Abstract
Introduction and hypothesis
Stress urinary incontinence (SUI) is in part attributed to qualitative and quantitative changes in connective tissue of the urogenital tract. We examined the association of collagen type I a1 (COLIA 1) Sp1 polymorphism with the risk of SUI.
Methods
Forty-five postmenopausal women suffering from urodynamically verified SUI (study group) were compared to 45 healthy volunteers (control). DNA was extracted from peripheral blood. The genotyping concerning the type 1 a1 collagen gene Sp1 polymorphism was performed with polymerase chain reaction.
Results
The polymorphic T allele was overrepresented in the SUI patients (63.2% versus 36.8%, p = 0.016). Odds ratio for SUI in women harboring the T allele was 2.19 (95% CI 1.149–4.176) compared to women with the wild-type genotype.
Conclusions
The COLIA1 Sp1 polymorphism is associated with increased prevalence of stress urinary incontinence in postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence and pelvic floor disorders are frequent problems among women, with a serious impact on health and quality of life. Urinary incontinence is the involuntary loss of urine. It affects about 30% of the female population. Among the kinds of incontinence (stress, urge, and mixed urinary incontinence), stress urinary incontinence (SUI) is the most frequent, with a prevalence of 50%. Urge and mixed type incontinence prevalence are 11% and 36%, respectively [1].
The prevalence of SUI is significantly increased after menopause, particularly in women with coexisting risk factors such as vaginal and instrumental deliveries, multiparity, obesity, and metabolic syndrome [2]. SUI can be attributed to insufficient bladder base support and neck or urethral sphincter dysfunction [3]. Continence also depends on the adequate function of the muscles of the pelvic floor as well as the connective tissue supporting structures of the paraurethral fascia and pubocervical ligaments [4]. Recent studies show an association between qualitative and quantitative changes in connective tissue and the presence of genuine SUI [5]. The main structural protein of connective tissue is type 1 collagen. The quality and organization of collagen fibers can affect the tensile strength of the pubocervical fascia which provides support to the bladder neck and base [6, 7].
Collagen type1 is a protein consisting of two a1 (I) and one a2 (I) chain {a1 (I)2a2(I)} [8]. The expression of genes encoding the collagen a1 chain (COLIA1) and a2 chain (COLIA2) is coordinated and efficiently regulated. Polymorphisms of the COLIA1 gene can affect its expression rate [9]. COLIA1 Sp1 is a single nucleotide polymorphism at the regulatory region of the COLIA 1, where guanine is substituted by thymine at the first intron of the gene. This polymorphism results in three different groups (homozygotes GG, heterozygotes G/T, and homozygotes T/T). These variations can affect a recognition site for the transcription factor Sp1, changing the affinity of Sp1 binding to the DNA chain. This leads to an increased production of COLIA1 relative to COLIA2 protein. Sp1 polymorphism may be responsible for reduced bone mechanical strength and mineral density [10]. There are also recent data implicating the COLIA1 polymorphism in the pathogenesis of SUI [11] or pelvic organ prolapse [12]. The aim of the present study was to examine the association between COLIA1 Sp1 polymorphism and the presence of stress urinary incontinence.
Materials and methods
Ninety postmenopausal women, aged 47 to 69 years old, were enrolled in the study. Patients were followed on a regular basis in the Menopause Clinic of the Second Department of Obstetrics and Gynecology in Aretaieio Hospital. Exclusion criteria were: medical history of urge or mixed type of incontinence, hysterectomy, neurological disorders, or cancer. All patients signed an informed consent form. The study was approved by the institution’s ethics committee.
The patients were divided into two groups: the first group involved 45 women suffering from SUI, while the second group involved 45 healthy women who served as controls. SUI was diagnosed by medical history and verified by urodynamic examination (uroflowmetry, cystometry, abdominal leak point pressure, and profilometry), according to the International Continence Society standards [13]. An ICS standardized 1-hour pad test was used to assess SUI severity [14]. All SUI patients had a positive (pad weight gain >2 gm) 1-hour pad test and were scheduled for operational treatment with tension-free vaginal tape.
All patients were examined in the morning in light clothing. Weight and height were recorded and body mass index (BMI) was calculated by the formula: weight (kg)/height2 (m). Fasting blood was drawn in all participants and samples were stored at −80°C until assessment. Bone mineral density (BMD) of the lumbar spine was measured using a Norland-Excell Plus-XR-36 densitometer (Norland Medical Systems, Fort Atkinson, WI, USA) by dual energy X-ray absorptiometry. Within-subject coefficient of variation was 1.1%.
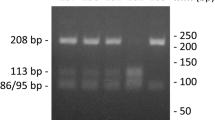
DNA was extracted from blood samples collected in EDTA, using Macherey–Nagel NucleoSpin® blood kit. The detection of the regulatory variant (G→T mutation, Sp-1 polymorphism) in intron 1 of the type 1 alpha 1 collagen gene (COL1A1) encoding for the alpha 1 (I) protein chain of type I collagen was performed using MutaGel® Collagen (AS) kit of Immunodiagnostik AG. Two sets of primers, (Mix 1, Mix 2) for both (wild type or variant) allele specific sequences within the human collagen gene COL1A1, were used directly for PCR with extracted DNA (150 μg), using the following amplification protocol (94°C for 5 min, 94°C, 30 s/58°C, 30 s/72°C, 90 s for 37 cycles, and final hold at 74°C for 5 min) and generate amplificates only in case of presence of one from both sequence possibilities (wild type or variant). The resulting amplification products were subsequently identified with gel electrophoretic methods using E-Gel® 4% agarose (HR) from Invitrogen™. The presence of normal gene variant (S) was indicated by detection of the DNA fragment (270 bp) of Mix 1 whereas the presence of pathogen gene variant (s) was indicated by the presence of the DNA fragment (270 bp) of Mix 2 (Fig. 1).
Based on a previous study of Skorupski et al. [11], a sample size of 45 women in each group would be required in order to detect an absolute difference of 30% or higher in the prevalence of the mutant genotype (G/T or T/T), with power of 80% and a p value <0.05. The χ 2 test was used to compare the prevalence of various genotypes among patients from the study and control groups. The association between each genotype and the presence of SUI was calculated using odds ratios and 95% confidence intervals. A p value <0.05 was considered statistically significant for all comparisons.
Results
Demographic characteristics of the 90 postmenopausal women participating in the study are shown in Table 1. Patients with SUI had a higher mean BMI (27.75 ± 3.29) compared to the control group (25.18 ± 3.32). Similarly, patients with SUI were older (58.5 ± 5.5) compared to women without SUI (55.8 ± 3.8, p = 0.008). Finally, patients with SUI had a higher frequency of multiparity compared to controls (33.3% versus 8.9%, p = 0.013).
The distribution of genotypes of the studied polymorphism in the 90 investigated women was GG 43.33%, GT 50%, and TT 6.7%. The comparison between the SUI and control groups showed higher prevalence of heterozygous state GT in the study group when compared to the control group (Table 2). The difference was statistically significant (p value = 0.041).
The odds ratio for SUI in patients with TT genotype was almost nine times higher when compared to women with the GG genotype (Table 3). However, the wide confidence limits indicate that this estimate has low precision. Patients with GT genotype had an odds ratio of 2.4 for SUI compared to women with the GG genotype (95% confidence interval 1.011 to 5.903). The T allele in the SP1 binding site of the COLIA1 gene was overrepresented in SUI group. Concerning the gene–dose effect, the overall odds ratio for SUI was 2.19 per copy of the T allele.
Discussion
SUI is a frequent disabling problem of postmenopausal women. Several studies attribute SUI to functional and quality changes of the fibrous connective tissue of the pelvic floor ligaments. Significant changes in the extracellular matrix may result to a stiffer and less supportive paraurethral connective tissue [6, 7]. Possible changes of the ratio between collagen I and III may lead to deterioration of the supporting properties of connective tissue. In a previous study, we demonstrated a significant reduction of collagen type I in about 53% patients with SUI, irrespectively of the presence or absence of genital prolapse [15]. This reduction can lead to inadequate support of the bladder neck, causing unequal transmission of the intraabdominal pressure between bladder neck and urinary bladder and a consequent development of SUI.
Numerous studies investigate genetic polymorphisms in the genes encoding the a1 and a2 chains of collagen type I and their influence in various pathological conditions. According to the Genetic Markers for Osteoporosis consortium (Genomos) framework project, COLIA 1 Sp1 polymorphism was associated with reduced bone mineral density and increased fracture risk [16]. Α meta-analysis, aiming to quantitate the association between collagen type I alpha 1 (COLIA1) Sp1 polymorphism and osteoporotic fracture risk in Caucasian postmenopausal women, showed that patients with vertebral fractures had a significantly higher frequency of the Ss genotype and a lower frequency of the SS genotype than controls. Additionally, patients with non-vertebral fractures had a significantly higher frequency of the ss genotype and a lower frequency of the SS genotype than controls (SS odds ratio [OR] 0.72; Ss OR 1.18; ss OR 1.97) [17]. These findings confirmed the results of a previous study, which showed that the “s” allele, associated with low BMD and increased fracture risk in Caucasians, is non-existent or very rare in the southern Chinese population. The absence of this “high risk” allele may in part account for the reduced fracture risk observed in the Chinese in comparison to western populations [18].
Data on the association of Sp1 polymorphism and pelvic floor disorders are sparse. A recent study found an increased prevalence of the heterozygous (GT) genotype of COLIA 1 Sp1 polymorphism among women with pelvic organ prolapse (POP) compared to the control group (33.3% and 19.3%, respectively). The age-adjusted odds ratio for POP was two times higher for patients carrying the T allele compared to women who had the wild-type GG genotype. This difference, however, did not reach statistical significance [12]. Furthermore, Rodrigues et al. conducting a case–control study in 107 patients with POP and 209 control women could not demonstrate a statistically significant association between Sp1 polymorphism and POP [19]. Finally, two small studies in Korean and Polish women failed to show an association between Sp1 polymorphism and POP [20, 21].
In contrast to POP, Sp1 polymorphism may be associated with SUI. Our study demonstrated that the G to T substitution in transcription factor Sp1-binding site is associated with the development of stress urinary incontinence, as the prevalence of the T allele was statistically significantly higher in women with SUI in comparison to healthy women. To the best of our knowledge, there is only one study in the literature examining the association between Sp1 polymorphism and SUI. Skorupski et al. [11] evaluated 50 women with SUI with a mean age of 53.4 and compared them to 50 healthy women with a mean age of 52.7. In accordance with our study, the odds ratio for SUI was 4.98 in women with the GT genotype and 2.23 for the TT genotype, while the T allele-specific odds ratio for SUI was 2.0. Supporting the hypothesis that COLIA1 polymorphism associates with SUI rather than POP, Goepel et al. showed a decreased immunohistochemical staining of types I, III, and VI collagen in periurethral tissue of patients with both POP and SUI over patients with POP alone. This finding suggests that collagen deficiency is more pronounced in SUI than in POP [22].
Regarding the results of our study, there are certain limitations; the small sample size can result in bias. It was also expected that women suffering from SUI have higher values of weight, BMI, and parity. This difference could also result into bias of the results, however to the best of our knowledge there is no genotype association to collagen polymorphism.
In conclusion, COLIA1 Sp1 polymorphism is associated with increased prevalence of stress urinary incontinence. Since genetic predisposition represents a considerable part of SUI etiology, further prospective studies involving larger populations should be conducted in order to confirm the contribution of COLIA1 Sp1 polymorphism in the etiology of SUI.
References
Hannestad YS, Rortveit G, Sandvik H, Hunskaar S (2000) Norwegian EPINCONT study. Epidemiology of incontinence in the county of Nord-Trøndelag. A community- based epidemiological survey of female urinary incontinence: the Norwegian EPINCONTstudy. Epidemiology of incontinence in the county of Nord-Trøndelag. J Clin Epidemiol 53(11):1150–1157
Melville JL, Katon W, Delaney K, Newton K (2005) Urinary incontinence in US women: a population-based study. Arch Intern Med 165(5):537–542
DeLancey JO, Starr RA (1990) Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med 35(8):765–771
Petros PE, Ulmsten UI (1993) An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand J Urol Nephrol Suppl 153:1–93
Rechberger T, Postawski K, Jakowicki JA, Gunja-Smith Z, Woessner JF Jr (1998) Role of fascial collagen in stress urinary incontinence. Am J Obstet Gynecol 179(6 Pt 1):1511–1514
Ulmsten U, Ekman G, Giertz G, Malmström A (1987) Different biochemical composition of connective tissue in continent and stress incontinent women. Acta Obstet Gynecol Scand 66(5):455–457
Falconer C, Ekman G, Malmström A, Ulmsten U (1994) Decreased collagen synthesis in stress-incontinent women. Obstet Gynecol 84(4):583
Keane DP, Sims TJ, Abrams P, Bailey AJ (1997) Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol 104(9):994–998
Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH (1996) Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet 14(2):203–5
Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP et al (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107(7):899–907
Skorupski P, Król J, Starega J, Adamiak A, Jankiewicz K, Rechberger T (2006) An alpha-1 chain of type I collagen Sp1-binding site polymorphism in women suffering from stress urinary incontinence. Am J Obstet Gynecol 194(2):346–350
Feiner B, Fares F, Azam N, Auslender R, David M, Abramov Y (2009) Does COLIA1 SP1-binding site polymorphism predispose women to pelvic organ prolapse? Int Urogynecol J Pelvic Floor Dysfunct 20(9):1061–1065
Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A et al (2002) International Continence Society. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21(3):261–274
Ghoniem G, Stanford E, Kenton K, Achtari C, Goldberg R, Mascarenhas T, Parekh M, Tamussino K, Tosson S, Lose G, Petri E (2008) Evaluation and outcome measures in the treatment of female urinary stress incontinence: International Urogynecological Association (IUGA) guidelines for research and clinical practice. Int Urogynecol J Pelvic Floor Dysfunct 19(1):5–33
Liapis A, Bakas P, Pafiti A, Hassiakos D, Frangos-Plemenos M, Creatsas G (2000) Changes in the quantity of collagen type I in women with genuine stress incontinence. Urol Res 28(5):323–326
Ralston SH, Uitterlinden AG, Brandi ML, Balcells S, Langdahl BL, Lips P, Lorenc R et al (2006) GENOMOS investigators. Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med 3(4):e90
Ji GR, Yao M, Sun CY, Zhang L, Han Z (2009) Association of collagen type I alpha1 (COLIA1) Sp1 polymorphism with osteoporotic fracture in Caucasian post-menopausal women: a meta-analysis. J Int Med Res 37(6):1725–1732
Lambrinoudaki I, Kung AW (2001) Absence of high-risk “s” allele associated with osteoporosis at the intronic SP1 binding-site of collagen Ialpha1 gene in Southern Chinese. J Endocrinol Investig 24(7):499–502
Rodrigues AM, Girão MJ, da Silva ID, Sartori MG, Martins Kde F, Castro Rde A (2008) COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J Pelvic Floor Dysfunct 19(11):1471–1475
Cho HJ, Jung HJ, Kim SK, Choi JR, Cho NH, Bai SW (2009) Polymorphism of a COLIA1 gene Sp1 binding site in Korean women with pelvic organ prolapse. Yonsei Med J 50(4):564–568
Skorupski P, Miotła P, Jankiewicz K, Rechberger T (2007) Polymorphism of the gene encoding alpha-1 chain of collagen type I and a risk of pelvic organ prolapse—a preliminary study. Ginekol Pol 78(11):852–5
Goepel C, Hefler L, Methfessel HD, Koelbl H (2003) Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand 82(7):659–664
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sioutis, D., Economou, E., Lambrinoudaki, I. et al. Sp1 collagen I A1 polymorphism in women with stress urinary incontinence. Int Urogynecol J 22, 835–839 (2011). https://doi.org/10.1007/s00192-011-1372-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-011-1372-9