Abstract
Purpose
The purpose of this study was to evaluate the shape and shift of the medial meniscus before and after meniscal repair concurrent with anterior cruciate ligament (ACL) reconstruction using magnetic resonance imaging (MRI) at 90° of knee flexion.
Methods
This study included 18 patients with ACL-deficient knees without meniscus tears (group A), 11 patients with medial meniscus tears alone (group M), and 15 patients with ACL-deficient knees complicated with medial meniscus tears (group AM). The posterior segment shape was evaluated using open MRI at 90° of knee flexion preoperatively and at 3 months postoperatively. The length, height, width, and posterior extrusion of the medial meniscus and posterior tibiofemoral distance were measured. These measurements were compared between the three groups.
Results
On preoperative MRI, a significant difference was observed in the posterior extrusion of the medial meniscus (group A, 1.2 ± 0.5 mm; group M, 1.7 ± 0.3 mm; group AM, 4.1 ± 1.5 mm, p < 0.001). All parameters did not differ between the three groups on postoperative MRI. In addition, the posterior width and extrusion of the medial meniscus were decreased significantly after meniscal repair concurrent with ACL reconstruction.
Conclusions
This study demonstrated that the medial meniscus shifted posteriorly at 90° of knee flexion in ACL-deficient knees complicated with medial meniscus tears. Medial meniscal repair concurrent with ACL reconstruction improved the deformed morphology and posterior extrusion. MRI measurements of the posterior extrusion at the knee-flexed position may be clinically useful to assess the functional improvement of the medial meniscus following meniscal repair combined with ACL reconstruction.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The anterior cruciate ligament (ACL) is the main restriction to anterior tibial loads [2, 14], whereas the posterior segment of the medial meniscus (MM) acts as a secondary stabiliser of anterior tibial translation [21, 27]. ACL injuries cause abnormal tibial translations and excessive shearing forces to the posterior segment of the MM [18, 26]. Compared to that in the extended position, the contact pressure between the meniscus and the femoral condyle is increased in the knee-flexed position [20]. An open magnetic resonance imaging (MRI) study reported that at 90° of knee flexion in ACL-deficient knees, the MM posterior segment was deformed because of the compression against the medial femoral posterior condyle [12]. Therefore, the posterior segment is susceptible to knee injuries with chronic ACL failure [3, 10]. MM tears associated with ACL injuries are considered among the main risk factors for progressive osteoarthritis [25].
Osteoarthritis is more frequently present in cases of concurrent ACL reconstructions with meniscectomy than in cases involving intact or repaired menisci [23]. Concurrent meniscectomy with ACL reconstruction can induce degenerative changes of the knee joint, as seen on radiographs [17]. In contrast, meniscal repair with concomitant ACL reconstruction has been associated with good clinical outcomes and a lower risk of reoperation during the follow-up periods [32, 34]. A clinical study reported that long-term knee functional scores were better among patients undergoing meniscal repair concurrent with ACL reconstruction than among those undergoing ACL reconstruction and partial meniscectomy [19]. However, very few studies have evaluated the effects of these procedures using MRI [8, 24]. The relationships between meniscal repair concurrent with ACL reconstruction and MM morphology also remain unclear.
This is the first MRI study to compare the shape and shift of the MM posterior segment at 90° of knee flexion in three cohorts: ACL-deficient knees without MM tears, ACL-intact knees with MM tears, and ACL-deficient knees complicated with MM tears. The purpose of this study was to evaluate the postoperative change of the MM morphology after ACL reconstruction, isolated MM repair, and MM repair concurrent with ACL reconstruction. Our study may help clarify the clinical effects of the meniscal repair concurrent with ACL reconstruction objectively. The hypotheses were as follows: (1) MM posterior segment in ACL-deficient knees complicated with MM tears is deformed and shifted posteriorly at 90° of knee flexion and (2) MM repair concurrent with ACL reconstruction can restore the deformed shape and posterior shift of the MM.
Materials and methods
The Institutional Review Board of Okayama University Graduate School (no. 1857) approved all the study protocols. Forty-four patients were operated on by two senior authors (TF and SM) between 2014 and 2017; they gave written informed consent to participate in this study. There were 18 patients with ACL rupture without MM tears (group A), 11 patients with isolated MM tears (group M), and 15 patients with ACL rupture complicated with MM tears (group AM). In groups M and AM, peripheral and vertical tears in the posterior segment were included, whereas degenerative, complicated, and dislocated tears of the MM were excluded. Patients who had lateral meniscus tears associated with ACL rupture were also excluded. Patients in groups A, M, and AM underwent ACL reconstruction, isolated MM repair, and MM repair concurrent with ACL reconstruction, respectively. Postoperative MRIs were examined at 3 months after surgery. The median duration of follow-up was 15.0 (range 3–30) months. Patient demographics are shown in Table 1.
Surgical procedure
The MM tears in groups M and AM were detected by arthroscopic probing. The mean length of the tears was 18 (range 10–25) mm. Moreover, 16, 7, and 3 patients had repairs using the FasT-Fix all-inside suture device (Smith & Nephew, Andover, MA, USA) alone, inside-out suture technique with 2-0 Wayolax (Matsuda Medical Instruments, Tokyo, Japan), and a combination repair using the FasT-Fix and 2-0 Wayolax, respectively. All ACL reconstructions were accomplished by hamstring tendon autograft (semitendinosus and/or gracilis tendons for anatomic double-bundle ACL reconstructions) [5, 15]. Graft fixation was achieved using an Endobutton CL (Smith & Nephew) or ACL Tight-Rope (Arthrex, Naples, FL, USA) on the femoral side [6, 7, 9]. Graft fixation on the tibial side was performed using a Double Spike Plate and a screw (Meira, Nagoya, Japan). Initial forces of 30 and 20 N were applied to the graft for the anteromedial and posterolateral bundles, respectively, at 10° of knee flexion.
All patients started knee motion exercises and partial weight-bearing at 2 weeks postoperatively. Full weight-bearing and running were allowed after 1 and 3 months, respectively.
MRI measurements
The patients underwent open MRI evaluations using the Oasis 1.2 T (Hitachi Medical, Chiba, Japan) with a coil in the 90° knee-flexed position in a non-weight-bearing condition and with the following settings: 16-cm field of view with an acquisition matrix size of 320 (phase) × 416 (frequency) and 4-mm slice thickness with 0-mm gap. Standard sequences of the Oasis included a sagittal proton density-weighted sequence [repetition time (TR)/echo time (TE), 1718/12] using a driven equilibrium pulse and coronal T2-weighted multi-echo sequence (TR/TE, 4600/84) [13].
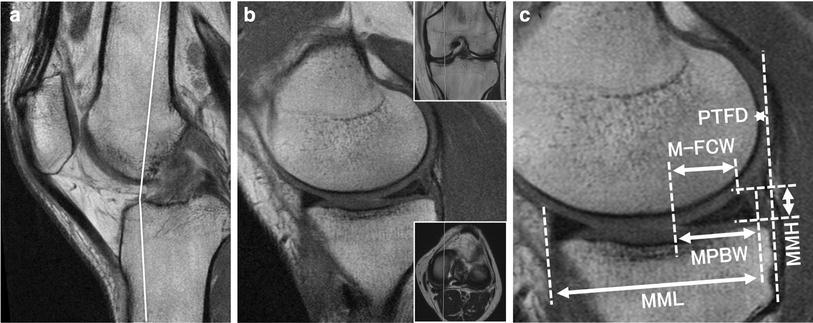
Analysis of the MM shape and shift was performed using a simple MRI-based meniscal sizing technique in the sagittal views as described previously [12]. The measurement plane was a sagittal plane that revealed the longest length of the MM (Fig. 1a). The medial meniscus length (MML) was defined as the distance from the anterior to the posterior border of the MM (Fig. 1b). The medial meniscus height (MMH) was defined as the distance from the lowest to the highest point of the MM posterior segment (Fig. 1c). The medial meniscus posterior body width (MPBW) was defined as the width from the anterior to the posterior border of the posterior segment (Fig. 1c). The distance from the posterior edge of the tibia to the posterior border of the MM was termed the medial meniscus posterior extrusion (MMPE), which was a measure of MM posterior shift. The anteroposterior distance between the tibial posterior edge and the posterior femoral condylar edge was termed the posterior tibiofemoral distance (PTFD) (Fig. 1b). These MRI parameters were compared between groups A, M, and AM. In addition, we evaluated postoperative changes of the parameters after each surgical procedure.
Magnetic resonance imaging-based measurements. a Sagittal view at 90° knee-flexed position. Inlet shows a white line projecting the measurement from the sagittal plane to the axial plane. b The sagittal measurement plane with the longest medial meniscus length (MML) and posterior tibiofemoral distance (PTFD). The green dashed line denotes the tibial posterior margin. c Measurement of MM posterior body width (MPBW), MM height (MMH), and MM posterior extrusion (MMPE)
Reliability evaluation
Inter-rater and test–retest reliabilities were assessed using the intraclass correlation coefficient (ICC) with the 95% confidence interval (CI). To determine the inter-rater reliability, four orthopaedic surgeons (YO, YK, TH, and TO) retrospectively examined the MRI scans in a blinded manner. The ICC was calculated for each MRI parameter by two-way, random, single measures with absolute agreement. Test–retest reliability was evaluated after 10–12 weeks in 44 stable knees. This time interval between test and retest was chosen because we believe it is long enough to prevent recall of the previous answer. ICCs were calculated using the Shrout and Fleiss method [30]. An ICC of ≥ 0.75 was considered excellent, ≥ 0.60 to < 0.75 was good; ≥ 0.40 to < 0.60 was fair, and < 0.40 was poor.
Statistical analysis
The differences in the MRI measurements between the three groups were evaluated using a one-way analysis of variance, followed by Tukey’s post-hoc pairwise comparisons. Pre- and postoperative examinations were assessed using the Wilcoxon signed-rank test. Data are presented as mean ± standard deviation. Significance level was set at p < 0.05. Power and statistical analyses were performed using EZR (Saitama Medical Center), which is a graphical user interface for R (The R Foundation for Statistical Computing). Effective statistical power of 80% (α = 0.05) was calculated for all parameters. The required sample size of MMPE was 8 in group AM. The effect sample sizes of MPBW for statistical significance between pre- and postoperative MRI were 18, 11, and 10 in groups A, M, and AM, respectively.
Results
Reliability evaluation
The overall inter-rater and test–retest reliability data are shown in Table 2. Excellent reliabilities were demonstrated in all MRI measurements. The inter-rater reliability (ICC 0.96) and test–retest reliability (ICC 0.96) in preoperative MMPE were the highest among all measurements.
Differences of MRI measurements
There were no significant differences in MML, MMH, MPBW, and PTFD between the three groups on preoperative MRIs (Table 3). However, a significant difference in the MMPE was observed (group A, 1.2 ± 0.5 mm; group M, 1.7 ± 0.3 mm; group AM, 4.1 ± 1.5 mm, p < 0.001). The mean MMPE was significantly higher in group AM than in groups A (p < 0.001) and M (p < 0.001). Although not significant, the MMPE in group M was larger than that in group A.
There were no significant differences in all parameters after the arthroscopic procedures on postoperative MRIs.
Postoperative change in MM shape and shift
The MPBW in all groups decreased significantly after each procedure (Table 4). The MMH in group AM also decreased postoperatively. Although the MMPE in groups M and AM decreased significantly, the MMPE in group A did not decrease. The postoperative PTFD in group AM increased.
Preoperatively, in the representative cases, the anterior tibial translation after ACL injury compressed the MM posterior segment (Fig. 2a). In group M, there was a tear gap in the posterior segment (Fig. 2b). In group AM, the tear gap widened and the posterior segment shifted posteriorly (Fig. 2c). The PTFD was reduced after the ACL reconstruction, and the compressed MM shape improved (Fig. 2d). The meniscal repair closed the tear gap (Fig. 2e). The meniscal repair concomitant with ACL reconstruction restored the posterior shift of the MM posterior segment in group AM (Fig. 2f).
The shape and shift of the medial meniscus (MM) on magnetic resonance imaging; green dashed lines, posterior margins of the tibia. A 32-year-old female patient in group A (a, d), a 41-year-old male patient in group M (b, e), and a 35-year-old male patient in group AM (c, f). a Preoperative image showing the compressed shape of the MM posterior segment. b Preoperative image presenting the vertical line of the torn MM and subtle MM posterior shift. c Preoperative image showing the posterior shift of the torn MM and the white arrow presenting the MM posterior extrusion. d The compressed shape was restored after anterior cruciate ligament reconstruction. e The vertical tear line was reduced after meniscal repair. f The posterior extrusion and the tear gap were decreased postoperatively
Discussion
The most important finding in the present study was that the torn MM posterior segment in ACL-deficient knees shifted posteriorly at 90° of knee flexion, and the posterior shift was reduced after MM repairs concurrent with ACL reconstructions. In addition, the posterior shift decreased postoperatively in ACL-intact knees with MM tears. These results suggest that the postoperative decrease in posterior shift could be due to the combined effect of meniscal repair and ACL reconstruction.
In this study, the MMPE in group A was the smallest among the three groups. This fact is supported by previous studies, which revealed the movement of the MM during knee joint flexion. Previous MRI studies showed that the posterior shift of the meniscus during knee flexion was the least in the MM posterior segment [28, 35]. A comparative study of ACL-injured and normal knees demonstrated that the meniscal posterior shift in the knee-flexed position was almost the same [29]. Therefore, it is conceivable that the ACL injury does not cause the posterior shift of the MM posterior segment (Fig. 3a). On the contrary, Amano et al. performed a three-dimensional MRI study to evaluate the deformations of torn MM posterior segments during knee flexion and reported that all torn menisci moved posteriorly, and the vertical gap of the torn MM increased from 0.9 to 1.2 mm at 60° of knee flexion [1]. Furthermore, ACL rupture causes excessive shearing forces to the MM posterior segment [26]. Thus, the marked MM posterior shift in group AM might be due to the increased vertical tear gap and the posterior extrusion of a piece of torn MM at 90° of knee flexion (Fig. 3b). The MM posterior shift is probably associated with losing the meniscal function as a secondary stabiliser.
Schematic illustrations of the medial meniscus (MM) posterior segment at 90° of knee flexion. a The ACL-deficient knee. The medial femoral condyle compresses the MM posterior segment by anterior translation force. b ACL-deficient knee with MM posterior segment tear. The MM posterior segment shifts posteriorly with increased vertical tear gap. c The ACL-reconstructed knee. ACL reconstruction improves the compressed shape of the MM posterior segment by repressing the anterior tibial translation. d The ACL-reconstructed knee with MM repair. The concurrent meniscal repair closes the vertical tear gap and restores the posterior shift of the MM posterior segment; black dashed lines, posterior margins of the tibia
ACL reconstruction is well known to be effective in repressing abnormal anterior tibial translation [14, 16]. In addition, some biomechanical studies demonstrated that ACL reconstructions could change the contact areas and pressures of the tibiofemoral joint postoperatively [11, 22]. Imhauser et al. showed that ACL deficiencies increased the contact pressure in the posterior area of the medial compartment under anterior loads, whereas ACL reconstruction reduced the contact pressure in the posterior area [11]. Moreover, Inoue et al. reported that the height and length of the MM posterior segment decreased after ACL reconstruction. They also reported a decrease in the contact area between the femoral posterior condyle and the MM posterior segment at 90° knee-flexed positions [12]. In this context, ACL reconstruction can reduce the contact area and pressure in the posteromedial compartment and restore the compressed shape of the MM posterior segment in the knee-flexed position (Fig. 3c).
MM repair concurrent with ACL reconstruction has achieved high healing rates as observed in the second-look arthroscopic evaluations (89%) [31]. In addition, a recent systematic review showed that the clinical failure rate was 10% for the inside-out technique and 16% for the all-inside technique at a 2-year follow-up [34]. It was conceivable that the procedure could create a favourable environment for meniscal healing due to fibrin clot formation associated with intra-articular hemarthrosis after drilling tibial and femoral tunnels [4, 33]. On the contrary, an MRI study by Furumatsu et al. revealed that MML was increased through MM repair concurrent with ACL reconstruction in the extended position, suggesting that the procedure could restore meniscal function by adjusting the torn MML [8]. In our present study, MRI-based MPBW and MMPE in group AM were decreased and equal to the values of groups A and M postoperatively. The decrease in MPBW could be explained by the fact that meniscal repair closed the vertical gap of the torn MM in group M (Fig. 2b, e). The decrease in MMPE is considered to be associated with the increase in PTFD and reduction in the contact pressure due to ACL reconstruction, in addition to the diminished vertical gap by the meniscal repair (Fig. 3d). Taken together, these results suggest that MM repair concurrent with ACL reconstruction could restore the shape and shift of the torn MM because of the combination of the two procedures.
There were several limitations to this study. First, our study had a small sample size for Wilcoxon signed-rank test to determine significant differences in MML, MMH, and PTFD in groups A and M. Second, we evaluated the MRI-based meniscal posterior translation under non-weight-bearing conditions. To assess the real function of the MM posterior segment in ACL-deficient knees, thin-slice MRI under loading conditions will be necessary. Third, MRI measurements at 90° of knee flexion are not representative of dynamic MM morphology. Three-dimensional reconstruction of the meniscus using dynamic MRI at various knee angles may be useful to visualize postoperative meniscal shift and morphological changes clearly. In addition, further investigations will be required to determine the precise improvements of MM morphology by meniscal repair, such as comparing it with concurrent meniscectomy with ACL reconstruction.
This study is clinically relevant in that the MRI measurements of MMPE at 90° flexed position are useful for surgeons because they can provide clinical information to help better understand how meniscal repair concurrent with ACL reconstruction can restore the function of MM as a secondary stabiliser.
Conclusions
This study demonstrated that the posterior segment of the MM was deformed and shifted posteriorly in ACL-deficient knee complicated with MM tear at 90° of knee flexion. MRI-based MPBW and MMPE were decreased after MM repair concurrent with ACL reconstruction, indicating that the surgical procedure restores the MM posterior shift and MM shape due to the combined effect of meniscal repair and ACL reconstruction.
References
Amano H, Iwahashi T, Suzuki T, Mae T, Nakamura N, Sugamoto K et al (2015) Analysis of displacement and deformation of the medial meniscus with a horizontal tear using a three-dimensional computer model. Knee Surg Sports Traumatol Arthrosc 23(4):1153–1160
Bell KM, Rahnemai-Azar AA, Irarrazaval S, Guenther D, Fu FH, Musahl V et al (2018) In situ force in the anterior cruciate ligament, the lateral collateral ligament, and the anterolateral capsule complex during a simulated pivot shift test. J Orthop Res 36(3):847–853
Chhadia AM, Inacio MC, Maletis GB, Csintalan RP, Davis BR, Funahashi TT (2011) Are meniscus and cartilage injuries related to time to anterior cruciate ligament reconstruction? Am J Sports Med 39(9):1894–1899
Dean CS, Chahla J, Matheny LM, Mitchell JJ, LaPrade RF (2017) Outcomes after biologically augmented isolated meniscal repair with marrow venting are comparable with those after meniscal repair with concomitant anterior cruciate ligament reconstruction. Am J Sports Med 45(6):1341–1348
Fujii M, Furumatsu T, Miyazawa S, Tanaka T, Inoue H, Kodama Y et al (2016) Features of human autologous hamstring graft elongation after pre-tensioning in anterior cruciate ligament reconstruction. Int Orthop 40(12):2553–2558
Furumatsu T, Fujii M, Tanaka T, Miyazawa S, Ozaki T (2015) The figure-of-nine leg position for anatomic anterior cruciate ligament reconstruction. Orthop Traumatol Surg Res 101(3):391–393
Furumatsu T, Kodama Y, Maehara A, Miyazawa S, Fujii M, Tanaka T et al (2016) The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res 57(2):91–98
Furumatsu T, Miyazawa S, Tanaka T, Okada Y, Fujii M, Ozaki T (2014) Postoperative change in medial meniscal length in concurrent all-inside meniscus repair with anterior cruciate ligament reconstruction. Int Orthop 38(7):1393–1399
Furumatsu T, Ozaki T (2018) Iatrogenic injury of the lateral meniscus anterior insertion following anterior cruciate ligament reconstruction: a case report. J Orthop Sci 23(1):197–201
Granan LP, Bahr R, Lie SA, Engebretsen L (2009) Timing of anterior cruciate ligament reconstructive surgery and risk of cartilage lesions and meniscal tears: a cohort study based on the Norwegian National Knee Ligament Registry. Am J Sports Med 37(5):955–961
Imhauser C, Mauro C, Choi D, Rosenberg E, Matthew S, Nguyen J et al (2013) Abnormal tibiofemoral contact stress and its association with altered kinematics after center–center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med 41(4):815–825
Inoue H, Furumatsu T, Miyazawa S, Fujii M, Kodama Y, Ozaki T (2018) Improvement in the medial meniscus posterior shift following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 26(2):434–441
Kashihara N, Furumatsu T, Kodama Y, Tanaka T, Ozaki T (2016) Concurrent lateral meniscal repair with anterior cruciate ligament reconstruction induces the extrusion of the lateral meniscus: assessments of magnetic resonance images. Acta Med Okayama 70(6):441–448
Kim D, Asai S, Moon CW, Hwang SC, Lee S, Keklikci K et al (2015) Biomechanical evaluation of anatomic single- and double-bundle anterior cruciate ligament reconstruction techniques using the quadriceps tendon. Knee Surg Sports Traumatol Arthrosc 23(3):687–695
Kodama Y, Furumatsu T, Miyazawa S, Fujii M, Tanaka T, Inoue H et al (2017) Location of the tibial tunnel aperture affects extrusion of the lateral meniscus following reconstruction of the anterior cruciate ligament. J Orthop Res 5(8):1625–1633
Kondo E, Merican AM, Yasuda K, Amis AA (2011) Biomechanical comparison of anatomic double-bundle, anatomic single-bundle, and nonanatomic single-bundle anterior cruciate ligament reconstructions. Am J Sports Med 39:279–288
Luc B, Gribble PA, Pietrosimone BG (2014) Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train 49(6):806–819
Markolf KL, Jackson SR, McAllister DR (2012) Force measurements in the medial meniscus posterior horn attachment: effects of anterior cruciate ligament removal. Am J Sports Med 40:332–338
Melton JT, Murray JR, Karim A, Pandit H, Wandless F, Thomas NP (2011) Meniscal repair in anterior cruciate ligament reconstruction: a long- term outcome study. Knee Surg Sports Traumatol Arthrosc 19(10):1729–1734
Muriuki MG, Tuason DA, Tucker BG, Harner CD (2011) Changes in tibiofemoral contact mechanics following radial split and vertical tears of the medial meniscus: an in vitro investigation of the efficacy of arthroscopic repair. J Bone Jt Surg Am 93(12):1089–1095
Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD (2010) The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med 38:1591–1597
Nagai K, Gale T, Irrgang JJ, Tashman S, Fu FH, Anderst W (2018) Anterior cruciate ligament reconstruction affects tibiofemoral joint congruency during dynamic functional movement. Am J Sports Med 46(7):1566–1574
Nakata K, Shino K, Horibe S, Tanaka Y, Toritsuka Y, Nakamura N et al (2008) Arthroscopic anterior cruciate ligament reconstruction using fresh-frozen bone plug-free allogeneic tendons: 10-year follow-up. Arthroscopy 24:285–291
Narazaki S, Furumatsu T, Tanaka T, Fujii M, Miyazawa S, Inoue H et al (2015) Postoperative change in the length and extrusion of the medial meniscus after anterior cruciate ligament reconstruction. Int Orthop 39(12):2481–2487
Neuman P, Englund M, Kostogiannis I, Friden T, Roos H, Dahlberg LE (2008) Prevalence of tibiofemoral osteoarthritis 15 years after nonoperative treatment of anterior cruciate ligament injury: a prospective cohort study. Am J Sports Med 36(9):1717–1725
Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH (2001) The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. Am J Sports Med 29(2):226–231
Seon JK, Gadikota HR, Kozanek M, Oh LS, Gill TJ, Li G (2009) The effect of anterior cruciate ligament reconstruction on kinematics of the knee with combined anterior cruciate ligament injury and subtotal medial meniscectomy: an in vitro robotic investigation. Arthroscopy 25:123–130
Scholes C, Houghton ER, Lee M, Lustig S (2015) Meniscal translation during knee flexion: what do we really know? Knee Surg Sports Traumatol Arthrosc 23(1):32–40
Shefelbine SJ, Ma CB, Lee KY, Schrumpf MA, Patel P, Safran MR et al (2006) MRI analysis of in vivo meniscal and tibiofemoral kinematics in ACL-deficient and normal knees. J Orthop Res 24(6):1208–1217
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86(2):420–428
Tachibana Y, Sakaguchi K, Goto T, Oda H, Yamazaki K, Iida S (2010) Repair integrity evaluated by second-look arthroscopy after arthroscopic meniscal repair with the FasT-Fix during anterior cruciate ligament reconstruction. Am J Sports Med 38(5):965–971
Walter RP, Dhadwal AS, Schranz P, Mandalia V (2014) The outcome of all-inside meniscal repair with relation to previous anterior cruciate ligament reconstruction. Knee 21(6):1156–1159
Wasserstein D, Dwyer T, Gandhi R, Austin PC, Mahomed N, Ogilvie-Harris D (2013) A matched-cohort population study of reoperation after meniscal repair with and without concomitant anterior cruciate ligament reconstruction. Am J Sports Med 41:349–355
Westermann RW, Duchman KR, Amendola A, Glass N, Wolf BR (2017) All-inside versus inside-out meniscal repair with concurrent anterior cruciate ligament reconstruction: a meta-regression analysis. Am J Sports Med 45(3):719–724
Yao J, Lancianese SL, Hovinga KR, Lee J, Lerner AL (2008) Magnetic resonance image analysis of meniscal translation and tibio-menisco-femoral contact in deep knee flexion. J Orthop Res 26(5):673–684
Acknowledgements
This study was supported by radiologic technologists in our university for taking accurate MRI and Ms. Ami Maehara for preparing schematic illustrations. Further, we would like to thank Editage (http://www.editage.jp) for English language editing.
Funding
No funding was required.
Author information
Authors and Affiliations
Contributions
YO, YK, TH, and TO measured the MRI-based parameters. TF and SM carried out the arthroscopic surgery. TF designed this study. YO and SM compiled the data. YO and TF prepared the manuscript, tables, and figures. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Ethical approval
The Institutional Review Board (Okayama University Graduate School, Okayama, Japan) approved this study.
Informed consent
Informed consent was obtained from all patients in this study.
Rights and permissions
About this article
Cite this article
Okazaki, Y., Furumatsu, T., Miyazawa, S. et al. Meniscal repair concurrent with anterior cruciate ligament reconstruction restores posterior shift of the medial meniscus in the knee-flexed position. Knee Surg Sports Traumatol Arthrosc 27, 361–368 (2019). https://doi.org/10.1007/s00167-018-5157-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-5157-2