Abstract
Introduction and hypothesis
There is a paucity of data evaluating the risk of de novo stress urinary incontinence (SUI) after surgery for pelvic organ prolapse (POP) in women with no preoperative occult SUI. We hypothesized that apical suspension procedures would have higher rates of de novo SUI.
Methods
This was a retrospective database review of women who had surgery for POP from 2003 to 2013 and developed de novo SUI at ≥6 months postoperatively. Preoperatively, all patients had a negative stress test and no evidence of occult SUI on prolapse reduction urodynamics. The primary objective was to establish the incidence of de novo SUI in women with no objective evidence of preoperative occult SUI after POP surgeries at ≥6 months.
Results
A total number of 274 patients underwent POP surgery. The overall incidence of de novo SUI was 9.9 % [95 % confidence interval (CI) 0.07–0.14]. However, the incidence of de novo SUI in those with no baseline complaint of SUI was 4.4 % (95 % CI 0.03–0.1). There was no difference in de novo SUI rates between apical [9.7 % (n = 57)] and nonapical [10.5 %, (n = 217] procedures (p = 0.8482). Multivariate logistic regression identified sacrocolpopexy [adjusted odds ratio (OR) 4.54, 95 % CI 1.2–14.7] and those with a baseline complaint of SUI (adjusted OR 5.1; 95 % CI 2.2–12) as risk factors for de novo SUI.
Conclusions
The incidence of de novo SUI after surgery for POP without occult SUI was 9.9 %. We recommend counseling patients about the risk of de novo SUI and offering a staged procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The prevalence of pelvic organ prolapse (POP) increases with age, and up to 20 % of women will require at least one surgery for correction of POP in their lifetime, with an estimated 30 % reoperation rate [1–3]. It is common for patients to be affected by more than one pelvic floor disorder, and 21.3 % women will require a concomitant stress urinary incontinence (SUI) procedure [1, 4]. The International Continence Society and International Urogynecological Association (ICS/IUGA) define SUI as a patient complaint of involuntary leakage of urine during physical exertion. When SUI is identified on urodynamic cystometry during increased abdominal pressure in the absence of a detrusor contraction it is referred to as urodynamic stress incontinence (USI) [5]. However, more advanced stages of POP are often not associated with symptomatic complaints of SUI due to the theoretical kinking of the urethra [6]. Surgical correction of prolapse can unmask SUI after surgery, referred to as de novo SUI, which is estimated to range from 16 to 51 % [7–11]. It has been recommended by the American Urological Association (AUA) that women with stage II or greater POP should have preoperative urodynamics (UDS) with prolapse reduction to assess for occult SUI [12]. IUGA and the ICS define occult SUI as evidence of USI when the prolapse is reduced [13]. There is no standard definition for de novo SUI by the IUGA/ICS. However, for this study, it is defined as the development of SUI after POP surgery in women with no preoperative evidence of occult SUI.
The Colpopexy and Urinary Reduction Efforts (CARE) trial reported that de novo SUI after surgery in previously continent women was as high as 44.1 % after abdominal sacrocolpopexy without a concomitant Burch procedure [14]. The Outcomes Following Vaginal Prolapse Repair and Mid-Urethral-Sling trial (OPUS) reported that 33.5 % (111/331) of patients had a positive prolapse-reduction test umasking occult SUI prior to surgery and that 71.9 % of those women reported de novo SUI symptoms after surgery in the sham group [8]. The authors of the OPUS trial concluded that a prophylactic sling procedure should be performed concomitantly with POP surgery, which would reduce de novo SUI from 49.4 % to 23.6 % (p < 0.001) [8]. In addition, Kasturi et al. demonstrated a 25 % risk of de novo SUI after total vaginal mesh procedures [15]. Although performing a prophylactic incontinence procedure on all women would reduce the risk of developing de novo SUI, it is debated whether exposing all patients to the additional risk is ethical and beneficial given the increased risk of complications and future surgeries. Although the literature demonstrates that placing an incontinence sling reduces the need for future SUI procedures, it also demonstrates that only a small proportion (5–17 %) of those who do not have a sling placed at the time of their POP surgery, will actually require a SUI surgery in the future [8, 14, 16].
To our knowledge, the majority of studies have evaluated the incidence of de novo SUI in those with evidence of occult incontinence on preoperative testing. However, there is a paucity of data evaluating patients who have no objective evidence of occult SUI preoperatively and develop de novo SUI after POP surgical correction. The aim of this study was to establish the incidence of de novo SUI following surgery for POP in patients with negative testing for occult SUI and explore the risk factors associated with this outcome.
Materials and methods
Institutional Board Review approval was obtained (IRB# FLA 13–130). This was a retrospective database review of patients with a diagnosis of POP who underwent surgical treatment from July 2003 to June 2013 at a single institution and developed de novo SUI at ≥6 months postoperatively. Patients were diagnosed with de novo SUI when there was no objective preoperative evidence of SUI or occult SUI and then reported subjective complaints or demonstrated objective evidence of SUI postoperatively. Patients underwent a thorough history and physical, including subjective assessment of SUI or urge urinary incontinence (UUI), total number of daily urine leaks, daily pad counts, and POP assessment by both the Baden-Walker and Pelvic Organ Prolapse Quantification (POP-Q) systems. Preoperative testing for objective parameters included a standardized supine stress test (SST) and a UDS with and without prolapse reduction. The standardized supine stress test was performed within 30 min of voiding, with the patient in the lithotomy position, with a strong Valsalva and cough. Patients were evaluated for SUI on UDS with and without prolapse reduction. Prolapse reduction was performed with a Sims half speculum that was elevated by hand during Valsalva and coughing to reduce POP. SUI was assessed during UDS through cough and Valsalva maneuvers at <100 cc, 150 cc, and at capacity with and without the prolapse reduced and with and without catheters in the urethra.
We included all women who had no evidence of SUI on preoperative testing and underwent a POP surgery without a concomitant incontinence surgery and had at least 6 months’ surgical follow-up. Patients with complaints of UI were included in the analysis as long as a complaint of SUI was not their primary complaint and they did not demonstrate SUI during testing or on prolapse reduction. Surgeries included total vaginal hysterectomy (TVH) with McCall’s culdoplasty, sacrocolpopexy, anterior vaginal wall repair, posterior vaginal wall repair, sacrospinous ligament fixation (SSLF), uterosacral ligament suspension (USLS), biologic apical mesh kit, trachelectomy, and Manchester procedure. Patients were excluded if they had a predominant complaint of SUI, objective evidence of SUI on either the SST or UDS, or had any prior incontinence procedure.
The primary objective was to assess the incidence of de novo SUI after POP surgeries at ≥6 months and determine whether specific procedures had higher rates of de novo SUI. The primary outcome measure was defined as de novo SUI in women with no evidence of occult SUI. The secondary outcome measure was defined as de novo SUI in women with no evidence of occult SUI and no baseline complaint of SUI. Our secondary objective was to evaluate for associated risks factors. We hypothesized that patients with apical POP surgery would have a higher incidence of de novo SUI. Additionally, those with evidence of intrinsic sphincter deficiency (ISD), defined by a maximal urethral closure pressure (MUCP) of <20 mm H20 on UDS testing, or those with urethral hypermobility but with no evidence of SUI would also be at risk for developing de novo SUI. Urethral hypermobility was defined as >30° or a change of >30° on Valsalva or cough when a Q-tip was placed inside the urethra.
Statistical analysis was performed using JMP Pro Version 10 (SAS Institute, Cary, NC, USA). Continuous data were checked for normality using the Shapiro-Wilks test prior to univariate analysis. Continuous data with normal distribution were analyzed using Student’ t test. Continuous data not normally distributed were analyzed using a Wilcoxon signed-rank test. Categorical data were analyzed using Pearson’s chi-square test or Fisher’s exact test if there were ≤5 subjects in 20 % of cells. Multivariate logistic regression for variables with significance at p < 0.05 at the univariate level was included in the final analysis. Ten outcome events were required per variable analysis for logistic regression. Variables identified to be potentially included in logistic regression included age, parity, body mass index (BMI), medical comorbidities, baseline complaints of UI, number of urinary leaks per day, number of pads used per day, POP-Q exam, and a diagnosis of urethral hypermobility or ISD.
Results
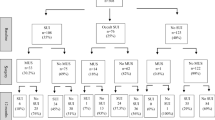
A total of 311 women were identified to have POP surgery without a concomitant sling and no objective evidence of occult SUI on preoperative testing. Thirty-seven women were excluded due to previous sling, Burch, or Marshall-Marchetti-Krantz procedures, leaving a total of 274 women in the final analysis. Baseline characteristics of the cohort are described in Table 1. When asked about UI, 23.3 % (n = 64) reported subjective complaints of SUI and 32.8 % (n = 90) reported having UUI. UDS findings demonstrated that the median MUCP was 69 (48–90), with only two (0.7 %) women having a component of ISD and with an MUCP <20. Almost all women (n = 270, 96.3 %) had urethral hypermobility.
Table 2 lists the incidence of de novo SUI by baseline examination findings and compares them with and without preoperative complaints of SUI. When evaluating those with no evidence of occult SUI, there was no difference in de novo SUI rates by age, BMI, parity, history of previous hysterectomy, diabetes, hypertension, pulmonary disease, or being a current smoker. There also was no difference in those with a baseline complaint of UUI or the number of pads used per day. There was, however, a significant rate of de novo SUI in 15 women who had a baseline complaint of SUI (55.5 % vs. 19.8 %, p < 0.001), as well as the number of UI leaks per day (p = 0.0486). In the univariate analysis evaluating those with no baseline complaint of SUI and no occult SUI, only being older was demonstrated to have a significantly higher rate of de novo SUI (74.6 years vs. 64.9 years, p = 0.0195).
A total of 27 women complained of SUI after 6 months. The incidence of occult SUI after surgery was 9.9 % (95 % CI 0.07–0.14) based on the primary outcome definition. Of these, 12 (4.4 %, 95 % CI 0.03–0.1) had new-onset de novo SUI defined by the secondary outcome measure (no baseline complaint of SUI and no preoperative occult SUI.) No patient who developed de novo SUI underwent additional SUI procedures. At 6 months, there was subjective improvement in both SUI (n = 49, 17.9 % vs. n = 15, 5.5 %, p < 0.001) and UUI (n = 65, 23.7 % vs. n = 25, 9.1 %, p = 0.0094) after POP surgery.
Table 3 lists the incidence of de novo SUI by procedure type based on the primary outcome measure. There was no difference in the rate of de novo SUI in apical procedures (n = 57, 9.7 %) vs. nonapical procedures (p = 217, 10.5 %, p = 0.8482) when using the primary outcome definition. There was a proportional difference of 0.008 (95 % CI −0.1 to 0.07), which did not reach statistical significance (p = 0.6972). There was significant de novo SUI after sacral suspensions procedures (24 %, p = 0.0247), which included sacrocolpopexy and sacrohysteropexy. However, when evaluating procedure type individually, only sacrocolpopexies had significant de novo SUI (29.4 %, p = 0.0172). There were five patients who underwent sacrocolpopexy and developed de novo SUI after having a negative test for occult SUI, with 60 % complaining of preoperative SUI (3/5, p = 0.0924). There was no difference in de novo SUI rates after SSLF (9.4 %), TVH (6.8 %), anterior (7 %), or posterior repairs (9.7 %), Manchester (0 %), trachelectomy (0 %), USLS (14.3 %), or LeFort colpocleisis (15 %).
Table 4 lists the incidence of de novo SUI by procedure type based on the secondary outcome measure. There was no significant difference in de novo SUI rates by any procedure type. or when using the secondary outcome definition comparing apical to nonapical procedures (n = 170, 5.8 % vs. n = 40, 5 %, p = 0.8287).
Multivariate logistic regression analysis was performed on variables with a p < 0.05, which included sacrocolpopexy, baseline complaint of SUI, and number of UI events per day. An analysis was performed for more than one, more than two, and more than three leaks per day. There was no increased risk for those who had daily incontinence events [adjusted odds ratio (OR) 0.5, 95 % CI 0.1–2.2]. Final multivariate logistical regression model demonstrated that there was an increased risk of de novo SUI only in those with no evidence of occult SUI before sacrocolpopexy (adjusted OR 4.54, 95 % CI 1.2–14.7) and those with a baseline complaint of SUI (adjusted OR, 5.1; 95 % CI 2.2–12). Logistic regression could not be performed in women who had no baseline complaint of SUI, since there were only 12 outcome events.
Discussion
The primary aim of this study was to establish the incidence of de novo SUI following surgery for POP in patients without evidence of occult SUI and identify possible risk factors. There was no difference in de novo SUI rates between apical suspension and nonsuspension procedures. The overall incidence of de novo SUI after POP procedures in those with no evidence of occult SUI on SST or UDS was 9.9 %. In addition, the incidence of de novo SUI, in those with no baseline complaints of SUI and no evidence of occult SUI, was 4.4 %.
It was hypothesized that apical procedures were going to be associated with a higher incidence of de novo SUI. Although there was no difference overall, sacrocolpopexies were identified as a risk factor for developing de novo SUI in the final logistic regression model. However, this was only true for patients who had a preoperative complaint of SUI. This is consistent with Leruth et al., who evaluated 50 women who had a negative stress test prior to laparoscopic sacrocolpopexy and found that the only identifiable risk factor was a preoperative complaint of SUI [relative risk (RR) 4.03, 95 % CI 1.16–14.09] [17]. In addition, Park et al. evaluated 70 women who had a negative prolapse reduction test prior to laparoscopic sacrocolpopexy and found that 18 % required a later sling procedure [18]. These studies support the concept that there is a high risk of developing de novo SUI and a risk of requiring later incontinence surgery after sacrocolpopexy. Based on this study and the literature, there does appear to be a higher risk of developing de novo SUI in patients who undergo a sacrocolpopexy and have a baseline complaint of SUI even if there is no evidence of occult SUI. It is recommended that future prospective studies further evaluate the development of de novo SUI and specifically focus on comparing suspension procedures to nonsuspension procedures.
Despite multiple studies reporting on the incidence of de novo SUI, very few studies have investigated de novo SUI in women with no evidence of occult SUI and compared suspension procedures to nonsuspension procedures. The most cited of studies for de novo SUI are the CARE and OPUS trials, which evaluated women with occult SUI who were randomized to POP surgery with and without concomitant incontinence procedures. The OPUS trial included both suspension and nonsuspension procedures, but the outcomes were not individually analyzed. Both studies concluded that a prophylactic incontinence procedure should be performed in prolapse repairs, since the rate of developing de novo SUI can be as high as 49.4 % [8, 14]. However, placement of a prophylactic sling in all patients might not be an appropriate solution, as doing so would place them at increased risk of mesh complications, future surgeries, and voiding dysfunction [19, 20]. These landmark studies differ from our study in that they evaluated a population with evidence of occult SUI. Given that our study demonstrates a small incidence of de novo SUI, and that no patient underwent additional procedures, the use of prophylactic slings in all patients should not be recommended as the standard of care.
The incidence of de novo SUI in our cohort of 4.4–9.9 % is comparable with current literature. Lo et al. evaluated women with no occult SUI by cough stress test and UDS (n = 637) and noted a de novo SUI rate of 11.1 %. However, in contrast to their study—in which findings of self-reported SUI and number of urinary leaks per day were risk factors for development of de novo SUI—Lo et al. noted that older age, diabetes, a lower MUCP, and a shorter functional urethral length were risk factors [21]. However, when evaluating patients with no baseline complaint of SUI, it was also found that older age was an associated risk factor for de novo SUI. Differences in study findings might be contributable to the large number of total vaginal mesh procedures included in the Lo et al. cohort (78 %) or the use of a pessary for prolapse reduction vs. a speculum. Columbo et al. evaluated women with no evidence of SUI on UDS after speculum reduction who were undergoing cystopexy (n = 52) vs. cystopexy with pubourethral ligament suspension (n = 50) [22]. Similar to our study findings, they found that the incidence of de novo SUI in both groups was small (8 %) by objective and subjective criteria.
Higher rates of de novo SUI have been quoted in the literature. In 2011, Al-Mandeel et al. evaluated 100 women 2 years after vaginal POP surgery who were objectively continent preoperatively based on a negative cough stress test and found 42 % had postoperative SUI [23]. They further mentioned 18/42 had a subjective complaint of SUI and that the true incidence of de novo SUI was 24 %. The authors commented that their incidence was higher than previously reported and was likely attributable to the higher POP stage in their patients, their longer follow-up interval (1–2 years), and the definition of postoperative SUI, which used the validated Urinary Distress Inventory (UDI-6) short form. In contrast, our study found much lower rates of de novo SUI (4.4 % vs. 24 %), even though it included the same procedures and a similar follow-up interval. A reason for the discrepancy may be in the technique of prolapse reduction, as overcorrection of the vault can lead to greater SUI, especially if direct pressure is applied to the anterior vaginal wall.
Multichannel UDS with prolapse reduction has become a standard practice for many surgeons for evaluating occult SUI but with variable methods [24, 25]. Visco et al. described several methods of prolapse reduction testing and found that the highest detection rate was with using a speculum (30 %) vs. manual (16 %), forceps (21 %), swab (20 %), or pessary (6 %) [7]. In our practices, postsurgical correction of POP is simulated by speculum reduction during UDS. We previously compared prolapse reduction using packing, speculums, and pessaries, and found that speculum and pessaries allowed the highest detection rates of occult SUI at our institution [26]. Based on our experience, if there is evidence of occult SUI, the patient is counseled about the risk of postoperative SUI and will likely receive an incontinence procedure. However, in patients with a baseline complaint of SUI who do not leak during UDS, a sling is not typically placed unless the patient reports significant bother from the symptoms.
This is one of the first studies that evaluated both vaginal and laparoscopic POP surgeries, compared apical suspension to nonapical suspension procedures, and evaluated for associated risk factors for development of de novo SUI. Additional strengths of the study include a large cohort evaluated over a 10-year period and that all patients had two objective tests—UDS and SST—to evaluate for occult SUI.
A limitation of the study is lack of a priori power calculation. A post hoc power calculation determined that the study had 80 % power with the current sample size to detect a difference of 23.8 %. Due to the small difference (0.8 %) between apical and nonapical procedures, it is possible that the nonsignificant result is secondary to an inadequate sample size. However, given that the confidence interval of the effect size is very narrow and crosses zero, it is more likely that the observed nonsignificance is true [27]. Addition weaknesses include the retrospective design using a database, which can results in missing or incomplete data. The study also did not use a validated survey or assess symptom severity when assessing preoperative or postoperative subjective complaints of SUI, which could have affected the true incidence of de novo SUI. In addition, the small sample size of sacrocolpopexies limited the ability to make conclusions or strong recommendations based on our data.
In conclusion, based on this cohort of women with and without suspension procedures, there appears to be no higher risk of de novo SUI after suspension procedures. The incidence of de novo SUI after POP procedures in women without preoperative evidence of occult SUI is only 9.9 %. Due to the low incidence of de novo SUI, even in women who complained of baseline SUI, we would not recommend concomitant sling procedures in this population. It is advised that these patients be counseled about their risk and offer a staged procedure if needed.
References
Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6. doi:10.1016/S0029-7844(97)00058-6.
Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–6. doi:10.1001/jama.300.11.1311.
Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123(6):1201–6. doi:10.1097/AOG.0000000000000286.
Smith FJ, Holman CD, Moorin RE, et al. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol. 2010;116(5):1096–100. doi:10.1097/AOG.0b013e3181f73729.
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26. doi:10.1007/s00192-009-0976-9.
Rosenzweig BA, Pushkin S, Blumenfeld D, et al. Prevalence of abnormal urodynamic test results in continent women with severe genitourinary prolapse. Obstet Gynecol. 1992;79(4):539–42.
Visco AG, Brubaker L, Nygaard I, et al. The role of preoperative urodynamic testing in stress-continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):607–14. doi:10.1007/s00192-007-0498-2.
Wei JT, Nygaard I, Richter HE, et al. A midurethral sling to reduce incontinence after vaginal prolapse repair. N Engl J Med. 2012;366(25):2358–67. doi:10.1056/NEJMoa1111967.
de Tayrac R, Gervaise A, Chauveaud-Lambling A, et al. Combined genital prolapse repair reinforced with a polypropylene mesh and tension-free vaginal tape in women with genital prolapse and stress urinary incontinence: a retrospective case–control study with short-term follow-up. Acta Obstet Gynecol Scand. 2004;83(10):950–4. doi:10.1111/j.0001-6349.2004.00499.x.
Liang CC, Chang YL, Chang SD, et al. Pessary test to predict postoperative urinary incontinence in women undergoing hysterectomy for prolapse. Obstet Gynecol. 2004;104(4):795–800. doi:10.1097/01.AOG.0000140689.90131.01.
Ennemoser S, Schonfeld M, von Bodungen V, et al. Clinical relevance of occult stress urinary incontinence (OSUI) following vaginal prolapse surgery: long-term follow-up. Int Urogynecol J. 2012;23(7):851–5. doi:10.1007/s00192-012-1765-4.
Winters JC, Dmochowski RR, Goldman HB, et al. Urodynamic studies in adults: AUA/SUFU guideline. J Urol. 2012;188(6 Suppl):2464–72. doi:10.1016/j.juro.2012.09.081.
Haylen BT, Maher CF, Barber MD, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int Urogynecol J. 2016;27(4):655–84. doi:10.1007/s00192-016-3003-y.
Brubaker L, Cundiff GW, Fine P, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. N Engl J Med. 2006;354(15):1557–66. doi:10.1056/NEJMoa054208.
Kasturi S, Diaz SI, McDermott CD, et al. De novo stress urinary incontinence after negative prolapse reduction stress testing for total vaginal mesh procedures: incidence and risk factors. Am J Obstet Gynecol. 2011;205(5):487 e481–484. doi:10.1016/j.ajog.2011.07.006.
van der Ploeg JM, Oude Rengerink K, van der Steen A, et al. Transvaginal prolapse repair with or without the addition of a midurethral sling in women with genital prolapse and stress urinary incontinence: a randomised trial. BJOG. 2015;122(7):1022–30. doi:10.1111/1471-0528.13325.
Leruth J, Fillet M, Waltregny D. Incidence and risk factors of postoperative stress urinary incontinence following laparoscopic sacrocolpopexy in patients with negative preoperative prolapse reduction stress testing. Int Urogynecol J. 2013;24(3):485–91. doi:10.1007/s00192-012-1888-7.
Park J, McDermott CD, Terry CL, et al. Use of preoperative prolapse reduction stress testing and the risk of a second surgery for urinary symptoms following laparoscopic sacral colpoperineopexy. Int Urogynecol J. 2012;23(7):857–64. doi:10.1007/s00192-011-1648-0.
Fatton B. Is there any evidence to advocate SUI prevention in continent women undergoing prolapse repair? An overview. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(2):235–45. doi:10.1007/s00192-008-0734-4.
van der Ploeg JM, van der Steen A, Oude Rengerink K, et al. Prolapse surgery with or without stress incontinence surgery for pelvic organ prolapse: a systematic review and meta-analysis of randomised trials. BJOG. 2014;121(5):537–47. doi:10.1111/1471-0528.12509.
Lo TS, Bt Karim N, Nawawi EA, et al. Predictors for de novo stress urinary incontinence following extensive pelvic reconstructive surgery. Int Urogynecol J. 2015;26(9):1313–9. doi:10.1007/s00192-015-2685-x.
Colombo M, Maggioni A, Zanetta G, et al. Prevention of postoperative urinary stress incontinence after surgery for genitourinary prolapse. Obstet Gynecol. 1996;87(2):266–71. doi:10.1016/0029-7844(95)00378-9.
Al-Mandeel H, Ross S, Robert M, et al. Incidence of stress urinary incontinence following vaginal repair of pelvic organ prolapse in objectively continent women. Neurourol Urodyn. 2011;30(3):390–4. doi:10.1002/nau.20947.
Roovers JP, Oelke M. Clinical relevance of urodynamic investigation tests prior to surgical correction of genital prolapse: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):455–60. doi:10.1007/s00192-006-0260-1.
Martin JL, Williams KS, Abrams KR, et al. Systematic review and evaluation of methods of assessing urinary incontinence. Health Technol Assess. 2006;10(6):1–132. iii-iv.
Lefevre R, Pollack J, Apostolis C, Aguilar V, Davila G. Unmasking occult incontinence in advanced vaginal prolapse: which reducing tool works best? Int Urogynecol J. 2008;19 Suppl 1:S1–166.
Hoenig JM, Heisey D. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat. 2001;55(1):19–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclaimer
None
Conflicts of interest
GW Davila received an honorarium from American Medical Systems, and CL Medical. He also is a consultant for American Medical Systems, and received research funding through Cololpast. The other authors have no disclosures or conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Alas, A.N., Chinthakanan, O., Espaillat, L. et al. De novo stress urinary incontinence after pelvic organ prolapse surgery in women without occult incontinence. Int Urogynecol J 28, 583–590 (2017). https://doi.org/10.1007/s00192-016-3149-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-016-3149-7