Abstract
Introduction and hypothesis
Pelvic organ prolapse has a multifactorial etiology. There is increasing evidence that genetic factors greatly impact its development. This study aimed to evaluate the possible relation of the collagenous polymorphism −1997 G/T with genital prolapse in Brazilian women.
Methods
A cohort study of 180 women with stage 0 or I (group A) pelvic organ prolapse disorder and 112 women with stage III or IV (group B) was conducted. Blood DNA was isolated, and the −1997 G/T polymorphism was identified by amplifying a region of the COLIA1 gene starting prior to the protein’s coding sequence.
Results
No significant difference in the prevalence of genotypes TG and TT was found between groups (p = 0.67); differences were not found even when patients were grouped by the presence of 0 or ≥ 1 polymorphic alleles (p = 0.46). Age and home birth were found to be independent risk factors for prolapse.
Conclusions
Our study could not find any association between the −1997G/T polymorphism and genital prolapse in Brazilian women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a common disorder that can disturb a woman’s quality of life. Women with POP have physical ailments and problems with their general health, personal relationships and sexual function [1].
More than 11% of American women will require operation for POP treatment or urinary incontinence (UI) during their life, and approximately 29% will need a reoperation [2].
POP has a multifactorial etiology. A variety of POP risk factors have been studied, including age, multiparity, vaginal delivery, forceps delivery, episiotomy, fetal macrosomia, overweight or obesity and increased intra-abdominal pressure conditions (e.g., chronic constipation and intense heavy lifting at work) [3,4,5,6,7]. There is increasing evidence that genetic factors and family history greatly impact the development of POP [3, 5, 8]. A large study with twin women showed that heredity significantly contributes to the occurrence of POP [9], and a recent systematic review found that women with a family history of POP have 2.58-times higher risk of developing the disease than women without a family history of the disease [10].
In recent years, several studies have reported variations in the expression of certain genes that can cause changes in the physical properties of connective tissue proteins, especially collagen fibers, and thus, hypothetically, lead to POP [3, 7,8,9].
Type I collagen is an important structural protein of connective tissue throughout the body, being the main constituent of this tissue in the pelvic floor. It consists of two chains of a1 (COLIA1) and one chain of a2 (COLIA2) [a1 (I) 2a2 (I)]. Polymorphisms of the COLIA1 gene may affect its rate of expression. In the −1997G/T (rs1107946) single nucleotide polymorphism (SNP), also called PCOL2, guanine (G) is replaced by thymine (T) prior to the start of the protein coding sequence. Consequently, three different genotypes are generated: G/G homozygotes, G/T heterozygotes and T/T homozygotes. This mutation is important because it affects the expression of the COLIA1 gene [11].
Many studies have shown the contribution of the SNP −1997G/T COLIA1 gene to susceptibility to osteoporosis in postmenopausal women [11, 12], but until recently, this polymorphism had not been studied in POP. All studies conducted thus far for POP have been limited to SNP +2045G/T (rs1800012), sometimes described as the “Sp1 binding site.” These studies observed qualitative and quantitative changes of type I collagen in patients with POP and stress urinary incontinence (SUI). In some studies, women with POP had a significantly lower amount of collagen than those without prolapse [13, 14]. Recently, Sioutis et al. found an association between the Sp1 polymorphism and increased risk of SUI [4].
POP and other collagen-associated disorders, such as osteoporosis, may have a common etiology, originating at the molecular level of the collagens [8, 11, 12]. Because the polymorphism of collagen is associated with other diseases, such as inguinal hernia, varicose veins, arterial aneurysm and joint hypermobility, it is plausible to believe that it is an underlying cause of all these disorders [8]. Type I collagen is one of the main constituents of the bone matrix and pelvic connective tissue. After the strong relationship of the −1997G/T polymorphism with bone mineral density (BMD) reduction and fracture was identified, we resolved to evaluate whether there is a relation between this polymorphism and POP since both diseases are genetically influenced [9, 11, 12]. Because collagen is of fundamental importance for pelvic floor support, and considering the limited existing knowledge about the relationship between −1997G/T and POP, we decided to carry out the present study. We have not found previous studies in Brazilian women on the association of −1997G/T with other collagen-associated diseases. Ultimately, the motivation for examining the −1997 G/T polymorphism was based on the hypothesis that this polymorphism is a risk factor for POP.
Materials and methods
This study was performed in the Department of Urogynecology and Vaginal Surgery within the Discipline of Gynecology at ABC Medical School (FMABC). The present study complied with the guidelines of Resolution 196/96 of the Brazilian National Health Council (Conselho Nacional de Saúde-CNS) and was previously submitted for evaluation and approved by the Research Ethics Committee of FMABC (approval no. 554.670/2014). All patients signed an informed consent form.
This was a cohort study carried out between 2014 and 2016. A total of 292 menopausal women were recruited; the women underwent anamnesis, physical gynecological examination and staging for genital prolapse using the Pelvic Organ Prolapse Quantification System (POP-Q) [15]. These women were separated into two groups. Group A consisted of women with POP stage 0 or I, while group B consisted of women with stage III or IV. All patients were asked about gynecological history (age at menopause, hormonal therapy, previous hysterectomy), obstetric history (parity, delivery method, newborn weight, episiotomy, labor analgesia and occurrence of home birth) and clinical history (smoking, hypertension, diabetes mellitus, dyslipidemia, chronic cough, constipation and activities with exaggerated physical exertion). Height and weight were measured and the body mass index (BMI) calculated. Regarding ethnicity, women were classified as white or non-white. The non-white term refers to the great miscegenation of the Brazilian population, as is difficult to precisely characterize the ethnic groups. Women in menacme, with neoplasias or with connective tissue disease were excluded from the study.
The patients were examined before the results of the genetic analysis; therefore, the doctors were blinded to the participants' genotypes.
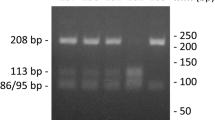
DNA from subjects was isolated from blood using the illustra blood genomicPrep Mini Spin Kit (GE Healthcare) following instructions from the supplier. Amplification of a 293-bp region from the COL1A1 gene promoter including the −1997G/T polymorphism (rs1107946) was conducted in a 10-μl reaction using approximately 100 ng of genomic DNA, PCR Master Mix (Promega) and primers described by Singh et al. (2011): 5′-CACCCTGCCCTAGACCAC-3′ and 5′-GAAAATATAGAGTTTCCAGAG-3′. The PCR protocol started with an initial step of 94 °C for 5 min followed by 45 cycles of three temperatures (94 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s) and final incubation at 72 °C for 10 min. Amplified DNA was digested by 1.0 U of BsaI restriction enzyme (Thermo Scientific) and analyzed in 3.0% agarose electrophoresis. Genotypes were determined by the observed pattern of digestion bands: a single 293 bp for homozygous TT, two bands of 212 and 81 bp for homozygous GG and three bands of 293, 212 and 81 bp for heterozygous TG (Fig. 1).
The normality of the quantitative data was verified using the Shapiro-Wilk test. Qualitative variables were compared using the chi-square and Fisher’s exact tests. An unpaired t-test was used to compare quantitative variables. The data were analyzed using GraphPad Prism 6 and SPSS version 23. After stratification of the groups, the influence of clinical characteristics on the risk of POP was estimated using odds ratios (ORs) obtained from the binary logistic regression model. The adopted significance level was 5% (p < 0.05), and the adopted confidence interval was 95% (95% CI).
Results
A total of 180 women with stage zero or I (group A) and 112 women with stage III or IV (group B) were selected for analysis. The groups were significantly different in terms of age, parity, number of pregnancies, number of vaginal deliveries and home deliveries (Table 1).
The frequency of the studied genotypes was consistent with the Hardy-Weinberg equilibrium in both groups (p = 0.45). In group A, 82 women presented the GG genotype, 81 the TG genotype and 14 the TT genotype. In group B, 57 women presented the GG genotype, 45 the TG genotype and 9 the TT genotype. No significant difference was observed between the groups in the prevalence of TG and TT genotypes (p = 0.67) (Table 2). There was also no significant difference when patients were stratified by the presence of at least one polymorphic allele compared with absence of polymorphic alleles (p = 0.46) (Table 3).
The characteristics associated with POP in the univariate analysis (Table 1) were included in a multivariate logistic regression model to characterize potential independent risk factors for POP. Age and home birth were identified as independent variables associated with POP (Table 4).
Discussion
SNPs are the most abundant type of DNA sequence variation in the human genome [7]. These variations are associated with population diversity, individuality and susceptibility to disease. Although the majority of SNPs do not have biological consequences, a fraction of substitutions have functional significance and are the basis for the physical diversity found among humans. Therefore, SNPs may play a direct or indirect role in phenotypic expression [16]. There is strong evidence that quantitative and qualitative changes in connective tissue, particularly collagen, may be responsible for defects in the pelvic floor support [8, 13, 14, 17]. This evidence may explain the growing interest in collagen polymorphisms.
The COLIA1 Sp1 polymorphism is the most frequently studied regarding POP and SUI. A recent study found a higher, but not statistically significant, prevalence of the COL1 Sp1 polymorphism in patients with advanced POP compared with those of the control group [18]. Rodrigues et al. carried out a case-control study with 107 patients with POP and 209 control women and failed to demonstrate a statistically significant association between the Sp1 polymorphism and POP [3]. Finally, two small studies in Korean and Polish women also showed no association between the Sp1 polymorphism and POP [19, 20]. Cartwright et al. conducted a systematic review and meta-analysis of genetic association studies of urinary symptoms and POP in women. This meta-analysis provides moderate epidemiological credibility for the association of COL1A1 Sp1 with POP. Although each individual study was insufficient, the size of the combined effect of the trials was significant (OR 1.33; 95% CI 1.02 to 1.73) [21]. However, the previous meta-analysis did not cover all eligible studies related to POP and COL1A1 Sp1. To clarify the authentic effect of the COL1A1 Sp1 polymorphism on POP susceptibility, Leng et al. performed another meta-analysis on this subject and demonstrated that the GT genotype is associated with an increased risk of POP (OR: 1.43; CI 95% 1.02–2.00) [22].
In agreement with POP, the Sp1 polymorphism is related to SUI. Sioutis et al. demonstrated that this polymorphism is associated with a 2.19 increased risk of developing SUI (95% CI 1.149–4.176) [4].
However, all of the studies conducted so far for collagen polymorphism and POP have been limited to the Sp1 SNP. The –1997G/T polymorphism has only recently been studied in POP. Numerous investigators have studied this polymorphism in relation to the risk of osteoporosis. Singh et al. showed that the –1997G/T polymorphism affects bone mineral density (BMD) in the lumbar spine and femoral neck and is associated with an increased risk of postmenopausal osteoporosis in women from northwestern India [11]. A meta-analysis of 32 studies involving 24,511 individuals with 7864 fractures included an analysis of three COL1A1 SNPs, i.e., rs1107946 (−1997G/T), rs1800012 (+1245G/T) and rs2412298 (-1663IndelT); the study found a strong association between polymorphisms and reduction of BMD or fracture. For the –1997G/T polymorphism, they identified five eligible studies, which together included 8257 women. The analysis showed a significant association between the –1997G/T polymorphism and reduction of BMD in the lumbar spine (p = 0.02). A similar difference was observed in the femoral neck, although it did not reach statistical significance (p = 0.09) [12].
Lee et al. investigated the association between POP and BMD of the lumbar spine and femoral neck in postmenopausal women. They selected 554 women and divided them into two groups (moderate to severe POP and mild or absent POP) and compared the BMDs between the groups. The lumbar spine BMD was inversely correlated with the severity of POP (p = 0.001). Knowing that a lower BMD is associated with an increased fracture risk, the authors postulated that women with severe POP would present an increased risk of osteoporotic fracture [23].
We conducted this pioneering study because of the limited existing knowledge on the effect of SNP –1997G/T with POP. In our study, we could not confirm the hypothesis that –1997G/T polymorphism is a risk factor for POP in Brazilian women. We believe that this result is influenced by the intense racial miscegenation of our population.
Although groups A and B were not completely homogeneous, a logistic regression model was applied to eliminate confounding factors and to identify independent risk factors. Our study showed that age, parity, vaginal delivery, home delivery and number of pregnancies were significantly different between groups. However, the multivariate logistic regression analysis identified only age and home birth as independent risk factors for the occurrence of POP, which are already well known as risk factors in the literature [5,6,7]. Other known risk factors, such as parity, vaginal delivery and number of pregnancies, were not confirmed, probably because of the small sample size.
Limitations of our study include the relatively small sample size and heterogeneity of the groups regarding clinical characteristics. Despite these limitations, this study is important because it is the first to investigate and try to demonstrate a relationship between the collagenous –1997G/T polymorphism and genital prolapse and will certainly contribute to the realization of meta-analyses in the future.
Based on the multifactorial etiology of POP, we believe that the discovery of new polymorphisms should help explain the complex pathophysiology of this disease, providing markers for clinical risk and prognosis as well as potentially providing a pathway to preventive measures and more effective treatments.
References
Digesu GA, Chaliha C, Salvatore S, Hutchings A, Khullar V. The relationship of vaginal prolapse severity to symptoms and quality of life. BJOG. 2005;112(7):971–6.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6.
Rodrigues AM, Girão MJ, da Silva ID, Sartori MG, Martins KF, Castro RA. COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:1471–5.
Sioutis D, Economou E, Lambrinoudaki I, Tsamadias V, Creatsa M, Liapis A. Sp1 collagen I A1 polymorphism in women with stress urinary incontinence. Int Urogynecol J. 2011;22:835–9.
Miedel A, Tegerstedt G, Mæhle-Schmidt M, Nyrén O, Hammarström M. Nonobstetric risk factors for symptomatic pelvic organ prolapse. Obstet Gynecol. 2009;113(15):1089–97.
Tegerstedt G, Miedel A, Mæhle-Schmidt M, Nyrén O, Hammarström M. Nonobstetric. Obstetric risk factors for symptomatic prolapse: a population-based approach. Am J Obstet Gynecol. 2009;194:75–81.
Martins Kde F, de Jármy-DiBella ZI, da Fonseca AM, et al. Evaluation of demographic, clinical characteristics, and genetic polymorphism as risk factors for pelvic organ prolapse in Brazilian women. Neurourol Urodyn. 2011;30(7):1325–8.
Lammers K, Lince SL, Spath MA, et al. Pelvic organ prolapse and collagen-associated disorders. Int Urogynecol J. 2012;23:313–9.
Altman D, Forsman M, Falconer C, Lichtenstein L. Genetic influence on stress urinary incontinence and pelvic organ prolapse. Eur Urol. 2008;54(4):918–22.
Lince SL, van Kempen LCLT, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J. 2012;23:1327–36.
Singh M, Singh P, Singh S, Juneja PK, Kaur T. A haplotype derived from the common variants at the 21997G/T and Sp1 binding site of the COL1A1 gene influences risk of postmenopausal osteoporosis in India. Rheumatol Int. 2013;33:501–6.
Jin H, Evangelou E, Ioannidis JPA, Ralston SH. Polymorphisms in the 5′ flank of COL1A1 gene and osteoporosis: meta analysis of published studies. Osteoporos Int. 2011;22:911–21.
Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347:1658–61.
Takano CC, Girão MJBC, Sartori MGF, et al. Analysis of collagen in parametrium and vaginal apex of women with and without uterine prolapse. Int Urogynecol J. 2002;13:342–5.
Haylen BT, de Ridder D, Freeman RM, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29(1):4–20.
Bortolini MAT, Rizk DEE. Genetics of pelvic organ prolapse: crossing the bridge between bench and bedside in urogynecologic research. Int Urogynecol J. 2011;22:1211–9.
Ward RM, Edwards DRV, Edwards T, Giri A, Jerome RN, WU JM. Genetic epidemiology of pelvic organ prolapse: a systematic review. Am J Obstet Gynecol. 2014;211(4):326–35.
Feiner B, Fares F, Azam N, Auslender R, David M, Abramov Y. Does COLIA1 SP1-binding site polymorphism predispose women to pelvic organ prolapse? Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(9):1061–5.
Cho HJ, Jung HJ, Kim SK, Choi JR, Cho NH, Bai SW. Polymorphism of a COLIA1 gene Sp1 binding site in Korean women with pelvic organ prolapse. Yonsei Med J. 2009;50(4):564–8.
Skorupski P, Miotła P, Jankiewicz K, Rechberger T. Polymorphism of the gene encoding alpha-1 chain of collagen type I and a risk of pelvic organ prolapse—a preliminary study. Ginekol Pol. 2007;78(11):852–5.
Cartwright R, Kirby AC, Tikkinen KA, et al. Systematic review and metaanalysis of genetic association studies of urinary symptoms and prolapse in women. Am J Obstet Gynecol. 2015;212(199):e1–24.
Leng B, Zhou Q, Zuo M. Association of COL1A1 Sp1-binding site polymorphism with susceptibility to pelvic organ prolapse: a meta-analysis. Int J Clin Exp Med. 2016;9(2):580–7.
Lee SW, Cho HH, Kim MR, et al. Association between pelvic organ prolapse and bone mineral density in postmenopausal women. J Obstet Gynaecol. 2015;35:476–80. Early Online: 1–5.
Acknowledgments
The authors thank the FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financially supporting this research under contract 2014/01107-6.
Author information
Authors and Affiliations
Ethics declarations
Conflicts of interest
None.
Additional information
Synopsis
In this cohort study, original in the literature, we could not identify any association between collagenous −1997G/T polymorphism and pelvic organ prolapse in Brazilian women.
Rights and permissions
About this article
Cite this article
Palos, C.C., Timm, B.F., de Souza Paulo, D. et al. Evaluation of COLIA1-1997 G/T polymorphism as a related factor to genital prolapse. Int Urogynecol J 31, 133–137 (2020). https://doi.org/10.1007/s00192-018-3833-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-018-3833-x