), and, for postmenopausal women, years since menopause, the means and 95% confidence intervals of log-transformed hsCRP concentrations were compared by analysis of covariance (ANCOVA) among subjects’ BMD status (normal vs. osteopenia vs. osteoporosis). Logarithmic transformation was used for serum hsCRP concentrations in the ANCOA analyses because of its positively skewed distribution. To examine the linearity of the log-transformed hsCRP levels according to subjects’ BMD status, the significance test for linearity ( P for trend test) was performed. Multiple regression analyses were used to explore the effect of hsCRP concentration on BMD and serum ALP after adjustment for covariates. We first considered several variables deemed of interest (hsCRP quintile levels, age, BMI, years since menopause), independent of their statistical significance. Health behavior variables (smoking habits, drinking habits, leisure time physical exercise) were included with the stepwise method, retaining only those that were statistically significant. In addition, the association between serum hsCRP levels and osteoporosis and/or osteopenia was determined by logistic regression analyses. Adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) were estimated. Age, BMI and years since menopause (for menopausal women) were included as covariates in this logistic regression. To examine the relationship between serum ALP levels and BMD, partial correlation coefficients were computed after adjustment for confounders, including age and BMI, as well as years since menopause in postmenopausal women. All statistical analyses were performed with SAS statistical software, with a P value of 0.05 considered statistically significant.
Results
Baseline clinical characteristics by menopausal status
The characteristics of the study subjects are shown in Table 1. The mean ± SD age of the premenopausal women was 42.6±5.1 years (range, 30–55 years), and the mean ± SD age of the postmenopausal women was 57.6±5.3 years (range, 41–76 years), with the mean ± SD years since menopause in the latter group being 7.7±5.7 years (range 1–31 years). The median hsCRP concentration in the premenopausal women was 0.7 mg/l, with the 25th, 75th and 95th percentiles being 0.5, 1.0 and 2.7 mg/l, respectively. In the postmenopausal women, the median hsCRP concentration was 1.0 mg/l, and the 25th, 75th and 95th percentiles were 0.6, 1.5 and 3.4 mg/l, respectively. These findings were similar to those previously observed in 1,399 healthy Korean women [23]. The concentrations of serum hsCRP, total ALP, calcium and phosphorus were significantly higher in the postmenopausal than in the premenopausal women (Table 1). Of the premenopausal women, 1,177 (32.1%) and 23 (0.6%) were osteopenic and osteoporotic, respectively. The number of subjects with osteoporosis was too small for separate analysis, thus osteopenic and osteoporotic groups were combined in the following analyses. Of the postmenopausal women, 594 (57.6%) and 232 (22.5%) were osteopenic and osteoporotic, respectively.
Relationship between serum hsCRP concentration and BMD status
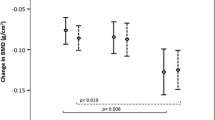
Median serum hsCRP concentrations (interquantile range) were 0.6 (0.4–0.9), 0.8 (0.5–1.3) and 1.1 (0.7–1.8) in normal, osteopenic and osteoporotic subjects, respectively. Using non-parametric test, there was a significant difference in the levels among the BMD status ( P <0.001), showing a tendency to increase in normal to oteoporotic subjects. We compared logarithmically transformed serum hsCRP concentrations with BMD status after adjustment for age, BMI and menopausal status because the hsCRP level was significantly correlated with age (γ=0.200, P <0.001) and BMI (γ=0.291, P <0.001). The log-transformed hsCRP levels were significantly higher in osteoporotic and osteopenic subjects than in normal subjects (F=13.861, P <0.001), and showed linearity ( P for trend <0.001) (Fig. 1a). Menopausal status did not have a significant interaction on the association ( P =0.457).
Serum hsCRP concentrations among normal, osteopenic and osteoporotic subjects. Osteopenia and osteoporosis were defined according to the WHO classification (–2.5< T -score ≤–1.0 SD and T -score ≤–2.5 SD, respectively). Serum hsCRP concentrations were logarithmically transformed because of its right-skewed deviation, and data were given as estimated mean ± 95% confidence intervals in all subjects after adjustment for age, body mass index and menopausal status (a), in premenopausal women after adjustment for age and body mass index (b), and in postmenopausal women after adjustment for age, body mass index and duration of menopause (c). In premenopausal women, the number of subjects with osteoporosis (n =23) was too small for separate analysis, thus osteopenic and osteoporotic groups were combined (“lower BMD”). There were significant differences in hsCRP levels in all subjects (F =13.861, P <0.001), premenopausal women (F =5.534, P =0.021), and postmenopausal women (F =3.097, P =0.046)
By menopausal status, we also compared logarithmically transformed serum hsCRP concentrations according to the BMD status. In premenopausal women, the log-transformed hsCRP levels were significantly higher in the subjects with osteopenia/osteoporosis than in normal subjects after adjustment for age and BMI (F=5.534, P =0.021) (Fig. 1b). In postmenopausal women, there was a significant difference in hsCRP level relative to BMD status (F=3.097, P =0.046) with linearity ( P for trend =0.008) after adjustments for age, BMI and years since menopause (Fig. 1c). The log-transformed hsCRP levels were significantly higher in osteoporotic than in normal subjects ( P =0.014).
Associations of serum hsCRP quintiles with BMD
When we categorized the subjects into five levels according to serum hsCRP concentration (Table 2), we found that the percentages of current or past smokers, drinkers and those who participated in leisure time physical exercise were similar in the hsCRP quintiles for both pre- and postmenopausal women. We found, however, that age, weight and BMI were higher in the higher hsCRP quintiles than in the lowest quintile for both groups of women ( P for trend <0.001). In postmenopausal women, height was lower in the 4th and 5th hsCRP quintiles than in the lowest quintile ( P for trend <0.001), and years since menopause was significantly longer in the highest hsCRP quintile than in the lowest quintile ( P for trend =0.001). Although there were no differences in serum calcium and phosphorus concentrations, serum total ALP levels tended to be higher in the higher quintiles in both pre- and postmenopausal women ( P for trend <0.001). Compared with those in the lowest quintile, premenopausal women in the 3rd to 5th quintiles, and postmenopausal women in the 3rd and 5th quintiles, had significantly higher serum total ALP concentrations. These differences in serum total ALP levels were persistently higher in both groups even after adjustments for confounding variables using multiple regression analysis (Table 3).
The multiple regression analysis also showed independent associations between each of these variables and BMD (g/cm2) (Table 3). In premenopausal women, age was negatively, and BMI was positively, correlated with BMD at both sites. In postmenopausal women, BMI was positively, and years since menopause was negatively, correlated with BMD. In the latter group, age was significantly correlated with BMD at the femoral neck, but not at the lumbar spine. In both groups, however, neither drinking habits nor leisure time physical exercise was significantly correlated with BMD at either site. Smoking habits were negatively associated with BMD only at the lumbar spine in postmenopausal women (β=–0.025, P =0.002). Importantly, compared with the lowest quintile, the 5th hsCRP quintile was significantly associated with lower BMD at the femoral neck in both pre- ( P =0.003) and postmenopausal ( P <0.001) women. At the lumbar spine, the 5th hsCRP quintile tended to be associated with lower BMD in both pre- ( P =0.060) and postmenopausal ( P =0.071) women. Smoking, which can influence the production of IL-6 [25], did not have a significant interaction on their associations of the hsCRP levels with serum ALP levels and BMD at all sites in both premenopausal and postmenopausal women ( P =0.086 to 0.856).
Partial correlation analysis revealed that serum ALP concentrations were negatively correlated with BMD at the femoral neck and lumbar spine both in premenopausal (γ=–0.159, P <0.001, and γ=–162, P <0.001, respectively) and postemenopausal women (γ=–0.242, P <0.001, and γ=–264, P <0.001, respectively). Therefore, we analyzed the association of the hsCRP levels with BMD values after additional adjustment for serum ALP concentrations. At the femoral neck, the 5th hsCRP quintile showed lower BMD in postmenopausal women (β=–0.025, P =0.008), although the significance disappeared in premenopausal women (β=–0.007, P =0.166).
Odds ratios (ORs) of the hsCRP quintiles for osteoporosis and/or osteopenia
Figure 2 presented the results of multiple logistic regression analyses with osteoporosis and/or osteopenia as the dependent variable. In premenopausal women, the OR for osteopenia/osteoporosis was significantly higher in the 5th hsCRP quintile than in the lowest quintile (OR=1.35, 95% CI =1.08 to 1.68) (Fig. 2a). In postmenopausal women, the OR for osteoporosis was 1.64 (95% CI =1.10 to 2.53) in the highest hsCRP quintile (Fig. 2b). After the additional adjustment for serum ALP concentrations, the significantly elevated ORs for osteoporosis and/or osteopenia disappeared in premenopausal (OR =1.17, 95% CI =0.93 to 1.46) and postmenopausal women (OR =1.31, 95% CI =0.78 to 2.20).
Adjusted odds ratios and 95% confidence intervals for osteoporosis and/or osteopenia relative to hsCRP quintile in premenopausal ( a) and postmenopausal women ( b). Logistic regression analyses were performed to determine the odds ratio of osteopenia/osteoporosis (in premenopausal women) or osteoporosis (in postmenopausal women) according to hsCRP quintile. Adjusted odds ratios were calculated along with 95% CI. The lowest quintile (quintile I) was the reference
Discussion
An association between circulating hsCRP level and BMD or biochemical bone turnover markers has been observed in several immune and inflammatory diseases. For example, in patients with rheumatoid arthritis, higher CRP was associated with lower BMD [26] and higher bone turnover, as measured by urinary excretion of pyridinoline and deoxypyridinoline collagen cross-linking [27]. In addition, elevated CRP was associated with increased urinary excretion of pyridinium crosslinks in patients with ankylosing spondylitis [28] and seronegative spondyloarthropathy [29]. To our knowledge, however, the hsCRP assay has not been used previously to determine the relationship between subclinical systemic inflammation and osteoporosis in healthy individuals without immune and inflammatory diseases.
We have shown here that pre- and postmenopausal women with osteopenia and/or osteoporosis had higher serum hsCRP levels than those with normal BMD, and that hsCRP levels higher than 1.2 mg/l and 1.8 mg/l were significantly associated with osteoporosis and/or osteopenia in pre- and postmenopausal women, respectively. Furthermore, hsCRP levels were positively correlated with total serum ALP levels. These findings suggest that higher hsCRP levels are associated with higher bone turnover rate, resulting in lower bone mass even in healthy women with normal hsCRP levels, and that a subclinical inflammatory process plays an important role in bone metabolism.
The mechanisms linking hsCRP and bone metabolism are not clear, but activated inflammatory cytokines are likely to be involved. Inflammatory processes can up-regulate many cytokines, such as IL-1, IL-6 and TNF-α, which strongly stimulate CRP production from the liver [18, 19] as well as induce bone resorption [3, 4, 10, 14], and increased bone resorption may result in increased bone turnover and decreased BMD. In support of this hypothesis, the production of IL-1, IL-6 and/or TNF-α by peripheral blood monocytes was positively correlated with bone resorption or spinal bone loss in healthy pre- [30] and postmenopausal [31] women, and serum IL-6 concentrations predicted femoral bone loss in healthy postmenopausal women [32]. In addition, serum concentrations of IL-6 and TNF-α could be positively correlated with serum hsCRP levels in healthy subjects [33] and those with myocardial infarction [34]. Another possibility is that the association between higher hsCRP levels and lower BMD comes from vitamin D deficiency [35]. In this study, however, the subjects were limited to those with normal serum levels of calcium, phosphorus and alkaline phosphatase in order to minimize any confounding effects of concomitant hyperparathyroidism or vitamin D deficiency. In addition, the serum levels of calcium and phosphorus did not differ among the hsCRP quintiles in pre- and postmenopausal women.
In the present study, we noted that height was significantly reduced in the 4th and 5th hsCRP quintiles, raising the question of vertebral fractures. However, we did not have a chance to examine spine X-ray for the presence of such fractures. In addition, we found that the association of hsCRP levels with BMD at the lumbar spine was weaker than at the femoral neck, especially in postmenopausal women (Table 3). The reason for this is not entirely clear, especially when it is considered that the spine is metabolically more active than the femoral neck due to its higher surface-to-volume ratio (largely trabecular) [36]. Osteoarthritis, which occurs more often at the spine in the elderly, may have falsely increased BMD measured by dual energy X-ray absorptiometry [37]. Further, subjects with osteoarthritis have been reported to have significantly higher hsCRP levels [22]. Thus, it is possible that osteoarthritic changes associated with aging may have ameliorated the association between hsCRP levels and BMD at the spine.
We excluded 26 subjects with the hsCRP levels >10 mg/l (10.1 to 26.5 mg/l) in order to eliminate the possibility of including subjects who may have had infectious or immune diseases [21]. Twenty were premenopausal and six were postmenopausal women. Of the 26 subjects, 21 were obese, 11 were hypertensive, 5 showed dyslipidemia, and 3 showed impaired fasting glucose. Addition of these subjects in the analyses strengthened the associations between serum hsCRP levels and BMD in premenopausal women. Compared with the lowest quintile, the highest hsCRP quintile showed lower BMD at the femoral neck (β= -0.016, P <0.001) and lumbar spine (β=–0.015, P =0.001), and the OR for osteopenia/osteoporosis in the highest quintile was 1.42 (95% CI =1.21 to 1.83). In postmenopausal women, the results were not changed by the addition of six subjects with high hsCRP levels (data not shown).
Although associations between atherosclerosis and osteopenia have been well documented [38, 39, 40], it is not clear whether one is the result of the other or whether they develop through common mechanisms. Recently, several lines of evidence have suggested that common or related mechanisms control both of these conditions from their early stages [41, 42]. The results shown here indicate that the risk of lower BMD is independently correlated with hsCRP concentration, a known marker for the risk of atherosclerosis [20]. Thus, it is feasible that a systemic inflammatory process may, at least partially, be a common mechanism for the development for lower bone mass and atherogenesis.
There were several potential limitations to this study. First, the study population was comprised of women who visited a health promotion center, and may not have been representative of the general population residing in a community, thus possibly resulting in selection bias. To minimize this, we intentionally applied strict exclusion criteria based on medical history and routine laboratory findings. Second, because this was a cross-sectional study, we cannot determine if there is a causal relationship between hsCRP levels and BMD values. Further prospective studies are necessary to determine whether this correlation is reflective of a causal relationship.
In conclusion, we have shown that increased serum hsCRP concentrations are associated with lower BMD and higher bone turnover rate in a large population of healthy women, and that hsCRP concentrations greater than 1.2 mg/l for premenopausal women and greater than 1.8 mg/l for postmenopausal women are significantly associated with osteoporosis and/or osteopenia. These findings suggest that subclinical systemic inflammation may be an important factor in bone turnover rate and bone mass.
References
Arron JR, Choi Y (2000) Bone versus immune system. Nature 408:535–536
Lorenzo J (2000) Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest 106:749–752
Muller B (2002) Cytokine imbalance in non-immunological chronic disease. Cytokine 18:334–339
Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC (1992) Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science 257:88–91
Roodman GD, Kurihara N, Ohsaki Y, Kukita A, Hosking D, Demulder A, Smith JF, Singer FR (1992) Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest 89:46–52
Yamamoto T, Ozono K, Kasayama S, Yoh K, Hiroshima K, Takagi M, Matsumoto S, Michigami T, Yamaoka K, Kishimoto T, Okada S (1996) Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest 98:30–35
Guise TA, Mundy GR (1998) Cancer and bone. Endocr Rev 19:18–54
Devlin RD, Bone HG 3rd, Roodman GD (1996) Interleukin-6: a potential mediator of the massive osteolysis in patients with Gorham-Stout disease. J Clin Endocrinol Metab 81:1893–1897
Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137
Gowen M, Mundy GR (1986) Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol 136:2478–2482
Pacifici R, Rifas L, Teitelbaum S, Slatopolsky E, McCracken R, Bergfeld M, Lee W, Avioli LV, Peck WA (1987) Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc Natl Acad Sci USA 84:4616–4620
Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA (1989) Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci USA 86:2398–2402
Johnson RA, Boyce BF, Mundy GR, Roodman GD (1989) Tumors producing human tumor necrosis factor induced hypercalcemia and osteoclastic bone resorption in nude mice. Endocrinology 124:1424–1427
Bertolini DR, Nedwin GE, Bringman TS, Smith DD, Mundy GR (1986) Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319:516–518
Walsh MC, Choi Y (2003) Biology of the TRANCE axis. Cytokine Growth Factor Rev 14:251–263
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, Wong T, Campagnuolo G, Moran E, Bogoch ER, Van G, Nguyen LT, Ohashi PS, Lacey DL, Fish E, Boyle WJ, Penninger JM (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402:304–309
Pepys MB, Baltz ML (1983) Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol 34:141–212
Weinhold B, Ruther U (1997) Interleukin-6-dependent and -independent regulation of the human C-reactive protein gene. Biochem J 327:425–429
Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, Kishimoto T, Nakatani T (2002) Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer 86:1396–1400
Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M (2003) Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 108:2317–2322
Yeh ET, Willerson JT (2003) Coming of age of C-reactive protein: using inflammation markers in cardiology. Circulation 107:370–371
Spector TD, Hart DJ, Nandra D, Doyle DV, Mackillop N, Gallimore JR, Pepys MB (1997) Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum 40:723–727
Chang JW, Yang WS, Min WK, Lee SK, Park JS, Kim SB (2002) Effects of simvastatin on high-sensitivity C-reactive protein and serum albumin in hemodialysis patients. Am J Kidney Dis 39:1213–1217
Orimo H, Sugioka Y, Fukunaga M, Muto Y, Hotokebuchi T, Gorai I, Nakamura T, Kushida K, Tanaka H, Ikai T, Oh-hashi Y (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM (2002) Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol 89:1117–1119
Devlin J, Lilley J, Gough A, Huissoon A, Holder R, Reece R, Perkins P, Emery P (1996) Clinical associations of dual-energy X-ray absorptiometry measurement of hand bone mass in rheumatoid arthritis. Br J Rheumatol 35:1256–1262
Oelzner P, Franke S, Muller A, Hein G, Stein G (1999) Relationship between soluble markers of immune activation and bone turnover in post-menopausal women with rheumatoid arthritis. Rheumatology (Oxford) 38:841–847
Marhoffer W, Stracke H, Masoud I, Scheja M, Graef V, Bolten W, Federlin K (1995) Evidence of impaired cartilage/bone turnover in patients with active ankylosing spondylitis. Ann Rheum Dis 54:556–559
MacDonald AG, Birkinshaw G, Durham B, Bucknall RC, Fraser WD (1997) Biochemical markers of bone turnover in seronegative spondylarthropathy: relationship to disease activity. Br J Rheumatol 36:50–53
Salamone LM, Whiteside T, Friberg D, Epstein RS, Kuller LH, Cauley JA (1998) Cytokine production and bone mineral density at the lumbar spine and femoral neck in premenopausal women. Calcif Tissue Int 63:466–470
Cohen-Solal ME, Graulet AM, Denne MA, Gueris J, Baylink D, de Vernejoul MC (1993) Peripheral monocyte culture supernatants of menopausal women can induce bone resorption: involvement of cytokines. J Clin Endocrinol Metab 77:1648–1653
Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J (2001) Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab 86:2032–2042
Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW (1999) C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol 19:972–978
Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E (2000) Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 101:2149–2153
Van den Berghe G, Van Roosbroeck D, Vanhove P, Wouters PJ, De Pourcq L, Bouillon R (2003) Bone turnover in prolonged critical illness: effect of vitamin D. J Clin Endocrinol Metab 88:4623–4632
Manolagas SC, Jilka RL (1995) Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med 332:305–311
Burger H, van Daele PL, Odding E, Valkenburg HA, Hofman A, Grobbee DE, Schutte HE, Birkenhager JC, Pols HA (1996) Association of radiographically evident osteoarthritis with higher bone mineral density and increased bone loss with age. The Rotterdam Study. Arthritis Rheum 39:81–86
Hak AE, Pols HA, van Hemert AM, Hofman A, Witteman JC (2000) Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 20:1926–1931
Kado DM, Browner WS, Blackwell T, Gore R, Cummings SR (2000) Rate of bone loss is associated with mortality in older women: a prospective study. J Bone Miner Res 15:1974–1980
Jorgensen L, Engstad T, Jacobsen BK (2001) Bone mineral density in acute stroke patients: low bone mineral density may predict first stroke in women. Stroke 32:47–51
Wallin R, Wajih N, Greenwood GT, Sane DC (2001) Arterial calcification: a review of mechanisms, animal models, and the prospects for therapy. Med Res Rev 21:274–301
Hirose K, Tomiyama H, Okazaki R, Arai T, Koji Y, Zaydun G, Hori S, Yamashina A (2003) Increased pulse wave velocity associated with reduced calcaneal quantitative osteo-sono index: possible relationship between atherosclerosis and osteopenia. J Clin Endocrinol Metab 88:2573–2578
Acknowledgements
This work was supported by a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (project no.: 01-PJ3-PG6–01GN11–0002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koh, JM., Khang, YH., Jung, CH. et al. Higher circulating hsCRP levels are associated with lower bone mineral density in healthy pre- and postmenopausal women: evidence for a link between systemic inflammation and osteoporosis. Osteoporos Int 16, 1263–1271 (2005). https://doi.org/10.1007/s00198-005-1840-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-005-1840-5