Abstract
Introduction and hypothesis
COLIA1 polymorphism is associated with increased risk for stress urinary incontinence. We hypothesize that a similar association exists with pelvic organ prolapse (POP).
Methods
Patients with advanced prolapse and healthy controls were evaluated by interview, validated questionnaires, and pelvic examination. DNA was extracted from peripheral blood, and polymerase chain reaction was performed to determine the presence or absence of the polymorphism. Power calculation indicated the need for 36 patients in each arm.
Results
The prevalence of the polymorphic heterozygous genotype (GT) in the study and control groups was 33.3% and 19.4%, respectively, leading to an odds ratio of 1.75. This difference, however, did not reach statistical significance (p = 0.27).
Conclusions
The COLIA1 polymorphism was not significantly associated with increased risk for POP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pelvic organ prolapse (POP) is a common and often debilitating group of conditions which frequently affect post-reproductive aged women, with considerable medical, social, and economical implications. The overall lifetime prevalence of this phenomenon ranges between 30% and 50% [1, 2], with the majority of women having stage 1 or 2 disease. In the elderly, stage 2 prolapse is as prevalent as 63% of all women [3]. Severe (stages 3 or 4) prolapse is less common, with a prevalence of around 2% in the adult female population [3, 4]. More than 11% of all American women will undergo at least one operation for POP or urinary incontinence during their lifetime, with a re-operation rate of 29% [5]. The annual cost of prolapse surgery in the USA is estimated to exceed one billion dollars [2]. There are certain recognized risk factors for developing POP including age, obesity, multiparity, increased weight of vaginally delivered babies, instrumental deliveries (especially forceps), extended second stage of labor, increased intra-abdominal pressure (such with chronic constipation and chronic obstructive lung disease), smoking, and various connective tissue disorders [4–8]. Ethnic and racial variations in the incidence of POP have also been described. Women of European and Hispanic ancestry may be at increased risk for developing POP and urinary incontinence as compared to women of Asian, African, and Native American ethnicity [6, 8, 9].
Since most multiparous women do not develop an advanced stage of prolapse [3, 4], and since this disorder has been reported in nulliparous women as well [10], one may hypothesize that genetic predisposition plays a role in the pathophysiology of POP. Jack et al. [11] studied ten patients younger than 55 years with a family history of prolapse. A genetic analysis of the inheritance pattern within these families demonstrated that POP segregated in a dominant fashion with incomplete penetrance. The relative risk for siblings of affected patients was found to be five times higher than that in the general population. In the last few years, several studies reported on variations in the expression of certain genes that could hypothetically lead to POP. Visco and Yuan [12] compared differential gene expressions in the pubococcygeus muscle in patients with advanced POP using a microarray analysis on 12,626 genes. They concluded that differences between patients with advanced POP and controls may be related to differential gene expression of structural proteins that are related to actin and myosin as well as extracellular matrix proteins in the pubococcygeus muscle. On the basis of this study, the same group then compared gene expression of two specific genes previously identified by the microarray analysis (skeletal muscle heavy chain polypeptide 3 and myosin binding protein H) in the pubococcygeus muscle of 17 patients with POP and 23 controls, using real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) analysis [13]. Significant differences in gene expression were observed for both genes between the two groups.
Another approach is to look for “errors” or polymorphism in the genetic code that might dictate alterations in the physical properties of tissue proteins. The only association was reported by Nikolova et al. [14], who found an increased prevalence of a single-nucleotide polymorphism (SNP) in the promoter to the gene LAMC1, encoding the γ1 chain of laminin, among women with an early onset and a family history of POP. Type I collagen is a major structural protein of connective tissue throughout the body. It is involved in innumerable essential processes within the cell apart from its role in providing support to the tissues. Its molecular structure is a heterotrimer, comprised of two α-1 chains and one α-2 chain which are encoded by the genes COLIA1 and COLIA2, respectively [15]. A thoroughly studied SNP in the COLIA1 gene is located at the regulatory region of the gene, affecting a transcription factor Sp1 binding site. It is caused by a substitution of guanidine for thymidine within its first intron, resulting in the occurrence of three different genotypes (homozygote G/G, heterozygote G/T, and homozygote T/T). As a consequence, the expression of the COLIA1 gene is altered, leading to abnormal production of the α-1 collagen chain relative to α-2 [15, 16]. An association between the COLIA1 polymorphism and osteoporosis was demonstrated in several studies within the last decade [16, 17]. Skorupski et al. [15] investigated the association between COLIA1 Sp1 polymorphism and stress urinary incontinence (SUI) in women and found that the odds ratio for developing SUI was 4.98 in subjects presenting the GT genotype and 2.23 for the TT genotype. Since it is believed that POP and SUI share a common etiologic basis, we aimed to assess the prevalence of COLIA1 Sp1 polymorphism among women with POP.
Materials and methods
The study protocol was approved by the institutional review board committee for human subjects, and all participants gave their written informed consent upon enrollment. This was a case–control study, and patients’ evaluations and genetic analysis occurred prospectively.
Subjects included in the study were women of Caucasian or Ashkenazi-Jewish origin who visited our gynecology outpatient clinic at Carmel and Lin Medical Centers, Haifa, Israel. Women with known connective tissue disorders (such as Marfan syndrome and Ehlers–Danlos syndrome), ongoing pregnancy, cancer involving reproductive or pelvic organs, or SUI were excluded. The study group consisted of women with advanced (stage 3 or 4) POP, and the control group consisted of women with no or mild (stage 0 or 1) prolapse. The diagnosis of advanced POP was based on physical examination of the external genitalia and vaginal canal, according to the POP quantification system (POPQ), as advocated by the International Continence Society, the American Urogynecologic Society, and the Society of Gynecologic Surgeons [18]. SUI was ruled out based on medical history, the results of a cough stress test, and two validated symptom-impact questionnaires-the urogenital distress inventory and the incontinence impact questionnaire [19, 20].
Blood samples were obtained from all subjects in tubes containing ethylenediaminetetraacetic acid. Genomic DNA was extracted from whole-blood leukocytes using a commercially available kit (high pure PCR template preparation kit, Roche, Mannheim, Germany). DNA was stored at −20°C until used. Determination of the COLIA1 polymorphism was performed by 2-step PCR (Eppendorf, Hamburg, Germany; Thermocycler) using 500 ng DNA as template. For amplification, the Taq DNA polymerase (Sigma, St. Louis, MO, USA) and commercially obtained oligonucleotide primers were used. The first step of PCR was carried out with the following primer sets: P1, 5′-GGAAGACCCGGGTTATTGCT-3′ (forward) and P2, 5′- CGCTGAAGCCAAGTGAAATA-3′ (reverse) [15, 21]. The 35 amplification cycles were preceded by denaturation at 94°C for 5 min. The repeated denaturations were performed at 94°C for 1 min, the annealing at 57°C for 1 min, and the elongation at 72°C for 1 min. A final primer extension was carried out at 72°C for 10 min. Undiluted PCR product (598 bp) from this reaction was used as template in nested PCR. The primers that were used for this reaction were P3, 5′- TAACTTCTGGACTATTTGCGGACTTTTTGG-3′ (forward) [15, 22] and P4, 5′-GTCCAGCCCTCATCCTGGCC-3′ (reverse) [15, 23].
The conditions of nested PCR were as follows: first denaturation at 94°C for 5 min and then 35 cycles of repeated denaturations at 94°C for 1 min, annealing at 58°C for 1 min, extension at 72°C for 45 s, and final extension at 72°C for 5 min.
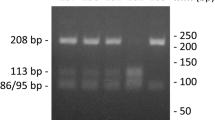
Reverse P4 primer was designed to introduce a restriction site for the enzyme BalI (MscI) only in alleles in which G is substituted by T [22, 23]. PCR products were digested with BalI according to manufacturer instructions (New England Biolabs, Beverly, MA, USA) and separated on 4% low-melting agarose gel. A single band (260 bp) corresponds to homozygote G/G, two bands (260 and 242 bp) to heterozygote G/T, and a single band (242 bp) enables the identification of homozygote T/T.
Power calculations (SPSS Inc. Chicago, IL, USA) were performed prior to recruitment, based on a previous report on genetic polymorphism and SUI [15], which found an increased prevalence of the GT genotype in the transcription factor Sp1-binding site in the gene encoding the alpha-1 chain of type I collagen among women with SUI as compared to healthy controls (58% versus 26%, respectively; p = 0.0023). Assuming a common etiology of POP and SUI, a sample size of 36 women in each group would be required in order to detect an absolute difference of 32% or higher in the prevalence of the mutant genotype (G/T or T/T), with power of 80% and a p value <0.05. The χ 2 test was used to compare the prevalence of various genotypes among patients from the study and control groups. The association between each genotype and the risk to develop advanced POP was calculated using odds ratios and 95% confidence intervals. A p value <0.05 was considered statistically significant for all comparisons.
Results
There were 36 participants in each group. The two groups were matched with respect to age, body mass index, and parity (overall parity, spontaneous vaginal deliveries, instrumentally assisted vaginal deliveries, and fetal macrosomy), as well as non-obstetric risk factors for POP including menopause, smoking, and chronic constipation (Table 1). All patients in the study group had POPQ St. 3 or 4 prolapse (most prolapsed component at more than +1 cm), and all patients in the control group had no more than St. 1 prolapse (most prolapsed component −1 cm or less; Table 2).
Genetic analysis of the entire studied population revealed the following distribution of genotypes at the SP1-binding site of the COLIA1 gene: GG, 72.2%; GT, 26.4%; and TT, 1.4%. There was a higher prevalence of the heterozygous state (GT) in the study group when compared to the control group (Table 3). Based on this finding, the calculated odds ratio for POP was 1.75 (2.0 when corrected for age) times higher for patients carrying any genotype other than GG compared to those who had the native GG genotype (Table 4). However, this difference in the prevalence of GT genotype did not reach statistical significance (p = 0.27). Moreover, the only participant who was found to carry the homozygous state (TT) was in the control group. The prevalence of a T allele at the Sp1-binding site of the COLIA1 gene did not differ significantly between the two groups (Table 3).
Discussion
Our results demonstrated a higher, albeit not statistically significant, prevalence of the COLIA1 polymorphism among patients with advanced POP over healthy controls. Genetic polymorphism in the genes encoding α-1 and α-2 chains of type I collagen and its influence on various pathological conditions have been thoroughly investigated. Yoneyama et al. [24] demonstrated a significantly higher prevalence of an exonic SNP in the COLIA2 gene among patients with intracranial aneurysms. During the last decade, several studies have reported the influence of the COLIA1 Sp1 polymorphism on bone density and osteoporosis. Recently, Suuriniemi et al. [16] found significantly reduced bone mineral density as well as reduction in other indices in association with COLIA1 polymorphism. Ralston et al. [17] reported results from a collaborative study involving 26,242 individuals from several European countries within the GENOMOS (Genetic Markers for Osteoporosis consortium) framework project. COLIA1 Sp1 polymorphism was found to be associated with reduced bone mineral density and increased risk for incidental fractures in women. While collagen is known as the major glycoprotein component of the extracellular matrix in the pelvic floor support system [25, 26], there is increasing evidence in the literature to connect impairments in collagen content and metabolism to POP [27–29]. Furthermore, the specific polymorphism explored in this study was found to be significantly more prevalent among women with SUI [15], a condition which is believed to share a common etiologic basis with POP, involving weakening of the tissues that normally provide support to the pelvic organs. Despite this assumption, a recent study by Rodrigues et al. [30] failed to demonstrate a significant association between the COLIA1 polymorphism and prolapse. This finding might be attributed to genetic heterogeneity between the Brazilian population (Rodrigues et al.) and the Polish population (Skorupski et al.) in the distribution of various mutations. However, although our study population consisted of Caucasian and Ashkenazi-Jewish Israeli women, who typically originate from Central or Eastern Europe, the higher prevalence of the polymorphic heterozygous genotype (GT) found in women with advanced POP did not reach statistical significance. Another possible explanation for the lack of statistical significance is that the association between the COLIA1 polymorphism and POP might not be as intense as between this polymorphism and SUI. Goepel et al. [27] showed a decreased immunohistochemical staining of types I, III, and VI collagen in periurethral tissue of patients with both SUI and POP over patients with POP alone, suggesting that deficiency in these subtypes of collagen is more pronounced in SUI than in POP. Of particular interest was the finding that the only case of polymorphic homozygosity (TT) was found in the control group (i.e., women without POP), suggesting that the GT genotype has a stronger association with POP than the TT genotype. A similar finding was reported in the study on COLIA1 polymorphism in SUI [15]. To determine whether this is mainly a result of a too small sample size or a true “protective effect” of the homozygous TT genotype for both SUI and POP, further research involving larger populations would be required.
References
Drutz HP, Alarab M (2006) Pelvic organ prolapse: demographics and future growth prospects. Int Urogynecol J Pelvic Floor Dysfunct 17(Suppl 1):S6–S9
Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS (2001) Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol 98:646–651
Nygaard I, Bradley C, Brandt D (2004) Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol 104:489–497
Samuelsson EC, Victor FT, Tibblin G, Svardsudd KF (1999) Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol 180:299–305
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89:501–506
Kim S, Harvey MA, Johnston S (2005) A review of the epidemiology and pathophysiology of pelvic floor dysfunction: do racial differences matter? J Obstet Gynaecol Can 27:251–259
Mant J, Painter R, Vessey M (1997) Epidemiology of genital prolapse: observations from the Oxford family planning association study. Br J Obstet Gynaecol 104:579–585
Swift S, Woodman P, O’Boyle A, Kahn M, Valley M, Bland D et al (2005) Pelvic organ support study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol 192:795–806
Dietz HP (2003) Do Asian women have less pelvic organ mobility than Caucasians? Int Urogynecol J Pelvic Floor Dysfunct 14:250–253; discussion 253
Harris RL, Cundiff GW, Coates KW, Bump RC (1998) Urinary incontinence and pelvic organ prolapse in nulliparous women. Obstet Gynecol 92:951–954
Jack GS, Nikolova G, Vilain E, Raz S, Rodriguez LV (2006) Familial transmission of genitovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct 17:498–501
Visco AG, Yuan L (2003) Differential gene expression in pubococcygeus muscle from patients with pelvic organ prolapse. Am J Obstet Gynecol 189:102–112
Hundley AF, Yuan L, Visco AG (2006) Skeletal muscle heavy-chain polypeptide 3 and myosin binding protein H in the pubococcygeus muscle in patients with and without pelvic organ prolapse. Am J Obstet Gynecol 194:1404–1410
Nikolova G, Lee H, Berkovitz S, Nelson S, Sinsheimer J, Vilain E et al (2007) Sequence variant in the laminin gamma1 (LAMC1) gene associated with familial pelvic organ prolapse. Hum Genet 120:847–856
Skorupski P, Krol J, Starega J, Adamiak A, Jankiewicz K, Rechberger T (2006) An alpha-1 chain of type I collagen Sp1-binding site polymorphism in women suffering from stress urinary incontinence. Am J Obstet Gynecol 194:346–350
Suuriniemi M, Kovanen V, Mahonen A, Alen M, Wang Q, Lyytikainen A et al (2006) COL1A1 Sp1 polymorphism associates with bone density in early puberty. Bone 39:591–597
Ralston SH, Uitterlinden AG, Brandi ML, Balcells S, Langdahl BL, Lips P et al (2006) Large-scale evidence for the effect of the COLIA1 Sp1 polymorphism on osteoporosis outcomes: the GENOMOS study. PLoS Med 3:e90
Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P et al (1996) The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol 175:10–17
Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA (1994) Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Continence program in women (CPW) research group. Qual Life Res 3:291–306
Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA (1995) Short forms to assess life quality and symptom distress for urinary incontinence in women: the incontinence impact questionnaire and the urogenital distress inventory. Continence program for women research group. Neurourol Urodyn 14:131–139
Vinkanharju A, Melkko T, Risteli J, Risteli L (2001) New PCR-based method for the Sp1 site polymorphism in the COL1A1 gene. Clin Chem Lab Med 39:624–626
Mann V, Hobson EE, Li B, Stewart TL, Grant SF, Robins SP et al (2001) A COL1A1 Sp1 binding site polymorphism predisposes to osteoporotic fracture by affecting bone density and quality. J Clin Invest 107:899–907
Grant SF, Reid DM, Blake G, Herd R, Fogelman I, Ralston SH (1996) Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet 14:203–205
Yoneyama T, Kasuya H, Onda H, Akagawa H, Hashiguchi K, Nakajima T et al (2004) Collagen type I alpha2 (COL1A2) is the susceptible gene for intracranial aneurysms. Stroke 35:443–448
Martin GR, Timpl R (1987) Laminin and other basement membrane components. Annu Rev Cell Biol 3:57–85
Ulmsten U, Falconer C (1999) Connective tissue in female urinary incontinence. Curr Opin Obstet Gynecol 11:509–515
Goepel C, Hefler L, Methfessel HD, Koelbl H (2003) Periurethral connective tissue status of postmenopausal women with genital prolapse with and without stress incontinence. Acta Obstet Gynecol Scand 82:659–664
Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ (1996) Changes in metabolism of collagen in genitourinary prolapse. Lancet 347:1658–1661
Wong MY, Harmanli OH, Agar M, Dandolu V, Grody MH (2003) Collagen content of nonsupport tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol 189:1597–1599; discussion 1599–1600
Rodrigues AM, Girao MJ, da Silva ID, Sartori MG, Martins Kde F, Castro Rde A (2008) COL1A1 Sp1-binding site polymorphism as a risk factor for genital prolapse. Int Urogynecol J Pelvic Floor Dysfunct 19:1471–1475
Acknowledgement
Mrs. Talma Rosen from the urogynecology outpatient department at the Carmel Medical Center contributed to the identification and recruitment of participants.
Funding
No funding was accepted for this project.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feiner, B., Fares, F., Azam, N. et al. Does COLIA1 SP1-binding site polymorphism predispose women to pelvic organ prolapse?. Int Urogynecol J 20, 1061–1065 (2009). https://doi.org/10.1007/s00192-009-0895-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-009-0895-9