Abstract
Introduction and hypothesis
The aim of the present systematic review and meta-analysis was to assess the effectiveness and safety of injections of the new bulking agent Urolastic® in the treatment of patients with stress urinary incontinence (SUI).
Methods
A systematic search was carried out to select observational and experimental studies on Urolastic® in female patients with SUI. Three different databases, Pubmed, the Cochrane Central Register of Controlled Trials, and Scopus, were used to retrieve scientific articles published from their inception to 31 January 2018.
Results
Eight full texts were evaluated but only five were selected for the qualitative and quantitative analyses. Duration of follow-up after Urolastic® injections was significantly heterogeneous, ranging from 6 to 24 months. Secondary injections were needed in 16.7%–35.0% of the treated patients. The pooled proportion of secondary injections was 20% (95% CI: 15%–24%; I2: 0%). Subjective improvement, measured by different means (i.e., patient global impression of improvement PGI-I score) was only assessed by 40% of the selected papers and was > 80% in two cohorts. The objective treatment success was evaluated by four (80.0%) papers and was achieved in all cohorts with a wide proportional range: from 32.7% (i.e., patients without objective SUI symptom cough tests and with a negative pad test) to 67.0%. Its pooled proportion was 57% (95% CI: 38%–75%; I2: 82.3%).
Conclusions
Urolastic® showed effectiveness in patients with SUI during a follow-up period of 6–24 months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence is a medical condition characterized by an involuntary loss of urine [1].
The most prevalent types of urinary incontinence are the following: stress urinary incontinence (SUI), characterized by an inadvertent loss of urine due to effort-, sneezing-, coughing-, or exertion-related increased intra-abdominal pressure; urge urinary incontinence (UUI), which is associated with urgency; mixed urinary incontinence (MUI), when both SUI and UUI occur. Their prevalence in non-institutionalized women is 49%, 21%, and 29%, respectively [2]. In particular, prevalence of SUI can range from 10 to 40% in the general population, increasing during the post-menopausal age with a negative impact on the quality of social, working, and affective life [2].
SUI therapy should be carefully selected following the assessment of its severity and of concomitant factors that could preclude surgical intervention [3, 4]. The most relevant effective options are exercises aimed at restoring the strength and tone of the pelvic floor and estrogenic therapy, along with the administration of Lactobacilli acidophilus [5]. In fact, pelvic floor muscle training (PFMT) is recommended as a first-line treatment for SUI (grade A evidence) [6].
Duloxetine has been approved in Europe for the treatment of SUI. The UK National Institute for Health and Care Excellence recommends that duloxetine should not be used as a first-line treatment or routinely offered as a second-line treatment for stress urinary incontinence, given that pelvic floor muscle training is more effective and less costly than duloxetine and that surgery is more cost-effective than duloxetine [7].
The surgical gold standard is represented by the placement of a mid-urethral sling (MUS) [8,9,10].
An even less invasive procedure is nowadays represented by the possibility of using injections of urethral bulking agents (UBAs), recommended in elderly patients, in those with a high anesthesiologic risk and in those reluctant to undergo surgery [11, 12]. It can be recommended in patients who were surgically treated without any benefit or with recurrence.
UBAs thicken the urethral wall and, elevating the urethral mucosa, restore the natural continence and urethral resistance [12, 13]. The ideal UBA should be easily injectable, highly cost-effective, and biocompatible and should not migrate from the injection site [14].
A new silicone-derived elastomer, Urolastic®, is an innovative UBA recently introduced on the market made of a vinyl dimethyl-terminated polydimethylsiloxane (PDMS) polymer, tetrapropoxysilane cross-linking agent, platinum divinyltetramethyl siloxane complex catalyst, and titanium dioxide radio-pacifying agent (Urolastic® Urogyn BV, Nijmegen, The Netherlands). This material polymerizes in situ, forming a uniform elastomer that adapts itself to the environment during injection; a few minutes after injection into the paraurethral tissue, it undergoes a change from the liquid to the solid state, thereby supporting the urethra. It has been proved to have high biocompatibility and little risk of migration from the injection site, offering long-term improvement in clinical symptoms [15, 16]. Urolastic® can be considered a hybrid between a UBA and a prosthesis, as it creates a soft-cuff effect by solidifying around the urethra, preventing migration [17].
We performed a systematic review and meta-analysis to assess the effectiveness and safety of Urolastic® in patients with SUI.
Materials and methods
Search strategy
A search to detect observational and experimental studies on Urolastic® in female patients with stress urinary incontinence was carried out.
Three different databases, Pubmed, the Cochrane Central Register of Controlled Trials, and Scopus, were evaluated to search for scientific articles published from their inception to 31 January 2018.
Furthermore, reviews or systematic reviews, as well as the list of references of the selected articles, were carefully evaluated to find other relevant articles.
Abstracts published in the main international conferences and gray literature documents were excluded owing to the scarce information provided and the lack of a quality-based peer-review process, respectively.
The following keywords were used and combined using different strings to increase the diagnostic sensitivity of the search: “Urolastic®,” “urinary incontinence,” “stress urinary incontinence,” and “bulking agents.”
Study selection
All trials and observational studies including information on efficacy and/or effectiveness and/or safety and/or tolerability of Urolastic® in women with stress urinary incontinence were selected.
Studies with at least one of the following characteristics were excluded: (1) case reports or series with less than five patients; (2) studies carried out in animals; (3) modeling-based studies; (4) studies not properly describing the primary and secondary outcomes; (5) studies written in languages other than English; (6) editorials, letters, correspondence, and reviews.
Titles and abstracts of the records were independently assessed by two study researchers (G.S. and L.S.); full texts of potentially significant studies were retrieved and evaluated by the above-mentioned authors. Discrepancies in the assessment of the articles and data extraction were resolved by a third investigator (G.C.).
Data extraction
An ad hoc electronic form was used to collect information related to the study aims. The search and data extraction results of the two above-mentioned authors were cross-checked for the analysis and for the detection of information inconsistencies. No relevant disagreements were found, and the inter-rater agreement was ~100%.
The form adopted to retrieve the most relevant variables included the following items: first author of the manuscript, title of the manuscript, year of publication of the manuscript, epidemiologic nature of the study, countries where the study was carried out, temporal period when the study was performed, sample size, age, gender, body mass index (BMI), parity, history of neurologic disorders, previous therapies for stress urinary incontinence, duration of the follow-up and number of individuals eligible for the follow-up, and treatment outcomes (i.e., second injection, subjective and objective improvement, and complications).
Data described in the manuscripts were anonymized, and no any ethical approvals to the ethical committee of the University of Sassari, Italy.
Study quality assessment
The process of the systematic review and meta-analysis was carried out according to guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [18]. The recruitment of studies and data extraction were associated with an inter-rater agreement of ~100%. The fewest discrepancies were resolved by consensus and the intervention of a third investigator (GC). Scientific quality was graded according to the Scottish Intercollegiate Guidelines Network (Table 1).
Statistical analysis
Descriptive and inference analyses were carried out. Qualitative and quantitative variables were summarized with absolute and relative (percentage) frequencies and means and standard deviations (DS), respectively.
Meta-analysis of the study variables was summarized with pooled and heterogeneity indicators as well as graphical computations (i.e., forest plots). In particular, forest plots described the between-study variability of point and 95% confidence interval (CI) estimates and the weight of selected sample sizes.
The choice of a fixed or random effects model was based on the level of between-study heterogeneity.
An inconsistency indicator (I2) was used to describe the relationship between true variability and overall variation; values < 25%, ≥ 25–< 50%, and ≥ 50% were regarded as low, medium, and high heterogeneity, respectively.
Bias assessment plots and the Egger weighted regression test method were used to evaluate potential publication bias.
A two-tailed p value < 0.05 was considered statistically significant.
The statistical computations were carried out with the statistical softwares STATA version 15 (StataCorp, Lakeway Drive, College Station, TX, USA) and StatsDirect version 3.1.12 (StatsDirect Ltd.).
Results
Study selection
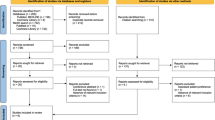
A total of 552 records were found (Fig. 1) [19,20,21]. Eight full texts were evaluated but only five were selected for the qualitative and quantitative analyses. Three were excluded: one (Zajda et al. 2013) [22] included the same cohort selected for the analysis in 2015 [19], whereas the others by Chan et al. in 2017 [23] and Renard et al. in 2017 [16] were a narrative review without any clinical outcomes and a study including male patients, respectively.
Characteristics of the selected studies
Selected manuscripts were published between 2015 and 2018 (Table 2). They described studies performed between 2011 and 2016. All (5, 100%) studies were carried out in Europe; one (20.0%) of them was performed in Poland and Qatar (multicentric). In particular, three (60%) were conducted in Poland and two (40.0%) in The Netherlands. The majority (4, 80.0%) were prospective cohort studies and only one (20.0%) a retrospective cohort study.
Characteristics of the study samples
Sample sizes ranged from 20 to 105 patients (Table 3). Their mean ranged between 56 and 65.5 years. The most represented gender was female (100%). The information on mean BMI was only included in two (40.0%) studies and ranged from 28.8 to 30.4. Parity was ≥ 2 in three (60.0%) studies. Four (80.0%) manuscripts described previous therapy for stress urinary incontinence: at least one out two (range: 45.0%–100.0%) were previously exposed to therapeutic interventions before Urolastic®. Only one (20.0%) paper reported on neurologic disorders of recruited individuals.
Duration of follow-up after Urolastic® injections was significantly heterogeneous, ranging from 6 to 24 months. Only two (40.0%) studies planned a long (i.e., 24-month) follow-up for their entire cohorts (Table 4). The mean and median durations of the follow-up were 16.9 and 18.5 months, respectively.
The same treatment outcomes were not assessed by all investigators (Table 5). Secondary injections (re-injections) were needed in 16.7%–35.0% of the treated patients. The mean time between primary and secondary injection was 9 weeks. The pooled proportion of secondary injections (re-injections) was 20% (95% CI: 15%–24%; I2: 0%) (Fig. 2).
Subjective improvement, measured by different means (i.e., patient global impression of improvement PGI-I score; completely dry, with complete dryness between catheterization), was only assessed by 40% of the selected papers and was > 80% in three cohorts (Fig. 3).
The objective treatment success was evaluated by four (80.0%) papers (Table 5) and was achieved in all cohorts with a wide proportional range: from 32.7% (Futyma et al. in 2016 [21], 16/49 with 24-month follow-up; i.e., patients without objective SUI symptom cough tests and with a negative pad test) to 67.0% (Futyma et al. in 2015 [20], 67/100). The pooled proportion was 57% (95% CI: 38%–75%; I2: 82.3%) (Fig. 4).
Complication rates, reported by all studies but one, significantly varied, ranging from 16.2 to 66.1% (Table 5). The pooled proportion was 36% (95% CI: 17%–57%; I2: 91.3%) (Fig. 5). De Vries et al. [17] studied 65 patients, but 3 were lost to follow-up, and complications were not documented. Thus, they had 41/62 (66.1) overall complications. However, the most relevant adverse events such as exposure or erosion occurred in 27.4% only in the study of De Vries et al. [17]. An exposure was considered a part of the implant that was not covered by tissue. Some of them were a trail of material that had solidified in the injection channel. The trails can often be removed with a forceps without the need for anesthesia. Erosions are a delayed complication; these arise when implants erode through the tissue. This was mainly observed at the anterior vaginal wall and was left untreated if there were no complaints. Futyma et al. [21] reported 17/66 (25.8%) complications overall but only specified the kind of complication in 11 cases. The most common (> 20%) complications were complaints of urgency, post-voiding residual > 150 ml, and exposure or erosion (Table 6).
Discussion
Several types of materials, including paraffin, autologous grease, polytetrafluoroethylene, glutaraldehyde combined with bovine collagen, porcine dermis, and hyaluronic acid plants, have been evaluated in patients with SUI. The majority of them have been abandoned because of adverse events, hypersensitivity reactions, and migration from the injection site [24].
UBAs are implanted in the urethral submucosa and exert a mass effect, increasing the urethral sphincter pressure and, consequently, improving urinary continence [25, 26]. Their placement is less invasive compared with conventional surgery.
The treatment can be repeated during the follow-up to consolidate the benefits obtained with the first injection and to improve persistent symptoms. UBAs are safe and recommended for patients with comorbidities and surgical contraindications [27].
Our meta-analysis showed its effectiveness; the selected studies showed subjective improvement (> 80% in three cohorts), measured by different means (i.e., patient global impression of improvement PGI-I score; completely dry, with complete dryness between catheterization); however, it was assessed only by 40% of the selected papers.
The objective treatment success was evaluated by 80.0% papers and was achieved in all cohorts, ranging from 32.7 to 67.0%. Its pooled proportion was 57%, thus supporting the potential benefits of the procedure.
Duration of follow-up after Urolastic® injections was significantly heterogeneous, ranging from 6 to 24 months. Only two studies planned a long (i.e., 24-month) follow-up. However, the proportion of patients followed up within 24 months was high (> 70%). Further studies should be focused on long-term outcomes, in terms of both safety and effectiveness.
The rate of secondary injections (re-injection) was low (20%) compared with other non-permanent bulking agents [23], reducing direct and indirect costs from a societal and health care perspective.
The complication rate ranged from 16.2 to 66.1%; however, the most relevant adverse events such as exposure or erosion occurred in 27.4% in the study of De Vries et al. [17].
Several limitations were detected: the same treatment outcomes were not assessed by all investigators. A possible bias of our study concerning the safety profile of the intervention is the selection criteria, where we excluded case reports or series, potentially citing side effects not mentioned in other studies. However, to reduce the heterogeneity between studies, we decided to have more restrictive exclusion criteria. Longer follow-up in future studies will help assess the safety and efficacy profile of the intervention. Unfortunately, most of the selected studies did not carry out a long-term follow-up. A consensus is needed to identify the best indicators that could assess the effectiveness. The heterogeneity should be significantly reduced to allow better inter-study comparisons. Moreover, it is crucial to perform comparative studies between Urolastic® and alternative materials as well as surgery. The added value of this systematic review and meta-analysis is the summary of the current evidence; strengths and limitations of Urolastic® underscored in the present study can help researchers to improve future study designs and to homogenize outcomes and end points for further comparisons.
Conclusions
The new silicone-derived elastomer Urolastic® was feasible and effective in patients with SUI after 6 months. However, bulking agents are not currently recommended as the first choice intervention in the international guidelines. More studies with larger sample sizes are needed to draw definitive conclusions.
References
Tantanasis T, Daniilidis A, Pantelis A, Chatzis P, Vrachnis N. Minimally invasive techniques for female stress urinary incontinence, how, why. when Arch Gynecol Obstet. 2013;288(5):995–1001.
Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjälmås K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62(4 Suppl 1):16–23.
Patel DA, Xu X, Thomason AD, Ransom SB, Ivy JS, DeLancey JO. Childbirth and pelvic floor dysfunction: an epidemiologic approach to the assessment of prevention opportunities at delivery. Am J Obstet Gynecol. 2006;195(1):23–8.
Alas AN, Chinthakanan O, Espaillat L, Plowright L, Davila GW, Aguilar VC. De novo stress urinary incontinence after pelvic organ prolapse surgery in women without occult incontinence. Int Urogynecol J. 2017;28(4):583–90.
Capobianco G, Wenger JM, Meloni GB, Dessole M, Cherchi PL, Dessole S. Triple therapy with lactobacilli acidophili, estriol plus pelvic floor rehabilitation for symptoms of urogenital aging in postmenopausal women. Arch Gynecol Obstet. 2014;289(3):601–8.
Dumoulin C, Hay-Smith EJ, Mac Habee-Seguin G. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database Syst Rev 2014;(5):CD005654.
Urinary Incontinence in Women: the Management of Urinary Incontinence in Women: NICE Clinical Guideline CG171, National Institute for Health and Care Excellence, London (UK), 2013 Available: http://guidance.nice.org.uk/CG171.
Capobianco G, Madonia M, Morelli S, Dessole F, De Vita D, Cherchi PL, Dessole S. Management of female stress urinary incontinence: a care pathway and update. Maturitas 2018; 109: 32–38.
Kurien A, Narang S, Han HC. Tension-free vaginal tape-Abbrevo procedure for female stress urinary incontinence: a prospective analysis over 22 months. Singap Med J. 2017;58(6):338–42.
Capobianco G, Dessole M, Lutzoni R, Surico D, Ambrosini G, Dessole S. TVT-ABBREVO: efficacy and two years follow-up for the treatment of stress urinary incontinence. Clin Exp Obstet Gynecol. 2014;41(4):445–7.
American Urological Association. Incontinence 2017. https://www.auanet.org/education/guidelines/incontinence.cfm.
Mamut A, Carlson KV. Periurethral bulking agents for female stress urinary incontinence in Canada. Can Urol Assoc J. 2017 Jun;11(6 Suppl 2):S152–4.
Herschorn S. Injection therapy for urinary incontinence. In: Wein A, Kavoussi LR, Novick AC, et al., editors. Campbell-Walsh urology. 11th ed. Philadelphia: Saunders; 2013. p. 2049–69.
Kotb AF, Campeau L, Corcos J. Urethral bulking agents: techniques and outcomes. Curr Urol Rep. 2009;10:396–400.
Kowalik CR, Casteleijn FM, van Eijndhoven HWF, Zwolsman SE, Roovers JWR. Results of an innovative bulking agent in patients with stress urinary incontinence who are not optimal candidates for mid-urethral sling surgery. Neurourol Urodyn 2018;37(1):339–345.
Renard J, Citeri M, Zanollo L, Guerrer C, Rizzato L, Frediani L, et al. Treatment of stress urinary incontinence in neurological patients with an injectable elastomer prosthesis: preliminary results. Int Neurourol J. 2017;21(1):75–9.
de Vries AM, van Breda HMK, Fernandes JG, Venema PL, Heesakkers JPFA. Para-urethral injections with Urolastic® for treatment of female stress urinary incontinence: subjective improvement and safety. Urol Int. 2017;99(1):91–7.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Zajda J, Farag F. Urolastic for the treatment of women with stress urinary incontinence: 24-month follow-up. Cent European J Urol. 2015;68(3):334–8.
Futyma K, Miotła P, Gałczyński K, Baranowski W, Doniec J, Wodzisławska A, et al. An open multicenter study of clinical efficacy and safety of Urolastic, an injectable implant for the treatment of stress urinary incontinence: one-year observation. Biomed Res Int. 2015;2015:851823.
Futyma K, Nowakowski Ł, Gałczyński K, Miotła P, Rechberger T. Nonabsorbable urethral bulking agent—clinical effectiveness and late complications rates in the treatment of recurrent stress urinary incontinence after 2 years of follow-up. Eur J Obstet Gynecol Reprod Biol. 2016;207:68–72.
Zajda J, Farag F. Urolastic-a new bulking agent for the treatment of women with stress urinary incontinence: outcome of 12 months follow up. Adv Urol 2013. 2013;724082:1–5.
Chan WKW, Chu PSK. Bulking agents in the management of urinary incontinence: dead or alive? Curr Bladder Dysfunct Rep. 2017;12(3):195–200.
Kasi AD, Pergialiotis V, Perrea DN, Khunda A, Doumouchtsis SK. Polyacrylamide hydrogel (Bulkamid®) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27(3):367–75.
Siddiqui ZA, Abboudi H, Crawford R, Shah S. Intraurethral bulking agents for the management of female stress urinary incontinence: a systematic review. Int Urogynecol J. 2017;28(29):1275–84.
Ghoniem G, Corcos J, Comiter C, Westney OL, Herschorn S. Durability of urethral bulking agent injection for female stress urinary incontinence: 2-year multicenter study results. J Urol. 2010;183(4):1444–9.
Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database Syst Rev 2012;(2): CD003881.
Acknowledgements
The PhD School of the Biomedical Sciences, Gender Medicine: Men, Women, and Children, Sassari University, Italy, supported the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None. The authors alone are responsible for the content and writing of the paper.
Additional information
Content
A meta-analysis was carried out on studies on the Urolastic® injectable elastomer prosthesis
Rights and permissions
About this article
Cite this article
Capobianco, G., Azzena, A., Saderi, L. et al. Urolastic®, a new bulking agent for treatment of stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J 29, 1239–1247 (2018). https://doi.org/10.1007/s00192-018-3703-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3703-6