Abstract
Purpose
Prediction of the risk of osteoarthritis in asymptomatic active patients with an isolated injury of the posterior cruciate ligament (PCL) is difficult. T1ρ magnetic resonance imaging (MRI) enables the quantification of the proteoglycan content in the articular cartilage. The purpose of this study was to evaluate subclinical cartilage degeneration in asymptomatic young athletes with chronic PCL deficiency using T1ρ MRI.
Methods
Six athletes with chronic PCL deficiency (median age 17, range 14–36 years) and six subjects without any history of knee injury (median age 31.5, range 24–33 years) were recruited. Regions of interest were placed on the articular cartilage of the tibia and the distal and posterior areas of the femoral condyle, and T1ρ values were calculated.
Results
On stress radiographs, the mean side-to-side difference in posterior laxity was 9.8 mm. The T1ρ values at the posterior area of the lateral femoral condyle and the superficial layer of the distal area of the medial and lateral femoral condyle of the patients were significantly increased compared with those of the normal controls (p < 0.05). At the tibial plateau, the T1ρ values in both the medial and lateral compartments were significantly higher in patients compared with those in the normal controls (p < 0.05).
Conclusion
T1ρ MRI detected unexpected cartilage degeneration in the well-functioning PCL-deficient knees of young athletes. One should be alert to the possibility of subclinical cartilage degeneration even in asymptomatic patients who show no degenerative changes on plain radiographs or conventional MRI.
Level of evidence
IV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The best treatment strategy for posterior cruciate ligament (PCL) injury and the operative indications remains controversial [3, 6, 15]. Non-operative treatment is generally recommended for isolated grade I and II PCL injuries. Treatment of isolated grade III PCL injuries is somewhat controversial. In general, operative treatment is recommended for chronic grade III PCL injuries in relatively young and active patients who become symptomatic because of pain or instability despite an appropriate rehabilitation program [3, 24]. However, non-operative treatment may be chosen even for young and active patients with grade III injuries if the patient remains complaint-free after initial rehabilitation [7]. A number of clinical studies suggest that the symptoms are not correlated with the grade of instability in isolated PCL injuries [7, 17, 19, 20].
Another concern in the treatment of PCL injury is the degenerative changes in articular cartilage during long-term follow-up. Although favourable subjective results of non-operative treatment on most isolated PCL injuries are reported to last up to 10 years, several studies suggest that articular cartilage deteriorates over time [2, 10, 17, 19, 20]. This also occurs in patients treated surgically [11, 16, 18, 24]. Most clinical studies utilise plain weight-bearing radiographs to assess the degenerative changes in articular cartilage [1]. These clinical studies have yielded little information on early degenerative changes in the articular cartilage matrix molecules. Furthermore, it is unclear what part of the articular cartilage of the knee joint would bear the brunt of the degenerative stress caused by kinematic loads in young athletes with PCL deficiency.
T1ρ magnetic resonance imaging (MRI) mapping enables the assessment of early degenerative changes in articular cartilage by detecting the proteoglycan content of the cartilage matrix [4, 8]. The clinical relevance of T1ρ MRI mapping has been reported in several in vivo studies [13, 14]. In this study, T1ρ MRI mapping was performed on six young athletes with chronic PCL deficiency. Although this is a preliminary study with a small sample size, the results showed unexpected cartilage damage in the asymptomatic knees of young athletes, suggesting a risk of cartilage deterioration in highly active patients with high instability.
Materials and methods
Six athletes with chronic PCL deficiency (median age 17, range 14–36 years) and six healthy volunteers (median age 31.5, range 24–33 years) without any knee problems were recruited for this study. The PCL deficiency was diagnosed using physical examinations, stress radiographs, and MRI findings. All patients participated in competitive team sports (soccer, baseball, rugby, and Japanese archery; the latter requires repetitive kneeling and squatting). All the patients had an isolated PCL injury without injury to any other ligaments including the posterolateral corner structures. All the patients were initially treated non-surgically with bracing for several months after the injury and given physical therapy for range of motion and muscle exercises. After the non-surgical treatment, they returned to their sports activities. One patient, who was injured 6 years ago, played on a competitive baseball team for 3 years and took part in Japanese archery for 3 years. Five patients had no complaints during sports activities, and one of them complained of occasional weakness and slight pain during sports activities. The median duration after PCL injury was 17 months (range 6–216 months). The mean Lysholm score was 98.3, and the mean Tegner score was 7.2.
Five of the healthy volunteers had participated in competitive or recreational sports (soccer, basketball, and baseball). The mean Tegner score of all the healthy volunteers was 6.3.
The study protocol was approved by the Institutional Ethics Board of Kyushu University (approval number: 23–75) and was carried out in accordance with the Tenets of the Declaration of Helsinki.
MRI scanning
The subjects underwent MRI of the knee, which was performed using a 3-Tesla MR system (Achieva 3.0T, Quasar Dual, Philips Healthcare, Best, The Netherlands) equipped with an 8-channel phased-array coil. The imaging protocol included a sagittal fat-suppression turbo spin echo T2-weighted imaging (FS-T2WI) sequence with the following parameters: repetition time (TR), 4,675 ms; echo time (TE), 71 ms; flip angle, 90°; turbo spin echo factor, 16; field of view (FOV), 140 × 140 mm; matrix, 400 × 400; slice thickness, 3 mm; slice gap, 0 mm; number of slices, 26; and number of excitations, 1. FS-T2WI was used as an anatomical reference as well as for diagnosis of injury to joint structures including ligaments, menisci, and cartilage.
Sagittal two-dimensional T1ρ mapping was performed without parallel imaging and each sequence used the following parameters: TR, 4.7 ms; TE, 2.4 ms; flip angle, 35°; FOV, 140 × 140 mm; matrix, 320 × 320; slice thickness, 3 mm; slice gap, 0 mm; number of slices, 26; NEX, 1; spin-lock pulse frequency, 500 Hz; and time of spin-lock (TSL), 1, 20, 40, 60, and 80 ms. Although flip angle was low, it did not affect T1ρ contrast because the shot interval was 6,000 ms between each slice acquisition and the k-space was filled using low–high ordering. T1ρ mapping was used for quantitative assessment.
Assessment of the T1ρ maps
T1ρ maps were estimated using pixel-by-pixel fitting of signals obtained from five different T1ρ-prepared images acquired with five different TSLs (1, 20, 40, 60, and 80 ms) and using the following exponential T1ρ decay equation: S(TSL) α exp (−TSL/T1ρ), where S(TSL) is the signal intensity in the T1ρ-prepared image with a given TSL. We produced T1ρ maps using Philips Research Integrated Development Environment (PRIDE) software written in Interactive Data Language (IDL 6.3, ITT Inc., Boulder, CO, USA).
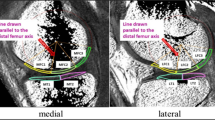
For the evaluation of T1ρ maps, regions of interest (ROIs) were drawn corresponding to parts of the full thickness of cartilage on the FS-T2WI, which was used after its in-plane resolution was adjusted to that of the T1ρ map. We picked up four adjacent sagittal slices at the centre of the medial and lateral compartment and evaluated the femoral condyle and tibial plateau in each slice. In the femoral condyle cartilage, ROIs were drawn around the distal and posterior areas. The distal area extends from the boundary adjacent to the anterior edge of the anterior meniscal body to the centre of the posterior meniscal body. The posterior area extends from the posterior edge of the distal area to an edge that is 90° posterior with respect to the femoral shaft axis. In the tibial plateau cartilage, ROIs were drawn to cover the entire cartilage area. ROIs were then divided into superficial and deep zones of equal thickness (Fig. 1). ROIs in the same locations were drawn on the T1ρ maps, and mean T1ρ values and standard deviations (SD) were calculated for each slice. The mean T1ρ values and SD of the ROIs of similar areas were calculated again from four slices. These analyses were performed by an experienced radiologist using “medical image processing, analysis, and visualisation” software (MIPAV, Biomedical Imaging Research Services Section, Center for Information Technology, National Institutes of Health, Bethesda, MD, USA). All measurements were performed by one observer (K. Okazaki) and were repeated in a blinded manner during the course of two sessions 1 month apart. Another observer (K. Osaki) independently made measurements of five randomly selected knees.
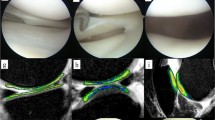
Regions of interest (ROIs) were drawn on the articular cartilage in fat-suppression turbo spin echo T2-weighted imaging (FS-T2WI) of the knee. ROIs were in the distal area (D) and posterior area (P) of the femoral condyle and in the tibial plateau (T). ROIs were then divided into superficial and deep layers. The image of the lateral compartment in a case with PCL deficiency is shown
Stress radiography
A stress radiography examination was performed by applying a 15-kg posterior load to the knee in 90° of flexion using a Telos arthrometer (Telos, Weiterstadt, Germany). Side-to-side differences in posterior translation of the tibia were recorded. A weight-bearing anterior-posterior radiograph was also taken for the knee in 30° of flexion.
Statistical analyses
For comparison of the T1ρ values between the PCL-deficient patients and the normal subjects, nonparametric comparisons were performed using a Mann–Whitney U test. p values of <0.05 were considered significant.
Results
All the PCL-deficient patients showed posterior laxity with an average of 9.8 mm (range 5.1–13.5 mm) in the stress radiographs. No patients showed osteoarthritic changes including joint space narrowing on weight-bearing anterior-posterior radiographs of the knees in 30° of flexion. These patients had no or mild symptoms during sports activities. The fat-suppression turbo spin echo T2-weighted images showed no severe degenerative changes in their cartilage including thinning, injury, or erosion (Fig. 2a, b). However, the T1ρ mapping MRI revealed an increase in T1ρ values in the articular cartilage of the femoral condyle and tibial plateau in both the medial and lateral compartments (Fig. 2c, d; Table 1). The differences in T1ρ values were significant in the tibial plateau in both the medial and lateral compartments (p < 0.05). The T1ρ values of the distal area of the femoral condyle (especially the superficial layer) and the posterior area of the lateral femoral condyle were also significantly higher in the patients. Relatively wide variations were also observed in the T1ρ values among patients. A very active soccer player (Tegner score = 9) showed a very high increase in T1ρ values in all areas, while relatively mild increases in T1ρ values were observed in the less active patients (i.e. Tegner score = 5) or the patients with a relatively recent injury. In contrast, although one normal volunteer was a very active athlete (Tegner score = 9), the T1ρ values were not high compared with others (Fig. 2e, f). Intraobserver reliability of the measurements was 0.89. Interobserver reliability of the measurements was 0.81.
Fat-suppression turbo spin echo T2-weighted imaging (FS-T2WI) of the medial compartment (a) and lateral compartment (b) in a case with PCL deficiency. c, d T1ρ maps of images corresponding to a and b, respectively. T1ρ maps of the medial compartment (e) and lateral compartment (f) in a normal control subject. The colour map indicates the T1ρ value
Discussion
The most important finding of this study was that degeneration of articular cartilage can occur in an asymptomatic PCL-deficient knee especially when the patient is a very active athlete and he/she has a significant instability of the knee. T1ρ MRI mapping enables us to evaluate the present integrity of cartilage matrix in vivo and may contribute towards research to predict the risk of osteoarthritis (OA).
Several MRI techniques have been developed to determine changes in the biochemical composition and macromolecular structure of hyaline cartilage. T1ρ mapping and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) techniques can be used to evaluate the proteoglycan content and distribution, while T2 mapping can be used to evaluate the extent of collagen fibre disorganisation by detecting the interaction between water and collagen molecules [4, 8]. Because T1ρ and T2 mappings require no injection of contrast agents, their potential for use in the detection of early degenerative changes of articular cartilage in OA has attracted attention. A comparative study of T1ρ and T2 mappings revealed that both techniques could detect cartilage degeneration in early OA using relaxation time, which is increased in affected subjects compared with that in normal control subjects. However, T1ρ has a larger range and higher effect size than T2, suggesting the superior sensitivity of T1ρ mapping in the detection of early cartilage degeneration [13, 21]. In fact, the loss of proteoglycans is considered to be an initiating event in early OA, while neither the content nor the type of collagen is altered in early OA [5]. The reliability of T1ρ mapping has been reported by showing the correlation between T1ρ values and the histological staining of proteoglycans in the corresponding area [23]. This is the first report to assess early cartilage degeneration in athletes with chronic PCL deficiency. Several studies investigated cartilage degeneration associated with ACL injury and ACL reconstruction [1, 9, 22]. Young et al. [25] reported a case of PCL injury, which was examined by dGEMRIC within 6 months of injury. This report focused on the effects of bone collision at the first injury or subsequent inflammation rather than on the effects on the cartilage of repetitive mechanical stress resulting from abnormal kinematics of the knee during sports activities.
The current study indicated that the T1ρ values in PCL-deficient patients increased up to 50 ms. Tsushima et al. [23] reported the T1ρ values and histology of articular cartilage in advanced OA patients who underwent total knee arthroplasty. They macroscopically rated the severity of cartilage degeneration on a 5-grade scale (from 0 to 4): Grade 0, normal–smooth surface; Grade 1, swelling and softening; Grade 2, superficial fibrillation; Grade 3, deep fibrillation—(coarse fissuring of the cartilage surface); and Grade 4, subchondral bone exposure. According to this study, a T1ρ value of 50 ms corresponds to Grade 2. There was an apparent loss of proteoglycan staining in the area that showed a T1ρ value of 50 ms. The T1ρ values of well-preserved areas (Grade 0) were rarely as high as 50 ms, even though these values were routinely obtained in severe OA patients who underwent arthroplasty.
A number of studies have reported the long-term clinical results of non-operative treatment for PCL injury [2, 10, 17, 19, 20]. Several studies reported relatively favourable long-term results. Shelbourne et al. [19] reported the radiographic results of 68 out of 133 consecutive patients treated non-surgically after an average follow-up of 5.4 years. Fifteen per cent of patients had degenerative arthritic changes in the involved knee only. Patel et al. [17] also reported that 17 % of 58 consecutive cases showed degenerative changes in the medial compartment at an average follow-up of 6.9 years. In both studies, the posterior laxity measured with a KT-1000 arthrometer was approximately 3–9 mm, and the grade of laxity was not correlated with either radiographic OA change or clinical scores such as the Lysholm and Noyes scores. In contrast, Boynton et al. [2] reported relatively poorer results in 38 untreated patients at an average follow-up of 13.4 years. Radiographic evidence of articular degeneration was seen in the medial tibiofemoral compartment in 53 %, in the lateral tibiofemoral compartment in 20 %, and in the patellofemoral compartment in 13 % of cases. As time from injury increased, articular degeneration seen on radiographs increased. In addition, the incidence of radiographic degeneration was higher in patients with greater instability than in those with lower instability. Keller et al. [10] also reviewed the records of 40 patients with an average follow-up period of 6 years and showed that the longer the interval between injury and evaluation, the greater the incidence of radiographic degeneration. As these studies suggest, the risk of articular degeneration in the PCL-deficient knee is controversial. This is probably because articular degeneration is influenced by many factors such as level and type of sports activity, degree of instability, body weight, muscle strength, time elapsed after the injury, and age. In this study, a very high T1ρ value was observed in patients with the greatest knee laxity and highest level of sports activity. Smaller increases in T1ρ values were seen in patients with less laxity and/or lower levels of sports activity even though the interval between the injury and evaluation was large. These results suggest that the influence on cartilage damage varies among the factors, and thus, some cases do not show any radiographic evidence of degeneration in long-term clinical studies.
Many clinical studies reported the tendency for radiographic degeneration to occur in the medial tibiofemoral compartment [2, 17, 19, 24]. Most previous clinical studies adopted the 45° flexion weight-bearing posterior-anterior radiograph. In the current study, T1ρ values increased in both the medial and lateral tibiofemoral compartments. Both medial and lateral tibial plateau showed significant increases in the T1ρ values. Squatting during sports activities can generate a shear stress on the articular cartilage around the posterior part of the femur, and the force applied to the tibia posteriorly in the squatting position might increase as knee flexion increases. Therefore, the finding of an effect on the posterior part of the femoral condyle in this study is reasonable and cannot be evaluated on plain radiographs. In fact, another advantage of an MRI study is that it allows a three-dimensional analysis. However, the patellofemoral joint, which is known as a predilection site of cartilage degeneration in PCL deficiency, was not examined in this study.
There were some limitations to this study. Since this was a cross-sectional study with small numbers of subjects, it could not be concluded that the cartilage degeneration detected by T1ρ MRI mapping would cause symptomatic OA in the near future. None of the cases showed an apparent radiographic OA finding even though the interval between injury and evaluation was long in two cases. It is unclear whether the findings observed in T1ρ MRI mapping had deteriorated over time since the injury. Even so, it could not be concluded that the MRI findings observed in this study were meaningless. Patients were enrolled because they did not show OA findings. As mentioned above, many factors influence the risk of OA. A longitudinal large-scale study could disclose significant risk factors for the development of OA. Nevertheless, since many clinical studies have proved that some patients actually develop OA, the detection of cartilage degeneration by T1ρ MRI mapping would help evaluate the present status of the injured knee. A bone scintigraph could be an alternative method to evaluate osteoarthritic change as it has been reported to be associated with the severity of OA [12]. The utilisation of single photon emission computed tomography would allow the three-dimensional localisation of cartilage damage. However, T1ρ MRI directly detects the degenerative changes in the cartilage, while bone scintigraphy detects the subchondral bone metabolisms; therefore, the latter might be less sensitive during the early stage of cartilage changes than T1ρ MRI.
The characteristics of patients are heterogeneous. The type and level of sports activity, age, level of joint laxity, and time elapsed after injury are all different. This makes it difficult to come to a definitive conclusion concerning risk factors. Nevertheless, all the patients showed similar increases in T1ρ values at the indicated area in this study. These results suggest that subclinical degeneration of the cartilage matrix is common in athletes with PCL deficiency.
Since this study did not include a group of patients who underwent PCL reconstruction, it is unclear whether surgical reconstruction would avert these cartilage damages.
Conclusion
This study suggests that T1ρ MRI mapping can detect unexpected cartilage degeneration in the well-functioning PCL-deficient knees of young athletes. One should be alert to the possibility of subclinical cartilage degeneration even when patients appear asymptomatic and have no degenerative findings on plain radiographs or conventional MRIs. This method is useful for the evaluation of lurking cartilage degeneration and the investigation of risk factors for the development of secondary OA.
References
Bolbos RI, Link TM, Ma CB, Majumdar S, Li X (2009) T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthritis Cartilage 17(1):12–18
Boynton MD, Tietjens BR (1996) Long-term followup of the untreated isolated posterior cruciate ligament-deficient knee. Am J Sports Med 24(3):306–310
Colvin AC, Meislin RJ (2009) Posterior cruciate ligament injuries in the athlete: diagnosis and treatment. Bull NYU Hosp Jt Dis 67(1):45–51
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Dijkgraaf LC, de Bont LG, Boering G, Liem RS (1995) The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. J Oral Maxillofac Surg 53(10):1182–1192
Fanelli GC, Beck JD, Edson CJ (2010) Current concepts review: the posterior cruciate ligament. J Knee Surg 23(2):61–72
Fowler PJ, Messieh SS (1987) Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med 15(6):553–557
Jazrawi LM, Alaia MJ, Chang G, Fitzgerald EF, Recht MP (2011) Advances in magnetic resonance imaging of articular cartilage. J Am Acad Orthop Surg 19(7):420–429
Hirose J, Nishioka H, Okamoto N, Oniki Y, Nakamura E, Yamashita Y, Usuku K, Mizuta H (2013) Articular cartilage lesions increase early cartilage degeneration in knees treated by anterior cruciate ligament reconstruction: T1rho mapping evaluation and 1-year follow-up. Am J Sports Med 41(10):2353–2361
Keller PM, Shelbourne KD, McCarroll JR, Rettig AC (1993) Nonoperatively treated isolated posterior cruciate ligament injuries. Am J Sports Med 21(1):132–136
Kim YM, Lee CA, Matava MJ (2011) Clinical results of arthroscopic single-bundle transtibial posterior cruciate ligament reconstruction: a systematic review. Am J Sports Med 39(2):425–434
Kraus VB, McDaniel G, Worrell TW, Feng S, Vail TP, Varju G, Coleman RE (2009) Association of bone scintigraphic abnormalities with knee malalignment and pain. Ann Rheum Dis 68(11):1673–1679
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil 15(7):789–797
Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majumdar S (2011) Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 29(3):324–334
Lopez-Vidriero E, Simon DA, Johnson DH (2010) Initial evaluation of posterior cruciate ligament injuries: history, physical examination, imaging studies, surgical and nonsurgical indications. Sports Med Arthrosc 18(4):230–237
Mariani PP, Adriani E, Santori N, Maresca G (1997) Arthroscopic posterior cruciate ligament reconstruction with bone-tendon-bone patellar graft. Knee Surg Sports Traumatol Arthrosc 5(4):239–244
Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT (2007) The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J 3(2):137–146
Sekiya JK, West RV, Ong BC, Irrgang JJ, Fu FH, Harner CD (2005) Clinical outcomes after isolated arthroscopic single-bundle posterior cruciate ligament reconstruction. Arthroscopy 21(9):1042–1050
Shelbourne KD, Davis TJ, Patel DV (1999) The natural history of acute, isolated, nonoperatively treated posterior cruciate ligament injuries. A prospective study. Am J Sports Med 27(3):276–283
Shelbourne KD, Muthukaruppan Y (2005) Subjective results of nonoperatively treated, acute, isolated posterior cruciate ligament injuries. Arthroscopy 21(4):457–461
Takayama Y, Hatakenaka M, Tsushima H, Okazaki K, Yoshiura T, Yonezawa M, Nishikawa K, Iwamoto Y, Honda H (2013) T1ρ is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: radiological-pathological correlation after total knee arthroplasty. Eur J Radiol 82(4):e192–e198
Theologis AA, Haughom B, Liang F, Zhang Y, Majumdar S, Link TM, Ma CB, Li X (2014) Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 22:298–307
Tsushima H, Okazaki K, Takayama Y, Hatakenaka M, Honda H, Izawa T, Nakashima Y, Yamada H, Iwamoto Y (2012) Evaluation of cartilage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int 32(9):2867–2875
Wu CH, Chen AC, Yuan LJ, Chang CH, Chan YS, Hsu KY, Wang CJ, Chen WJ (2007) Arthroscopic reconstruction of the posterior cruciate ligament by using a quadriceps tendon autograft: a minimum 5-year follow-up. Arthroscopy 23(4):420–427
Young AA, Stanwell P, Williams A, Rohrsheim JA, Parker DA, Giuffre B, Ellis AM (2005) Glycosaminoglycan content of knee cartilage following posterior cruciate ligament rupture demonstrated by delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC). A case report. J Bone Joint Surg Am 87(12):2763–2767
Conflict of interest
The authors declare that there was no conflict of interest regarding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okazaki, K., Takayama, Y., Osaki, K. et al. Subclinical cartilage degeneration in young athletes with posterior cruciate ligament injuries detected with T1ρ magnetic resonance imaging mapping. Knee Surg Sports Traumatol Arthrosc 23, 3094–3100 (2015). https://doi.org/10.1007/s00167-014-3469-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-014-3469-4