Abstract
Purpose
Early diagnosis of cartilage degeneration and longitudinal tracking of cartilage health including repair following surgical intervention would benefit from the ability to detect and monitor changes of the articular cartilage non-invasively and before gross morphological alterations appear.
Methods
Quantitative MR imaging has shown promising results with various imaging biomarkers such as T2 mapping, T1 rho and dGEMRIC demonstrating sensitivity in the detection of biochemical alterations within tissues of interest. However, acquiring accurate and clinically valuable quantitative data has proven challenging, and the reproducibility of the quantitative mapping technique and its values are essential. Although T2 mapping has been the focus in this discussion, all quantitative mapping techniques are subject to the same issues including variability in the imaging protocol, unloading and exercise, analysis, scanner and coil, calculation methods, and segmentation and registration concerns.
Results
The causes for variability between time points longitudinally in a patient, among patients, and among centres need to be understood further and the issues addressed.
Conclusions
The potential clinical applications of quantitative mapping are vast, but, before the clinical community can take full advantage of this tool, it must be automated, standardized, validated, and have proven reproducibility prior to its implementation into the standard clinical care routine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, non-surgical and surgical techniques exist with a goal to stabilize and improve cartilage health and based on clinical evaluation of pain symptoms and subjective outcomes scoring. It appears that these preventative and repair strategies experience some successes [52]. Arthroscopy is currently the gold standard for in vivo diagnosis of cartilage degeneration as it allows for the direct assessment of the cartilage surface integrity and the qualitative assessment and grading of cartilage stiffness [19]. However, subjecting a patient to a second arthroscopic procedure to directly evaluate cartilage surface integrity and stiffness is invasive with associated morbidity and avoided whenever possible. Currently, there are limited non-invasive imaging-based clinical tools available which are sensitive enough to assess the changes in cartilage associated with the earliest stages of osteoarthritis (OA). Radiographs are commonly used to evaluate decreased joint space associated with cartilage thinning, but this approach is limited to moderate to severe diffuse cartilage loss, which must occur before joint space narrowing will present radiographically [22]. Conventional magnetic resonance imaging (MRI) allows for macroscopic anatomic assessment of the cartilage but is less sensitive to the biochemical changes associated with early OA (i.e. proteoglycan depletion, changes in water content and collagen content and organization) [58]. As the prevention, delay and the potential reversal of cartilage damage have been the desired goals of OA treatment, the development of an appropriate method of assessing and tracking early OA is imperative.
Recently, quantitative MRI techniques such as T2, T2*, dGEMRIC (delayed gadolinium-enhanced MRI of cartilage), sodium imaging ((23)Na), chemical exchange saturation transfer (CEST), diffusion weighted imaging (DWI) and T1rho mapping have been shown to be sensitive to biochemical changes in cartilage [12]. Studies in the hip, knee, shoulder and ankle have demonstrated significant changes in quantitative mapping values associated with various stages of OA, surgical repair techniques and various pathologies [33, 39, 46]. Of these sequences, T2 mapping and dGEMRIC are currently the most widely used in the clinical setting and while many researchers have shown differences in dGEMRIC and T2 mapping values between healthy populations and those with OA, the specific values associated with healthy and damaged cartilage remain variable and not well understood [68]. The lack of understanding of factors that contribute to the variable values in the literature has hindered the inclusion of these techniques as part of standard clinical care. Potential sources of variation affecting T2 mapping results include scanner type and magnet strength, coil type, patient and control population, imaging protocol, T2 mapping sequence parameters, post-image processing, method of calculation and analysis/reporting of values. In order for quantitative MRI to be more clinically useful, it is important to first determine the factors affecting the reproducibility in efforts to create standardized and universally accepted protocols.
The purpose of this paper, therefore, is to review the current T2 mapping literature and highlight and discuss potential reasons for the lack of reproducibility among centres. By identifying these factors, we may be able to reach a consensus across centres to reduce variability and increase reproducibility. By doing so, we may better be able successfully to implement quantitative techniques, such as T2 mapping, into the standard clinical workflow, something that has eluded the orthopaedic community. The focus of this review will be on T2 mapping since it is currently the most widely used non-invasive cartilage mapping sequence. However, the sources of variability, in most cases, can be extrapolated to other quantitative MR imaging techniques.
Materials and methods
Electronic searches in PubMed (http://www.ncbi.nim.nih.gov/pubmed) were performed to identify relevant studies for this review. No starting date was entered for the electronic searches in order to obtain the entire span of literature available in PubMed. Search terms included “Asymptomatic”, “Osteoarthritis”, “Quantitative MRI”, “Magnetic Resonance Imaging” and “Cartilage T2”. The reference lists of relevant manuscripts were also screened during this process.
T2 mapping technique
T2 relaxation time/value refers to the spin–spin relaxation time which relates to the decay of magnetization in the transverse plane following radio frequency (RF) pulse excitation. T2 mapping has demonstrated sensitivity to water content and collagen content, structure and organization in cartilage [11]. Damage to the collagen-proteoglycan (PG) matrix and the increase of water content in degenerating cartilage has been shown to increase T2 values [35], and elevated values have been observed in patients with OA [15]. T2 mapping has proven to be a useful predictor of cartilage degeneration and repair tissue following surgical intervention in the knee [29], hip [6] and ankle joint [14]. Compared with other quantitative MRI techniques (shown in Table 1), T2 mapping has the advantage that it can be performed non-invasively without the injection of contrast agents and that T2 mapping sequence and post-processing software are available in many commercial MRI scanner systems. For any clinical tool, it is essential to appreciate and document reproducibility including the test–retest reliability. Such has been evaluated in the knee articular cartilage with Glaser et al. [20] reporting that precision errors are smaller in investigations of the patella than in the femoral or tibial cartilage. Additionally, Mosher et al. [44] found greater reproducibility in repeated T2 measurements in the patella compared to the femoral or tibial measurements. The reasons for variations in cartilage reproducibility should be better understood and such assessments should be extrapolated to other joints and tissues.
Study population
The chief clinical application for T2 mapping involves the detection of early cartilage damage prior to the onset of symptoms and prior to detection using conventional screening techniques; thus, the inclusion criteria for these subjects and patients are critical for the evaluation of the efficacy of the technique. It is necessary to understand normative T2 values in the joint in order to comprehend values associated with damage. Additionally, using normative areas of cartilage as a reference or a control is common within the literature for its application in longitudinal tracking and normalizing, and thus, the inclusion of such data should be better understood and standardized.
While it is currently not possible to be certain that no early cartilage degeneration exists in the subject without histology, inclusion as an asymptomatic individual has been performed using a variety of parameters ranging in intensity from acquiring a subjective history of the individuals’ joint to performing a clinical examination of the joint, lending itself to the variability observed in the literature. For the knee, subject reported questionnaires such as the Western Ontario and McMaster Universities Arthritis Index (WOMAC), the Lysholm Knee questionnaire and the Tegner Activity Scale may be useful in quantifying joint health when used in conjunction with objective semi-quantitative scores such as the Kelgren Lawrence radiographic score and morphologic semi-quantitative MRI scores [such as the following with their known acronyms: Whole-Organ Magnetic Resonance Imaging Score (WORMS), MRI Osteoarthritis Knee Score (MOAKS), Boston Leeds Osteoarthritis Knee Score (BLOKS) and Knee Osteoarthritis Scoring System (KOSS)]. Arthroscopically, macroscopically normal cartilage can be determined via scoring systems such as the Outerbridge Classification and the International Cartilage Repair Society (ICRS) arthroscopic grading system score, but the process is invasive and not practical for prospective healthy cohort studies. Highlighting the difficulty of classifying a subject as healthy, Joseph et al. [28] evaluated the difference in subjects at risk for OA and healthy cohorts and observed that in the patella and lateral tibia, there was a higher prevalence of cartilage defects in the healthy control group compared to the subjects at risk for OA. Apprich et al. [1] evaluated 43 patients via morphological MRI, 11 of who exhibited no cartilage damage or defects on the medial condyle. These 11 patients were given a grade 0 ICRS score and were deemed morphologically normal, but, upon analysis three of these “normal” patients exhibited T2 means higher than that for the patients with an ICRS score of grade I.
Apart from evaluating asymptomatic subjects, the use of an internal control (an area of morphologically “normal-appearing” cartilage) has been utilized in surgical repair patients serving as a reference to track repair tissue changes in T2 values within the patient. This method may be problematic as the steps to identify an internal control vary in the literature. Mamisch et al. [40] placed the internal control 0.5 cm from the transplant area in all patients, Welsch et al. [59] specified that the internal control had to be at least 2.0 cm away from the cartilage repair tissue and in a different paper, and Welsch et al. [60] utilized an internal control in the same anatomic region of interest (ROI) as the area of the cartilage repair tissue. Apprich et al. [2] observed that in patients with a focal cartilage defect, adjacent areas of morphologically appearing cartilage showed consistently higher mean T2 values than control volunteers without a defect. This finding may indicate the impact of focal defects on the surrounding cartilage and the joint as a whole. As discussed previously, “normal” cartilage definition, one without signs of early cartilage degeneration on standard morphological images, may not be sufficient [11]. The definition of the internal control cartilage should be standardized and, even if standardization is achieved, internal control may not be feasible or reproducible for longitudinal studies of an individual patient, as the “control” cartilage may change between time points.
Imaging protocol
Several scanning sequences have been developed for the purpose of determining T2 values of tissue, but the use of various T2 acquisition techniques may contribute to the variability observed in the literature. Such T2 acquisition sequences include the spin-echo (SE) and fast SE (FSE) techniques as well as several pulse sequences such as multi-echo SE (MESE) sequences and spiral sequences. 3D double-echo steady-state imaging (DESS), a gradient echo based sequence, has recently gained interest as it generates two signal echoes characterized by different contrast behaviour from which the underlying T2 value can be calculated [61]. The DESS approach allows for the morphological and quantitative MRI to be acquired in one scan, but less accuracy in the T2 value calculation has been predicted as the calculation is accomplished by using only two data points unlike most T2 acquisitions that fit based on upwards of six data points. Welsch et al. [64] reported high correlation between DESS derived T2 values and T2 mapping values, but values from the T2 mapping MESE sequence had a higher sensitivity for detecting damaged cartilage. Furthermore, Pai et al. [47] investigated the differences in T2 values of healthy patellar cartilage using SE, FSE, MESE and a spiral sequence at 3.0T and found the mean values to range between 27.0 and 41.0 ms. Considering that the differences in T2 values between healthy and damage cartilage have been shown to be in the range of 2.0–12.3 ms [35, 40, 59, 68], the findings by Pai et al. [47] are sobering as they highlight the variation that can result in using different T2 acquisition scans. It may be considered that the differences in T2 mapping values found in the literature will continue as newer acquisition scanning techniques (MESE and DESS) are being adopted while the current popular techniques such as the SE and FSE sequences are still being used and reported.
Field strengths and coils
The T2 mapping literature has utilized varying scanners from different manufacturers using magnet strengths between 1.5T and 7.0T for human articular cartilage in vivo. MRI at 3.0T has recently become perhaps the benchmark for clinical routine and research as it has twice the signal-to-noise ratio (SNR) and higher resolution than that of the standard 1.5T. More recently, MRI field strength of 7.0T has been used with preliminary success in the research setting as these systems are still investigational and not approved or widely available for standard clinical care. Imaging at 7.0T has been gaining interest as it allows for increased morphological detail and has functional imaging capacity coupled with faster acquisition times [57]. Signal-to-noise ratio is directly related to magnetic strength, and T2 mapping values have been shown to be highly affected by noise [30]. When comparing SNR at 3.0T and 7.0T, it was observed that SNR varied greatly between different tissues with some of the largest SNR increases found in articular cartilage and no SNR increases observed in the trabecular bone [45]. However, the increased field strength of the 7.0T may come at the cost of increased artefact (susceptibility and chemical shift) and acoustic noise [9].
The evolution of high-field MRI has been further aided by advancement of coil technology such as the introduction of the multichannel coil and the phased array coil. The type and condition of the coils used not only affect image quality but can affect the T2 calculation. Chang et al. [9] observed average differences in cartilage T2 reaching −6.8 ± 6.7 ms at the tibia and −8.2 ± 9.7 ms at the femur when comparing values from a 28-channel coil with a quadrature coil, consequently advising that coils should be switched with caution during quantitative MR studies.
Loading status
T2 values have demonstrated sensitivity to the patient’s activity level prior to scanning as it can relate to the time the joint is unloaded. These changes may be due to an influx/efflux of water or by a change in collagen fibre orientation within the loaded joint [43]. To reduce the effects of loading, T2 mapping acquisition is commonly performed following 30–45 min of unloading inside the scanner [40, 62]. Mamisch et al. [40] compared T2 mapping values of healthy and repair cartilage during early unloading and late unloading and observed significant differences between patients only in the late unloading condition. Furthermore, it was observed that after subjects rested for 40 min in the scanner, T2 values of the medial femoral condyle increased significantly with increasing ICRS cartilage defect grade while the unloaded state did not show a significant increase in the same measure [1]. T2 values in the knee cartilage between early unloading and late unloading conditions have shown to have an increase in T2 values of up to 9.1 ms with the largest change found in the superficial layer of cartilage [3]. Liess et al. [37] observed an increase in cartilage thickness and an increase in T2 values of the patellar cartilage 45 min post-exercise, suggesting the activity level before the scan should also be regulated. The unloading time and protocols prior to scanning/mapping in these reports vary, and the optimal procedure has not been determined.
T2 calculation
It has been demonstrated that different methods of T2 calculation cause significant differences in T2 values [32, 51]. Pixel-wise monoexponential non-negative least-squares fit analysis is currently the most common method of calculating T2 values. Commercialized software packages such as MapIt (Siemens Medical Solutions, Erlangen, Germany), OsiriX (Imaging Software, Geneva, Switzerland), MRIMapper (Beth Israel Deaconess and MIT 2006) and MPCURVEFIT (NASA/GSFC Code 662, Greenbelt, MD) have been used to generate T2 maps using a monoexponential fit algorithm. While each package uses the same general algorithm, it is unclear if there exists small differences in the algorithms that could affect the T2 calculation substantially therefore prospective analysis of these potential differences should be performed. Custom in-house software has been reported using similar monoexponential fit algorithms with some additions including discarding early or late echoes and the use of post-processing techniques such as noise correction [15]. While these additions may lead to more accurate T2 values, the specific algorithms are often not reported in custom in-house software leading to difficulty in interpreting and reproducing the results.
Koff et al. [32] compared T2 values of 10 healthy knees using a linear, a weighted, and a nonlinear fitting algorithm for a monoexponential decay equation and reported that all three methods resulted in significantly different T2 values. Although the average difference of T2 values between methods was around 5.0 ms, the nonlinear algorithm consistently exhibited the best fit to the acquired data [32]. Raya et al. [51] examined the accuracy and precision of the traditional linear least-squares regression fit and two new noise-corrected nonlinear fits: a noise-corrected exponential fit and a noise-corrected squared signal intensity exponential fit. Using a phantom and varying levels of artificially induced noise, it was found that the traditional fit methods led to lower accuracy at lower T2 values compared to two noise-corrected fit methods with overestimations up to 500 % (up to 60.0 ms over the known phantom value). Intuitively, noise reduces the accuracy of T2 values, and thus, noise correction should be further investigated.
Data exclusion is commonly reported within the literature including exclusion of T2 values with a poor fit, outliers, selected slices and echoes [15, 30, 32, 35]. T2 values above 150.0–200.0 ms have been excluded from analysis in order to omit synovial fluid and partial volume effect that may have been included within the region of interest [35]. Exclusion of full slices due to partial volume averaging, motion artefact, arterial pulse artefact and chemical shift artefact has been reported [32]. The magic angle effect, which occurs when collagen fibres are oriented at 55° relative to the applied static magnetic field (B 0), has been used to explain the aetiology of focally increased signal observations on short TE images of cartilage, especially curved cartilage surfaces such as the cartilage of the femoral condyle and the acetabular cartilage [42]. Because increases in T2 values have been associated with cartilage damage, this area of increased signal is believed to introduce a source of quantification error. Mosher et al. [42] observed that over a normalized distance, T2 profiles oriented at 50–60° to B 0 are statistically significantly longer (higher T2 values) than the T2 profiles oriented at 0–10°. The presence of stimulated echoes can introduce difficulty when analysing multiecho decay data or multiecho T2 data as these stimulated echoes lead to corruption of the exponential decay of the primary echoes [49]. This is thought to lead to inaccurate T2 values from invalid distributions. The first echo has been excluded from calculations due to the stimulated echo effect [38], which was well described by Hennig et al. [25]. This is a debated point as improved accuracy of T2 values has been demonstrated when the first echo is dropped [38] yet others have concluded that the lower SNR of the later echo times would cause more variation in the T2 values than leaving in the first echo [67] as the first echo is considered the only “correct” spin echo.
Segmentation methods
Currently, analysis of cartilage T2 values requires manual or semi-automatic segmentation (based on edge detection) which is not only time intensive but could potentially add variability. Segmentation accuracy is highly dependent on the sequence [18] with delineation between cartilage and bone most accurately performed on gradient echo sequences [17]. However, gradient echo sequences have smaller contrast dynamic range compared to sequences, such as turbo-spin-echo sequences, which could reduce the sensitivity to cartilage degeneration. Currently, there is no universally accepted sequence for cartilage segmentation.
Semi-automated segmentation techniques are performed on morphological images and require the trained user/rater to manually correct for areas of synovial fluid, chemical shift artefact and regions of partial voluming [56]. The morphologic images are then registered to the T2 mapping images, and the corresponding T2 values are extracted. The registration of images may also cause variation in T2 values depending on the registration algorithm. Manual segmentation techniques are performed either on morphologic images or directly on the T2 mapping images. Again, raters must be trained to perform manual segmentations to decrease variability and ensure accuracy. As previous work has indicated, the cartilage/bone interface can be difficult to delineate with manual segmentation due to factors such as partial volume averaging of cartilage voxels with bone voxels [55]. Since T2 mapping sequences are not fat suppressed, a chemical shift artefact is often present near the bone interface and can affect segmentation as well as calculated T2 values. Current Food and Drug Administration (FDA) approved T2 mapping software packages require manual segmentation of each slice which in turn is time consuming and not feasible in the clinical routine. Automated cartilage segmentation is needed for analysis of the full cartilage volume in the clinical environment in order to standardize the segmentations and reduce the post-processing time.
Region of interest (ROI)
The subregions/ROIs used for analysis following cartilage repair procedures are typically determined by evaluating the morphological MR image and recalling surgical reports. Due to the high prevalence of OA in the weight-bearing femoral/tibial joint, this is a common area for subregion analysis following surgical intervention in the knee, with two to three consecutive slices used for analysis [15]. Nonetheless, classification and detection of the weight-bearing region vary across studies. A zonal thickness of at least two pixels in the subregion has been set as a minimum requirement for analysis [1, 59]. Further studies are needed to validate the reproducibility of various methods of locating the cartilage repair sites and the effect on the T2 mapping values.
For additional analysis, a few institutions have presented further separation into two or three zones representing the deep, middle and superficial layers as they are believed to correspond with the anatomical structure of cartilage [65]. Apprich et al. [1] observed that the average T2 values for the deep, superficial and global cartilage layers significantly increase with severity of cartilage defect grade. T2 zonal variations have also been used to characterize cartilage repair tissue such as by Welsch et al. [59] who observed zonal increases from deep to superficial cartilage following MACT but not following MFX. The differences in T2 values between superficial and deep zones are likely due to the zonal variation of cartilage structure and composition within normal hyaline cartilage [41]. Deep zones of normal hyaline cartilage are marked by collagen fibres that run perpendicular towards the cortical surface, while the more superficial zones nearer the articular surface the collagen fibres have a more random and then tangential orientation that causes a dissimilar mobility of water protons in the partly anisotropic part of the cartilage tissue. The number of subregions used to describe normative or baseline cartilage in healthy cohorts has varied greatly as appreciated in Table 2. The most common approach in the knee has been subdividing the patella, medial and lateral femur, and the medial and lateral tibia [26, 28]. Other researchers have further divided these cartilage regions into anterior and posterior or lateral and medial subregions [23]. However, no standardized set of subregions has been adopted.
Results
The method of analysing and reporting T2 values results in the literature differs among institutions making comparison difficult if not entirely unfeasible, and as such, these techniques are visited. The mean and standard deviations of T2 values within subregions are most commonly reported; however, alternate methods have been adopted. Normalization of T2 mapping values has been proposed as a way to minimize variability between subjects. It has been suggested that normalizing T2 values to healthy appearing cartilage could aid in the assessment of the tissue’s heterogeneity and the tracking of OA in a clinical setting. Dunn et al. evaluated the T2 values in femoral and tibial cartilage in patients with varying degrees of OA and compared them with healthy cohorts based on the calculation of the T2 Z-score. The group observed higher Z-scores in the weight-bearing region both in patients with mild and severe OA when compared with healthy cohorts [15]. The Z-score is evaluated per pixel and is generated using the mean and STD of T2 values from each subregion by the following equation: Z = (voxel i−mean healthy)/STD healthy. Recent work by Lattanzi et al. [34] stressed the probable importance of standardizing quantitative MRI results on a patient-specific basis by using the Z-score for evaluating patients with femoroacetabular impingement (FAI). Although the work evaluated the Z-score using T1 values, the baseline or healthy T1 values varied significantly among patients. Therefore, the standardization of the T1 values to each patient was able to increase sensitivity in detecting abnormal cartilage in FAI [34].
The T2 index has been utilized to compare repair tissue to the normal internal control tissue [13, 63]. The T2 index aims to express the repair tissue relative to the normal tissue using the equation: T2 Index = (repair T2 (mean)/normal T2 (mean)) × 100 [13]. Significant differences have been found between scaffold techniques using the T2 index [63], and the T2 index has also been shown to correlate with the International Knee Documentation Committee (IKDC) subjective score following MFX [13].
Some researchers have presented more complex analysis called texture analysis, which involves comparing neighbouring pixels [24]. Grey-level co-occurrence matrix (GLCM) texture analysis determines the frequency that neighbouring grey-level values occur in an image. Recently, the literature has demonstrated the potential of this technique in the field of biochemical cartilage MRI using T2 maps of the knee joint and has been able to detect early degenerative changes in the cartilage matrix reflected by the spatial distribution of T2 values [28]. Parameters for GLMC texture analysis include contrast, variance, homogeneity, entropy and are typically calculated in each cartilage subregion. GLMC entropy of cartilage T2 has been shown to be elevated in patients with OA compared to healthy controls [7, 36] as well as more heterogeneous [28] and has higher variance [8], and higher contrast [8, 28]. Carballido-Gamio et al. [7] determined that texture analysis used in conjunction with mean T2 values could improve classification between subjects with and without OA.
Discussion
The most important finding of the present review was identification of the factors that impact the observed variability of T2 values in the current literature. Early diagnosis of cartilage degeneration and longitudinal tracking of cartilage health response to non-operative treatment as well as repair following surgical intervention would require the ability to non-invasively detect and monitor changes in the cartilage integrity prior to gross morphological changes. Factors that influence normal cartilage variation should be identified to improve our understanding of cartilage disease such as OA. Quantitative MR imaging has demonstrated promising results with various biomarkers, but acquiring accurate and clinically valuable quantitative data has proven challenging and variable.
Automated segmentation and registration of the full cartilage volume is needed for quantitative analysis of the mapping results and for tracking patient progress over time. Current FDA approved T2 mapping software packages require manual segmentation of each slice, which is not clinically practical and requires the MRI technologist to select the slices to segment. Optimally, cartilage should also be automatically divided into clinically relevant subregions using reproducible landmarks to allow for correlation with surgical findings and longitudinal tracking of cartilage health. Cartilage should be evaluated in subregions that are clinically relevant and reproducible as variations in the composition and structure of healthy cartilage, such as in the knee, have proven inconsistent and may lead to inhomogeneous distribution of cartilage T2 values [16]. Some institutes have chosen to evaluate only the weight-bearing regions of the cartilage, but this may not be as useful as degeneration can occur throughout the whole joint, highlighting the need for subregions that take this into consideration [54].
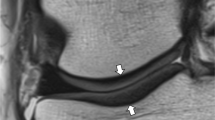
If successfully implemented into clinical routine, cartilage mapping has many potential uses. Clinically meaningful colour scales could be applied to cartilage mapping overlays for qualitative assessment (shown in Fig. 1), and quantitative data could be analysed to help in clinical decision making and outcome tracking. Quantitative mapping could be utilized in treatment planning and tracking a patient’s progress over time. The current biochemical state of the cartilage may also be related to clinical outcome; however, this has yet to be determined and few studies with histological correlation have been performed in vivo in the human. In addition, the health of the cartilage may help determine whether a conservative approach such as physical therapy can be attempted or if surgical intervention is needed. It may be theorized, however, as we currently do not fully understand the cartilage biochemical alterations that occur in each of these scenarios (i.e. osteoarthritic cartilage, early OA, damaged cartilage, post-operative, non-operative intervention, and pharmaceutical and therapeutic intervention), that each may have a different effect on the T2 value and that the research community should strive for understanding of this. It may also be reasonable, taking into account the parameters that may introduce variability and affect reproducibility, that a specific method of analysis (i.e. determination of internal healthy control cartilage; subregion/ROI analysis) may be appropriate for different situations (e.g. follow-up evaluation after cartilage repair surgery, monitoring cartilage following an injury for development of OA etc.) as determined by a surgeon or researcher. This notion may facilitate understanding of individual circumstances and reasons to optimize reproducibility.
There are many implications for the future inclusion of quantitative MRI techniques such as T2 mapping into the day-to-day standard clinical workflow. If the technique becomes reproducible and time effective, it may be possible for quantitative mapping to be used to monitor the effect that surgical or non-surgical treatment of other joint tissues has on the health of cartilage with near immediate feedback. For example, it has been shown that removal of the meniscus causes early progression to OA [4]. Furthermore, non-anatomic joint reconstruction could lead to abnormal loading within the joint and early cartilage degeneration [50]. Surgical approaches such as hip FAI surgery are thought to stabilize and even reverse the progression of cartilage damage, but this has not been able to be evaluated in a non-invasive manner. Physical therapy rehabilitation techniques such as muscle strengthening and bracing are thought to reduce pressure within joint compartments and possibly affect cartilage health [10]. Response of cartilage health to intervention strategies such as platelet-rich plasma (PRP) injections, stem cell injections or other pharmaceutical interventions could also be investigated with the use of quantitative MRI mapping. Currently, treatment response to PRP has been monitored using repeat morphological MRI, which may assess gross macroscopic chondral damage but may not be as sensitive to earlier degeneration and cartilage health [5].
Cartilage T2 mapping has a number of limitations which is reflected in the lack of implementation into the current clinical workflow as well as outlined in this review. Large multicenter trials paying particular attention to reproducible methodology should continue to be implemented. Understanding how these values react at different time points, geographical locations and various environmental circumstances would be of great importance in the literature. The Osteoarthritis Initiative (OAI) has made considerable advances in this direction with standardized calibration procedures and acquisition methods, which are compulsory processes in order for comparisons and longitudinal measurements to be made [53].
T2 mapping analysis is currently limited by its time cost. To reduce this time cost and further decrease error (i.e. human error) to be practical and reproducible in the normal clinical workflow, automated segmentation and registration routines should be implemented. If implemented, future studies would need to evaluate these automated routines in asymptomatic individual and clinical patients, at various time points longitudinally. This information will be vital to the literature to evaluate any increases in reproducibility when implementing a fully automated approach.
Conclusion
Quantitative MRI techniques, such as T2 mapping, may provide the basis for diagnosis and follow-up evaluation of cartilage injury and response to cartilage treatment and repair and may provide valuable information regarding the composition of the tissue and the state of the cartilage repair tissue. Initial results have been promising and as the field continues to evolve, reproducibility of the quantitative mapping technique and its values are essential. The causes for variability between time points and among centres need to be understood and the issues addressed. An imaging protocol for cartilage health quantification should be agreed upon within the clinical community. The protocol must fit within the routine clinical imaging time and include the images needed for cartilage quantification without sacrificing clinical diagnostic ability for the entire joint. Once a set of standardized protocols are developed and proposed, subsequent studies are needed to determine specific values or distribution of values, which are associated with healthy and damaged cartilage. The potential clinical applications of T2 mapping are vast but before the clinical community can take full advantage of this tool, it must be reproducible, automated, standardized and validated prior to its implementation into standard clinical care routine.
References
Apprich S, Welsch GH, Mamisch TC, Szomolanyi P, Mayerhoefer M, Pinker K, Trattnig S (2010) Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthr Cartil 18(9):1211–1217
Apprich S, Mamisch TC, Welsch GH, Stelzeneder D, Albers C, Totzke U, Trattnig S (2011) Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81(4):e438–e443
Apprich S, Mamisch TC, Welsch GH, Stelzeneder D, Albers C, Totzke U, Trattnig S (2012) Quantitative T2 mapping of the patella at 3.0T is sensitive to early cartilage degeneration, but also to loading of the knee. Eur J Radiol 81(4):e438–e443
Bedi A, Kelly N, Baad M, Fox AJ, Ma Y, Warren RF, Maher SA (2012) Dynamic contact mechanics of radial tears of the lateral meniscus: implications for treatment. Arthroscopy 28(3):372–381
Campbell K, Boykin R, Wijdicks C, Erik Giphart J, LaPrade R, Philippon M (2012) Treatment of a hip capsular injury in a professional soccer player with platelet-rich plasma and bone marrow aspirate concentrate therapy. Knee Surg Sports Traumatol Arthrosc 21(7):1684–1688
Carballido-Gamio J, Link TM, Li X, Han ET, Krug R, Ries MD, Majumdar S (2008) Feasibility and reproducibility of relaxometry, morphometric, and geometrical measurements of the hip joint with magnetic resonance imaging at 3T. J Magn Reson Imaging 28(1):227–235
Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM (2009) Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 36(9):4059–4067
Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S (2011) Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med 65(4):1184–1194
Chang G, Wiggins GC, Xia D, Lattanzi R, Madelin G, Raya JG, Finnerty M, Fujita H, Recht MP, Regatte RR (2011) Comparison of a 28-channel receive array coil and quadrature volume coil for morphologic imaging and T2 mapping of knee cartilage at 7T. J Magn Reson Imaging 35(2):441–448
Chew KT, Lew HL, Date E, Fredericson M (2007) Current evidence and clinical applications of therapeutic knee braces. Am J Phys Med Rehabil 86(8):678–686
Choi JA, Gold GE (2011) MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am 19(2):249–282
Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, Baum T, Mosher TJ, Carrino JA, Guermazi A (2011) Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 31(1):37–61
Domayer SE, Kutscha-Lissberg F, Welsch G, Dorotka R, Nehrer S, Gabler C, Mamisch TC, Trattnig S (2008) T2 mapping in the knee after microfracture at 3.0 T: correlation of global T2 values and clinical outcome—preliminary results. Osteoarthritis Cartilage 16(8):903–908
Domayer SE, Apprich S, Stelzeneder D, Hirschfeld C, Sokolowski M, Kronnerwetter C, Chiari C, Windhager R, Trattnig S (2012) Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthr Cartil 20(8):829–836
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232(2):592–598
Eckstein F, Winzheimer M, Hohe J, Englmeier KH, Reiser M (2001) Interindividual variability and correlation among morphological parameters of knee joint cartilage plates: analysis with three-dimensional MR imaging. Osteoarthritis Cartilage 9(2):101–111
Friedrich KM, Reiter G, Kaiser B, Mayerhofer M, Deimling M, Jellus V, Horger W, Trattnig S, Schweitzer M, Salomonowitz E (2011) High-resolution cartilage imaging of the knee at 3T: basic evaluation of modern isotropic 3D MR-sequences. Eur J Radiol 78(3):398–405
Fripp J, Crozier S, Warfield SK, Ourselin S (2010) Automatic segmentation and quantitative analysis of the articular cartilages from magnetic resonance images of the knee. Med Imaging IEEE Trans 29(1):55–64
Galea A, Giuffre B, Dimmick S, Coolican MRJ, Parker DA (2009) The accuracy of magnetic resonance imaging scanning and its influence on management decisions in knee surgery. Arthroscopy 25(5):473–480
Glaser C, Mendlik T, Dinges J, Weber J, Stahl R, Trumm C, Reiser M (2006) Global and regional reproducibility of T2 relaxation time measurements in human patellar cartilage. Magn Reson Med 56(3):527–534
Goto H, Iwama Y, Fujii M, Aoyama N, Kubo S, Kuroda R, Ohno Y, Sugimura K (2012) A preliminary study of the T1rho values of normal knee cartilage using 3T-MRI. Eur J Radiol 81(7):e796–803
Guermazi A, Roemer F, Burstein D, Hayashi D (2011) Why radiography should no longer be considered a surrogate outcome measure for longitudinal assessment of cartilage in knee osteoarthritis. Arthritis Res Ther 13(6):247
Hannila I, Susanna Räinä S, Tervonen O, Ojala R, Nieminen MT (2009) Topographical variation of T2 relaxation time in the young adult knee cartilage at 1.5T. Osteoarthr Cartil 17(12):1570–1575
Haralick RM, Shanmugam K, Dinstein I (1973) Textural features for image classification. IEEE Trans Biomed Eng SMC 3(6):610–621
Hennig J (1991) Echoes—how to generate, recognize, use or avoid them in MR-imaging sequences. Concepts Magn Reson 3:125–142
Hovis KK, Stehling C, Souza RB, Haughom BD, Baum T, Nevitt M, McCulloch C, Lynch JA, Link TM (2011) Physical activity is associated with magnetic resonance imaging–based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis Rheum 63(8):2248–2256
Hovis JJ, Alizai H, Tham SC, Souza RB, Nevitt MC, McCulloch CE, Link TM (2012) Non-traumatic anterior cruciate ligament abnormalities and their relationship to OA using morphological grading and cartilage T2 relaxation times data from the Osteoarthritis Initiative (OAI). Skelet Radiol 41(11):1435–1443
Joseph G, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, Lynch J, McCulloch C, Majumdar S, Link T (2011) Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls—data from the osteoarthritis initiative. Arthritis Res Ther 13(5):R153
Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, Lynch JA, Nevitt MC, McCulloch CE, Majumdar S, Link TM (2012) Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years—data from the Osteoarthritis Initiative. Osteoarthr Cartil 20(7):727–735
Juras V, Zbyn S, Szomolanyi P, Trattnig S (2010) Regression error estimation significantly improves the region-of-interest statistics of noisy MR images. Med Phys 37(6):2813–2821
Kai B, Mann SA, King C, Forster BB (2011) Integrity of articular cartilage on T2 mapping association with meniscal signal change. Eur J Radiol 79(3):421–427
Koff MF, Amrami KK, Felmlee JP, Kaufman KR (2008) Bias of cartilage T2 values related to method of calculation. Magn Reson Imaging 26(9):1236–1243
Krause FG, Klammer G, Benneker LM, Werlen S, Mamisch TC, Weber M (2010) Biochemical T2* MR quantification of ankle arthrosis in pes cavovarus. J Orthop Res 28(12):1562–1568
Lattanzi R, Petchprapa C, Glaser C, Dunham K, Mikheev AV, Krigel A, Mamisch TC, Kim YJ, Rusinek H, Recht M (2012) A new method to analyze dGEMRIC measurements in femoroacetabular impingement: preliminary validation against arthroscopic findings. Osteoarthr Cartil 20(10):1127–1133
Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, Majumdar S (2007) In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthr Cartil 15(7):789–797
Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, Majumdar S (2009) Spatial distribution and relationship of T1ρ and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 61(6):1310–1318
Liess C, Lüsse S, Karger N, Heller M, Glüer CC (2002) Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthr Cartil 10(12):907–913
Maier CF, Tan SG, Hariharan H, Potter HG (2003) T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging 17(3):358–364
Maizlin ZV, Clement JJ, Patola WB, Fenton DM, Gillies JH, Vos PM, Jacobson JA (2009) T2 mapping of articular cartilage of glenohumeral joint with routine MRI correlation—initial experience. HSS J 5(1):61–66
Mamisch TC, Trattnig S, Quirbach S, Marlovits S, White LM, Welsch GH (2010) Quantitative T2 mapping of knee cartilage: differentiation of healthy control cartilage and cartilage repair tissue in the knee with unloading—initial results1. Radiology 254(3):818–826
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8(4):355–368
Mosher TJ, Smith H, Dardzinski BJ, Schmithorst VJ, Smith MB (2001) MR imaging and T2 mapping of femoral cartilage: in vivo determination of the magic angle effect. AJR Am J Roentgenol 177(3):665–669
Mosher TJ, Liu Y, Torok CM (2010) Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis Cartilage 18(3):358–364
Mosher TJ, Zhang Z, Reddy R, Boudhar S, Milestone BN, Morrison WB, Kwoh CK, Eckstein F, Witschey WR, Borthakur A (2011) Knee articular cartilage damage in osteoarthritis: analysis of MR image biomarker reproducibility in ACRIN-PA 4001 multicenter trial. Radiology 258(3):832–842
Nordmeyer-Massner JA, Wyss M, Andreisek G, Pruessmann KP, Hodler J (2011) In vitro and in vivo comparison of wrist MR imaging at 3.0 and 7.0 tesla using a gradient echo sequence and identical eight-channel coil array designs. J Magn Reson Imaging 33(3):661–667
Oneto J, Ellermann J, LaPrade R (2010) Longitudinal evaluation of cartilage repair tissue after microfracture using T2-mapping: a case report with arthroscopic and MRI correlation. Knee Surg Sports Traumatol Arthrosc 18(11):1545–1550
Pai A, Li X, Majumdar S (2008) A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging 26(9):1215–1220
Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, Link TM (2011) Knee cartilage T2 characteristics and evolution in relation to morphologic abnormalities detected at 3T MR imaging: a longitudinal study of the normal control cohort from the Osteoarthritis Initiative. Radiol 261(2):507–515
Prasloski T, Mädler B, Xiang Q-S, MacKay A, Jones C (2012) Applications of stimulated echo correction to multicomponent T2 analysis. Magn Reson Imaging 67(6):1803–1814
Prisk VR, Imhauser CW, O’Loughlin PF, Kennedy JG (2010) Lateral ligament repair and reconstruction restore neither contact mechanics of the ankle joint nor motion patterns of the hindfoot. J Bone Joint Surg Am 92(14):2375–2386
Raya JG, Dietrich O, Horng A, Weber J, Reiser MF, Glaser C (2010) T2 measurement in articular cartilage: impact of the fitting method on accuracy and precision at low SNR. Magn Reson Imaging 63(1):181–193
Rodriguez-Merchan EC (2013) Regeneration of articular cartilage of the knee. Rheumatol Int 33(4):837–845
Schneider E, Nessaiver M (2013) The Osteoarthritis Initiative (OAI) magnetic resonance imaging quality assurance update. Osteoarthr Cartil 21(1):110–116
Slauterbeck JR, Kousa P, Clifton BC, Naud S, Tourville TW, Johnson RJ, Beynnon BD (2009) Geographic mapping of meniscus and cartilage lesions associated with anterior cruciate ligament injuries. J Bone Joint Surg Am 91(9):2094–2103
Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, Schmithorst VJ, Smith MB (2001) Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14(1):50–55
Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, Majumdar S, Link TM (2011) A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging—data from the Osteoarthritis Initiative. Osteoarthr Cartil 19(8):984–989
Trattnig S, Zbyn S, Schmitt B, Friedrich K, Juras V, Szomolanyi P, Bogner W (2012) Advanced MR methods at ultra-high field (7 Tesla) for clinical musculoskeletal applications. Eur Radiol 22(11):2338–2346
Wang YX, Griffith JF, Ahuja AT (2010) Non-invasive MRI assessment of the articular cartilage in clinical studies and experimental settings. World J Radiol 2(1):44–54
Welsch GH, Mamisch TC, Domayer SE, Dorotka R, Kutscha-Lissberg F, Marlovits S, White LM, Trattnig S (2008) Cartilage T2 assessment at 3-T MR imaging: in vivo differentiation of normal hyaline cartilage from reparative tissue after two cartilage repair procedures—initial experience. Radiology 247(1):154–161
Welsch G, Mamisch T, Quirbach S, Zak L, Marlovits S, Trattnig S (2009) Evaluation and comparison of cartilage repair tissue of the patella and medial femoral condyle by using morphological MRI and biochemical zonal T2 mapping. Eur Radiol 19(5):1253–1262
Welsch GH, Scheffler K, Mamisch TC, Hughes T, Millington S, Deimling M, Trattnig S (2009) Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn Reson Med 62(2):544–549
Welsch G, Trattnig S, Hughes T, Quirbach S, Olk A, Blanke M, Marlovits S, Mamisch T (2010) T2 and T2* mapping in patients after matrix-associated autologous chondrocyte transplantation: initial results on clinical use with 3.0-Tesla MRI. Eur Radiol 20(6):1515–1523
Welsch GH, Mamisch TC, Zak L, Blanke M, Olk A, Marlovits S, Trattnig S (2010) Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping. Am J Sports Med 38(5):934–942
Welsch GH, Mamisch TC, Zak L, Mauerer A, Apprich S, Stelzeneder D, Marlovits S, Trattnig S (2011) Morphological and biochemical T2 evaluation of cartilage repair tissue based on a hybrid double echo at steady state (DESS-T2d) approach. J Magn Reson Imaging 34(4):895–903
White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R (2006) Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 241(2):407–414
Xu J, Xie G, Di Y, Bai M, Zhao X (2011) Value of T2-mapping and DWI in the diagnosis of early knee cartilage injury. J Radiol Case Rep 5(2):13–18
Yamamoto S, Watanabe A, Nakamura J, Ohtori S, Harada Y, Kishida S, Wada Y, Takahashi K (2011) Quantitative T2 mapping of femoral head cartilage in systemic lupus erythematosus patients with noncollapsed osteonecrosis of the femoral head associated with corticosteroid therapy. J Magn Reson Imaging 34(5):1151–1158
Zarins ZA, Bolbos RI, Pialat JB, Link TM, Li X, Souza RB, Majumdar S (2010) Cartilage and meniscus assessment using T1rho and T2 measurements in healthy subjects and patients with osteoarthritis. Osteoarthr Cartil 18(11):1408–1416
Acknowledgments
The Steadman-Philippon Research Institute is a 501(c)(3) non-profit institution supported financially by private donations and corporate support from the following entities: Smith and Nephew Endoscopy, Inc., Arthrex, Inc., Siemens Medical Solutions USA, Inc., ConMed Linvatec, Inc., Össur Americas, Inc., Small Bone Innovations, Inc., Opedix, Inc., Evidence Based Apparel, and Sonoma Orthopedics, Inc.
Conflict of interest
The authors report no conflict of interest in regard to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Surowiec, R.K., Lucas, E.P. & Ho, C.P. Quantitative MRI in the evaluation of articular cartilage health: reproducibility and variability with a focus on T2 mapping. Knee Surg Sports Traumatol Arthrosc 22, 1385–1395 (2014). https://doi.org/10.1007/s00167-013-2714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2714-6