Abstract
T1 rho and T2 mapping are magnetic resonance imaging (MRI) techniques to detect early degenerative changes in cartilage. Recent advancements have enabled 3D acquisition for both techniques. The objective of the present study was to examine the correlation of 3D T1 rho and 3D T2 mapping with macroscopic and histological characteristics of knee cartilage. Twenty-one patients who underwent total knee arthroplasty due to osteoarthritis with involvement of the medial compartment but with minimum involvement of the lateral compartment were enrolled. Prior to surgery, five series of MRI were acquired with a 3-T scanner. 3D T1 rho/T2 analyses were performed following determination of regions to be assessed using in-house software that incorporated three series of MRI acquisitions data (3D-MERGE, 3D-SPGR, and 3D-CUBE). During surgery, the cartilage of the lateral compartment was macroscopically assessed with the International Cartilage Research Society (ICRS) articular classification system. The extracted specimens were histologically assessed using the OARSI histology score. Three regions of interest (ROI) were assessed for each slice (two slices per knee): the central lateral femoral condyle (cLFC), the posterior portion of the lateral femoral condyle (pLFC), and the lateral tibia plateau (LTP). For each ROI, the mean T1 rho and T2 relaxation time, the ICRS grade, and the OARSI score were compared. Neither the T1 rho nor the T2 reflected the macroscopic grading. The T1 rho could discriminate between histological grades 1 and 2. However, the T2 could not. The T1 rho relaxation time was higher in the pLFC than in the cLFC even in the same grade. Compared to T2 mapping, T1 rho mapping may have an advantage in differentiating grades I and II cartilage degeneration on OARSI histological grading system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing number of patients with knee osteoarthritis (OA) is one of the biggest medical problems to be solved in a geriatric society. The development of disease-modifying OA drugs (DMOAD) has been anticipated but no officially approved DMOADs are available currently. However, earlier detection of cartilage degeneration might change the situations and may lead to a reduction in the incidence of OA [1, 2]. In addition, biomarkers to detect subtle changes of an early phase of the disease are requisite as outcome measurements for interventions including DMOADs. For these purposes, techniques to detect early cartilage degeneration have been sought and specially designed MRI techniques have been developed. T2 mapping, delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC), and T1 rho mapping are representative of these efforts [3]. All three techniques have been reported as promising. Distinct difference among them is that dGEMRIC requires use of a contrast agent and T1 rho and T2 do not. Knowledge and technical improvement of both T1 rho and T2 mapping have been increasing and researchers have found out that these two techniques reflect different major components of the cartilage, i.e., T1 rho reflects glycosaminoglycan (GAG) content and T2 reflects collagen anisotropy. In the process of cartilage degeneration, the loss of GAG content and the progression of collagen anisotropy are not sequential events but rather progress simultaneously [4,5,6,7]. Thus, the role of T1 rho and T2 mapping in the early cartilage degeneration are closely related to each other and further understanding and technical refinement are still necessary to use these imaging tools in a clinical setting. When comparing T1 rho and T2 mapping, some papers have reported nearly equal capacity to detect early changes of cartilage degeneration but several other papers have reported a greater sensitivity with T1 rho over T2 mapping [3, 7,8,9,10]. Two of these papers used histology and/or measurement of GAG content as a gold standard, but neither examined both the femoral cartilage and tibial cartilage independently. In this present study, the authors examined the capability of T1 rho, as well as T2 mapping to detect early degeneration of the femoral and tibial cartilage using specimens retrieved during total knee arthroplasty. For that purpose, T1 rho and T2 relaxation times were calculated from three-dimensionally acquired data, which corresponded histologically with the examined region of interest (ROI).
Our hypothesis is that T1 rho is superior to T2 in detecting early cartilage degeneration.
Materials and methods
Subjects
The study was approved by the institutional review board of the authors’ institution. A total of 21 patients who met the following inclusion criteria were enrolled: (1) diagnosed with primary medial-type knee osteoarthritis and scheduled for total knee arthroplasty (TKA) during a period from April 2012 to November 2013, (2) had not had any previous surgery of the index knee, and (3) with a lateral femorotibial compartment of no more than grade one on the Kellgren and Lawrence scale on a pre-operative X-ray [11]. Patient demographics were recorded at the time of the TKA. All patients provided written consent before participating in this study.
MRI acquisition
All MR examinations were conducted on a Discovery MR750 3.0T MR scanner (Discovery® MR750 MR System, GE Healthcare, Japan) using an 8-channel knee coil. T1 rho and T2 acquisitions were performed with the Research pack 3.0 from GE (GE Healthcare, Japan), which was distributed to several academic institutions for research purposes. Before processing the 3D T1 rho or T2 maps, 3D MERGE (multiple echo recombined gradient-echo), 3D SPGR (spoiled gradient-echo), and 3D Cube were acquired to create precise cartilage mask images.
Each sequence for acquisition was as follows:
-
1)
Sagittal 3D MERGE images with a repetition time/echo time (TR/TE) = 30/13.0 ms, flip angle (FA) = 5 degrees, bandwidth (BW) = 50 kHz/pixel, echo train length (ETL) = 3, field of view (FOV) = 15 cm, slice thickness = 3.0 mm and matrix = 320 × 320. Acquisition time was 4 min 53 s.
-
2)
Sagittal 3D SPGR images with a TR/TE = 15.1/4.1 ms, FA = 20 degrees, BW = 31.2 kHz/pixel, FOV = 15 cm, slice thickness = 3 mm and matrix = 512 × 512. Acquisition time was 2 min 4 s.
-
3)
Sagittal 3D Cube images with a TR/TE = 1500/18.1 ms, FA = 90 degrees, BW = 62.5 kHz/pixel, ETL = 50, FOV = 15 cm, slice thickness = 3 mm and matrix = 320 × 320. Acquisition time was 2 min 59 s.
-
4)
T1 rho and T2 relaxation times were obtained using a sagittal 3D fast spin-echo CUBE sequence.
The imaging parameters for the T1 rho and T2 acquisitions were as follows: T1 rho (TR/TE, 9/2.6 ms, time of recovery, 1500 ms, field of view, 15 cm, matrix 320 × 320, slice thickness, 3 mm, bandwidth, 62.5 kHz, time of spin-lock (TSL), 1/10/30/60/80 ms, frequency of spin-lock, 500 Hz, acquisition time, 10 min), and T2 (same as the T1 rho quantification except for TE, 13.4/27/40.7/54.4/67.8, and acquisition time, 9 min 59 s) The imaging parameters were nearly identical to those previously reported by Souza et al. [12].
T1 rho and T2 mapping
To obtain the T1 rho and T2 mapping images of each knee, a position adjustment was performed between the SPGR, MERGE, and Cube images in order to compensate for slight changes of knee position that may occur as time elapsed during the image acquisition. Briefly, two cuboid volumes of interest (VOIs) were set that were large enough to include the femur and tibia separately for each 3D MR image acquisition. Then, registration between the different MR image acquisitions was performed using normalized mutual information as a similarity measure between the images. Then, the optimization of the position adjustment was performed according to Powell’s method [13, 14]. Finally, segmentation was performed in a semi-automatic fashion based on Bezier splines and edge-detection from the SPGR sequence, as reported previously but modified for this study [15].
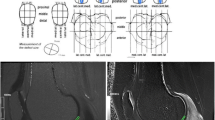
More specifically, approximately 20 control points were placed inside the cartilage area (Fig. 1a) followed by a connection of the points by Bezier splines (Fig. 1b). Then, perpendicular lines were created from each point toward the cartilage surface (Fig. 1c) and the points of the surface were determined. Line profiles were computed in the opposite direction and, based on the maximums of the first derivatives of the intensity the positions of the articular surfaces were identified (Fig. 1d). A Bezier spline was used to create the cartilage surface by connecting the surface points (Fig. 1e) and, when necessary, a fine adjustment was performed manually by moving the surface points (Fig. 1f). In the opposite direction, i.e., from the control points to the bone marrow, the same procedure was performed to determine the bone to cartilage boundary. In cases where the cartilage border was not clear, which often occurred between the femoral and tibial cartilage boundary or between the cartilage and joint effusion boundary, we redefined the points manually using the better images from the MERGE or Cube sequences to confirm the cartilage border. For this purpose, the three types of images were easily tabbed by simply clicking the select button.
Semi-automatic segmentation of the cartilage. Approximately 20 control points were placed inside the cartilage (a). A Bezier spline connected these points (b). Perpendicular lines were created from each point toward the cartilage surface (c). Line profiles were computed and the positions of the articular surface were found based on the maximums of the first derivatives of the intensity (d). A Bezier spline was used to create the cartilage surface by connecting the surface points (e) and an adjustment was performed manually by moving the surface points when necessary (f)
Finally, the T1 rho and T2 maps were matched to the segmented cartilage images, allowing for the production of the T1 rho and T2 cartilage mapping images (Fig. 2). The mean T1 rho and T2 values were obtained in each ROI. All these processes were performed using in-house software developed with Matlab (Mathworks, Natick, MA, USA). The cartilage segmentation was performed by a single orthopedic surgeon. The image matching, and the T1 rho and T2 relaxation times for each ROI were calculated by engineering faculty investigators. All investigators were different from those performing the surgeries.
Comparison of T1 rho and T2 mapping. Sagittal images of the T1 rho and T2 maps. From this image, the average T1 rho and T2 values for the three ROIs were calculated. Note that the relaxation time of the pLFC (yellow arrows) with T1 rho mapping was higher than that of the cLFC (white arrows) whereas those of both regions (cLFC and pLFC) were almost identical in both of mapping in this case.
Macroscopic assessment
At the time of the TKA, a macroscopic assessment of the affected articular cartilage was performed by three orthopedic surgeons, according to the International Cartilage Repair Society (ICRS) cartilage injury assessment system (ICRS grading score). This system includes the following grades: grade 0 = normal; grade 1 = nearly normal (1a = soft indentation on superficial lesions, 1b = superficial fissures and cracks); 2 = abnormal (lesions extending down to <50% of cartilage depth); 3 = severely abnormal (3a = cartilage defects extending down >50% of cartilage depth, 3b = cartilage defects extending down to the calcified layer, 3c = cartilage defects extending down to but not through the subchondral bone); 4 = full thickness defects exposing the underlying subchondral bone [16]. Three ROIs were assessed for each knee, i.e., two from the femoral condyle (the central lateral femoral condyle; cLFC and the posterior portion of the lateral femoral condyle; pLFC) and one from the lateral tibia plateau (LTP).
Histology
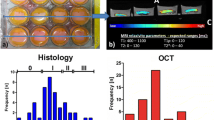
The osteochondral specimens were obtained from the resected joint surface and subdivided into 3 mm thinner specimens with a flat chisel (indicated with black boxes in Fig. 3, right) that were set perpendicular to the epicondylar line (the upper purple line drawn by a surgeon and indicated with a red arrow head in Fig. 3, right) on the femoral side. For the tibial side, specimens parallel to the AP axis of the tibia as defined by Akagi were obtained (the dotted yellow line in the tibial plateau in Fig. 3, right) [17, 18]. Samples were then processed for histological assessment (Fig. 3 right). Specimens were fixed with 4% paraformaldehyde, decalcified with 20% ethylenediaminetetraacetate, paraffin-embedded through routine procedures and then sections were stained with safranin O. The histological evaluation was done employing the Osteoarthritis Research Society International (OARSI) OA cartilage histopathology assessment system (OARSI grade): grade 1 = surface intact, grade 2 = surface discontinuity, grade 3 = vertical fissures (clefts) and grade 4 = erosion [19].
Determination of the slices and ROIs to be assessed. The reference lines used to determine the MRI images and to obtain osteocartilagenous specimens are shown. Two parallel lines (orange and green lines) indicate the sagittal image of MR that was perpendicular to the epicondylar line (dotted red line). Three regions of interest (ROIs) were placed: (1) the central lateral femoral condyle (cLFC, red) (2) the posterior lateral femoral condyle (pLFC blue), and (3) the central lateral tibia plateau (cLTP, yellow). Black boxes in the right photo show the locations of the osteocartilagenous specimens. The red arrowhead indicates the epicondylar line. The yellow dotted line indicates the “Akagi line” that is perpendicular to the epicondylar line of the femur [17, 18]
ROI placement
Two sagittal slices were set to compare the imaging and histology. First, a reference line was drawn through the deepest point of the groove and the deepest point of the femoral notch (white line in Fig. 3, left), which was perpendicular to the epicondylar line (dotted red line in Fig. 3, left). The first sagittal slice was defined as being parallel to the reference line and passing through the lateral supracondylar ridge (green line in Fig. 3, left). The second slice was 4.5 mm lateral and parallel to the first slice (orange line in Fig. 3, left).
For each slice, three ROIs were assessed: (1) the central lateral femoral condyle (cLFC), the femoral cartilage region between the most posterior margin of the anterior horn of the lateral meniscus (LM) and the posterior margin of the posterior horn of the LM, (2) the posterior lateral femoral condyle (pLFC), the cartilage region of the posterior portion of the LFC to the cLFC, and (3) the central lateral tibia plateau (cLTP), the tibial cartilage region between the most posterior edge of the anterior horn of the LM and the most anterior edge of the posterior horn of the LM (Fig. 3, center). Osteocartilaginous specimens are shown (Fig. 3, right): (The distal portion of the femoral condyles (upper), the posterior portion of the femoral condyles (middle), and the tibial plateau (bottom)). Two purple lines in the distal femur (the epicondylar; red arrow head, and the posterior condylar; lines) were drawn by the surgeon for surgical reference. The locations of the specimens for the histological assessment are indicated by the long black boxes and they were obtained perpendicular to the epicondylar line. Note that the dotted red line and the upper purple line (red arrow head) in Fig. 3 comprise the epicondylar line. The yellow dotted line (the Akagi line) on the tibial plateau is known to be perpendicular to the epicondylar line of the femur [17, 18]. Thus, theoretically the osteocartilagenous specimens and the sagittal slice of MRI were accordant. The locations of microscopic grading were determined by taking convex prominent of the femur and tibia into account.
Validation study
1) The inter-observer reliability and intra-observer reliability were examined by picking 10 randomly selected cases (i.e., 20 slices). A single examiner placed the ROI twice with an interval of 2 weeks for intra-observer reliability and two observers placed the ROIs independently for inter-observer reliability. Calculation of the T1 rho and T2 relaxation times was conducted by another examiner. The ICC (1,2) and ICC (2,2) were calculated.
Statistical analysis
-
1)
As a first round analysis for the total of 44 sites (two slices from each knee), the mean T1 rho and T2 relaxation times of ROIs were calculated for each macroscopic (ICRS) and microscopic grade (OARSI). The three locations (cLFC, pLFC, and cLTP) were assessed independently. In addition, the T1 rho and T2 relaxation times for each grade were compared separately between the three different ROI locations (cLFC, pLFC, and cLTP).
The difference of the mean relaxation times for the inter-grades for each ROI and that of the intra-grades among the location of the ROIs was statistically analyzed using the Kruskal-Wallis test and a post hoc Steel-Dwass test. A p value of less than 0.05 was considered to be statistically significant.
-
2)
As a second round analysis to exclude intrinsic correlations, a single slice from each knee was selected to reduce the intrinsic correlation. The lower OARSI grade slice or the more medial slice of the two when the grades were the same was selected from each knee. Then, using a mixed effect model analysis, the effects of histological grades and locations of the ROI were analyzed. A p value of less than 0.01 was considered to be statistically significant. All statistical analyses were performed with SAS software version 9.3 (SAS Institute, Cary, NC).
Results
Patient demography
The mean age of the participants was 72.1 ± 8.2 (59–87) years old (18 women and 3 men, 12 right and 9 left knees). The mean body mass index (BMI) was 25.7 ± 3.6 (21.4–32.8) kg/m2, and the mean interval between MRI scan and surgery was 10.8 ± 8.5 (1–28) days. Nineteen knees were classified as grade IV on Kellgren and Lawrence scale and two knees were grade III due to degenerative changes of medial compartment.
Reproducibility of ROI placement
The ICC (1, 2) for T1 rho was from 0.885 to 0.982 in the three ROIs whereas that for T2 was from 0.993 to 0.997. The ICC (2, 2) for T1 rho was from 0.916 to 0.974 in the three ROIs whereas that for T2 was from 0.815 to 0.982.
Comparisons of T1 rho and T2 values among macroscopic grades
The mean T1 rho and T2 relaxation times of different ICRS grades from each ROI are shown in Table 1. Significant differences of the T1 rho relaxation times were observed between ICRS grade 1a and grade 3a in the cLFC, grade 0 and grade 3a in the cLTP, and grade 1b and grade 3a in the cLTP. A significant difference of the T2 relaxation times was observed only between ICRS grades 1b and 3a in the cLTP. In terms of location difference, the T1 rho relaxation times of the pLFC were higher than those of the cLFC in ICRS grades 1a, 2, and 3a and that of the cLTP in ICRS grade 1b. The T2 relaxation times of the pLFC were higher than that of the cLFC in ICRS grades 1a, and those of the cLTP in grades 1a, 1b, and 2.
Comparisons of the T1 rho and T2 values among histological grades
-
1)
The mean T1 rho and T2 relaxation times for the different OARSI histological grades in each ROI are shown in Table 2. Significantly lower T1 rho relaxation times for OARSI grade 1, compared to the other grades, were consistently found in all three ROIs. The T1 rho relaxation times for grade 2 were significantly lower than OARSI grade 4 in all three ROIs. On the other hand, the T2 relaxation times differed only between OARSI grades 1 and 4 in all three ROIs and between OARSI grades 1 and 3 in the pLFC and the cLTP. Thus, T2 could not discriminate between OARSI grades 1 and 2 as well as between grades 2 and 3. The T1 rho relaxation times of the pLFC were significantly higher than those of the cLFC in OARSI grades 1, 2, and 3 and those of the cLTP in OARSI grades 1 and 2. The T2 values of the pLFC were significantly higher than those of the cLFC in OARSI grades 1 and 2, and those of the cLTP in OARSI grades 1, 2, and 3.
-
2)
Ten, 11, and 9 slices of OARSI grade 1 and 6, 6, and 7 slices of OARSI grade 2 were picked up from the cLFC, cLTP, and pLFC, respectively. As the number of OARSI grades 3 or 4 was less than 4 in each location, they were excluded.
The OARSI grade and location affected the variability of the T1 rho relaxation time, but only location had effect on the T2 (Tables 3 and 4). The relaxation time of T1 rho for OARSI grade 1 was lower than that of grade 2 but the relaxation time of T2 did not differ between the grades (Tables 3 and 4), whereas both relaxation times for the pLFC were higher than those for the cLFC and cLTP (Tables 3 and 4).
Discussion
In this study, we showed that 3D T1 rho mapping was able to differentiate as early as grade 1 and II cartilage degeneration on OARSI histological grade by measuring T1 rho relaxation times. The rational for pursuing quantifiable MR techniques for cartilage is because morphological assessments are insufficient to detect early cartilage degeneration [20,21,22]. Palmer et al. reported morphological MRI was capable of detecting no less than grade III cartilage degeneration on OARSI histological grading system but requirement of physiological (=quantifiable) MRI to discriminate less than grade III cartilage [23]. Thus, the importance of quantifiable MRI is to detect a subtle degenerative change that is difficult for morphological MRI to detect.
T1 rho vs. T2
Whether T1 rho or T2 mapping is superior to detect early cartilage degeneration is controversial. This study showed that 3D T1 rho mapping of articular cartilage could differentiate early cartilage degeneration, comparable to histological examination. Discrimination between grades 1 and 2 on the OARSI cartilage histopathological assessment system was indicated in all three ROIs. Thus, this technique meets the need to detect early cartilage changes and identify candidates for early intervention, which was not achievable by 3D T2 mapping.
Several studies have reported the superiority of T1 rho to detect early cartilage degeneration [3, 7,8,9,10]. Consistent with our results, Nishioka et al. reported that T1 rho mapping correlated well with OARSI histological grade, using the lateral tibial plateau obtained at the time of surgery [7]. Although lacking histological confirmation, Hirose et al. and Nishioka et al. reported the superiority of T1 rho using a whole organ magnetic resonance imaging score (WORMS) [24] as a standard reference [3, 9]. In the present study, T1 rho could not differentiate macroscopic ICRS grading but Takayama et al. reported that T1 rho could differentiate macroscopically normal versus mildly denatured cartilage, which T2 mapping could not [4]. Wang et al. reported that T1 rho, along with dGEMRIC, was more sensitive to detect early cartilage degeneration than T2 [10].
Contrary to the above studies, Wong et al. reported that the T2 value was more reliable in that it correlated better to glycosaminoglycan (GAG) content, compared to T1 rho [25]. As indicated in the limitations, the whole lateral plateau was used for the GAG assessment, which presumably included various degrees of degenerated cartilage.
Macroscopic analysis vs. T1 rho
This study shows that T1 rho could not consistently differentiate macroscopic changes. Only 1a vs. 3a on ICRS grading in the cLFC, and 0 vs. 3a and 1b vs. 3a in the cLTP were discriminated, which is in line with previous studies [8, 24]. Takayama et al. reported no significant differences in the T1 rho values of mild to moderate degeneration using five-point macroscopic grading as a reference. Observer-dependent differences were also reported in their paper [8]. Tsushima et al. also reported no significant differences between T1 rho values from macroscopic grade 1 and those of grade 2, when employing the five-point grading system [26]. This may suggest that the composition of the cartilage differed little between some adjacent macroscopic grades, which made discrimination by T1 rho difficult.
Site-specific differences among the ROIs
In the present study, the T1 rho values of the pLFC were higher than those of the cLFC in all three grades of the ICRS and grades 1 to 3 on the OARSI (Tables 1 and 2). The same tendency was seen by Okazaki et al., reporting that the T1 rho value of the deep zone of the posterior area of the femur was higher compared to that of the distal area of the femur in PCL injured knees versus normal knees, although no statistical analysis was performed [27]. This finding might be due to the magic angle phenomenon, or unknown artifacts, or may simply be a site-specific feature of cartilage, such as differences in proteoglycan content [28]. Li et al. reported a higher GAG content in the inferior femoral condyle than in the posterior femoral condyle of similar severity of osteoarthritic knees to the present study [29]. Goto et al. also reported a site-dependent difference of the T1 rho value, using smaller ROIs in knees of asymptomatic volunteers with an average age of 41.6 years. A positive correlation of the T1 rho value and age was also reported [30]. Nozaki et al. reported that the type of pulse sequence (SPGR vs. b-FFE) affected the T1 rho values [28]. Therefore, before applying T1 rho mapping in a clinical setting, several issues need to be addressed, including age-related differences, site-specific differences, and standardization of the sequences employed. Once these problems are resolved, T1 rho mapping may be promising. Using the same modality and knee coils at different institutions, Li et al. reported that T1 rho values showed minimal differences and concluded that, with careful quality control and cross-calibration, multi-site studies were possible [28].
Compositional parameters of cartilage
Articular cartilage is composed of 90% type-II collagen, 5–10% proteoglycan (PG) and bound water [32]. Among them, the GAG concentration has been reported to have the largest influence on T1 rho values [7, 25], although it is not a single decisive parameter. T1 rho may reflect non-specific changes in the cartilage extracellular matrix [33]. van Tiel et al. reported that the T1 rho relaxation time did not correlate with either the GAG content or the collagen content of human cartilage, whereas dGEMRIC correlated highly with both parameters [34]. The authors speculated that T1 rho mapping might measure other elements of cartilage such as water content or a combination of composites of the cartilage extracellular matrix [34]. Considering that cartilage degeneration does not depend on a single compositional change but rather a combination of multiple parameters proceeding simultaneously [35, 36], use of this technique to detect early changes in articular cartilage would be possible, but further studies are required to determine the parameters affecting T1 rho values. This study demonstrates that T1 rho distinguishes histological changes, which might reflect multiple parameters (i.e., loss of GAG, derangement of collagen, surface irregularities and other compositional changes) [19]. Despite the previously mentioned uncertainty of T1 rho values, the clinical application of this technique may prove useful. Early changes of cartilage have been detected in patients with a posterior root tear of the medial menisci [37], in a lower-limb alignment dependent fashion [38], in ACL injured knees [31, 39], in asymptomatic PCL injured knees [26], in mal-aligned patella [40] and in hip joints with a cam deformity [41].
Need for further improvement
Although there is growing evidence that T1 rho use in clinical settings is preferable [3, 7,8,9,10, 33, 37, 38], several problems need to be addressed for further technical refinement, in addition to defining the characteristic features of T1 rho. (1) A standard way to acquire and process images is lacking. (2) The MRI protocols and methodology of segmentation in the T1 rho studies should be standardized [28]. (3) Site-specific differences, even with the same histological grade of cartilage, shown in the present study, indicate the necessity for determining adequate placement of the ROIs in terms of size and location. (4) In this study, 3 ROIs were set per slice, i.e., the cLFC, pLFC, and cLTP, which was similar to other reports [27, 30]. However, although it was taken into account that the mechanical property of the cartilage differed whether it was adjacent to the menisci or not [42], it was not possible to verify if ROIs were adequate. Compositional or biomechanical differences between the femoral and tibial cartilage [43] and site-specific differences even in the same femur or tibia [38, 44] might help determine adequate placement of the ROIs but this has not yet been achieved. One alternative way to determine the ideal ROIs would be to construct 3D images of the whole cartilage. However, methods to evaluate the status of whole cartilage based on 3D images have not been developed.
Limitations
Several limitations regarding our study need to be considered. First, samples from osteoarthritic knees that required joint replacement were used and the less degenerated area of cartilage from the lateral compartment was examined. Thus, even cartilage composition of a macroscopic or histological grade 1 might be different from healthy cartilage. If this is the case, our study lacked control samples although the T1 rho and T2 values were within the reference range.
Second, we obtained osteochondral specimens with chisels referencing surgically used lines in order to match them to the MRI slices as much as possible, but inevitably the manual procedure may have resulted in discordance. Third, we employed as a standard reference, the macroscopic ICRS grade and microscopic OARSI grade, where the grades were basically dependent upon the surface irregularities. However, the MR images referred to the composition of the cartilage. The close relationship between the status of the surface and the cartilage composition of the mid to Opnedeep zones could make them an acceptable standard [19]. The OARSI histological grading system has been widely used in this type of study [4, 7, 45, 46]. Adding dGEMRIC might improve our study but lengthy acquisition time in total was a concern for ethical committee and we were not allowed to perform. Fourth, we did not perform a validation study between the 2D and 3D acquisitions. However, the sequences we used in the present study were in accordance with previous studies and our goal was to compare the 3D T1 rho and the 3D T2 acquisitions, so we believe that the lack of validation should be excused [12, 47].
Lastly, our study lacked a biochemical analysis of the cartilage.
Conclusion
Compared to T2 mapping, T1 rho mapping may have an advantage in differentiating grades I and II cartilage degeneration on OARSI histological grading system.
References
Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20:407–422
Mosher TJ, Walker EA, Petscavage-Thomas J, Guermazi A (2013) Osteoarthritis year 2013 in review: imaging. Osteoarthr Cartil 21:1425–1435
Nishioka H, Hirose J, Okamoto N, Okada T, Oka K, Taniwaki T, Nakamura E, Yamashita Y, Mizuta H (2015) Evaluation of the relationship between T1 and T2 values and patella cartilage degeneration in patients of the same age group. Eur J Radiol 84:463–468
Wei B, Du X, Liu J, Mao F, Zhang X, Liu S, Xu Y, Zang F, Wang L (2015) Associations between the properties of the cartilage matrix and findings from quantitative MRI in human osteoarthritic cartilage of the knee. Int J Clin Exp Pathol 8:3926–3936
Raya JG, Melkus G, Adam-Neumair S, Dietrich O, Mützel E, Reiser MF, Putz R, Kirsch T, Jakob PM, Glaser C (2016) Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology 266:831–841
Rautiainen J, Nissi MJ, Liimatainen T, Herzog W, Korhonen RK, Nieminen MT (2014) Adiabatic rotating frame relaxation of MRI reveals early cartilage degeneration in a rabbit model of anterior cruciate ligament transection. Osteoarthr Cartil 22:1444–1452
Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y (2012) T1r and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging 35:147–155
Takayama Y, Hatakenaka M, Tsushima H, Okazaki K, Yoshiura T, Yonezawa M, Nishikawa K, Iwamotod Y, Honda H (2015) T1 is superior to T2 mapping for the evaluation of articular cartilage denaturalization with osteoarthritis: radiological–pathological correlation after total knee arthroplasty. Eur J Radiol 85:1098–1104
Hirose J, Nishioka H, Nakamura E, Oniki Y, Yamashita Y, Mizuta H (2012) T1 and T2 mapping of the proximal tibiofibular joint in relation to aging and cartilage degeneration. Eur J Radiol 81:2776–2782
Wang L, Regatte RR (2014) Quantitative mapping of human cartilage at 3.0T: parallel changes in T2, T1r, and dGEMRIC. Acad Radiol 21:463–471
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Souza RB, Kumar D, Calixto N, Singh J, Schooler J, Subburaj K, Li X, Link TM, Majumdar S (2014) Response of knee cartilage T1 rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthr Cartil 22:1367–1376
Studholme C, Hill DLG, Hawkes J (1996) Automated 3-D registration of MR and CT images of the head. Med Image Anal 1:163–175
Press WH, Teukolsky SA, Vetterling VT, Flannery BP (1994) Numerical recipes, the art of scientific computing (2nd ed). Cambridge University Press, Cambridge, pp 282–306
Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, Majumdar S (2008) Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 12:120–135
Brittberg M, Peterson L (1998) Introduction of an articular cartilage classification. ICRS Newsletter 1:5–8
Akagi M, Mori S, Nishimura S, Nishimura A, Asano T, Hamanishi C (2005) Variability of extraarticular tibial rotation references for total knee arthroplasty. Clin Orthop Relat Res 436:172–176
Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C (2004) An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res 420:213–219
Pritzker KPH, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14:13–12
Bittersohl B, Mamisch TC, Welsch GH, Stratmann J, Forst R, Swoboda B, Bautz W, von Rechenberg B, Cavallaro A (2009) Experimental model to evaluate in vivo and in vitro cartilage MR imaging by means of histological analyses. Eur J Radiol 70:561–569
McGibbon CA, Trahan CA (2003) Measurement accuracy of focal cartilage defects from MRI and correlation of MRI graded lesions with histology: a preliminary study. Osteoarthr Cartil 11:483–493
Kijowski R, Blankenbaker DG, Munoz DRA, Baer GS, Graf BK (2013) Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology 267:503–513
Palmer AJR, Brown CP, McNally EG, Price AJ, Tracey I, Jezzard P, Carr AJ, Glyn-Jones S (2013) Non-invasive imaging of cartilage in early osteoarthritis. Bone Joint J 95-B:738–746
Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D et al (2004) Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil 12:177–190
Wong CS, Yan CH, Gong NJ, Li T, Chane DQ, Chu YC (2013) Imaging biomarker with T1 and T2 mappings in osteoarthritis—in vivo human articular cartilage study. Eur J Radiol 82:647–650
Tsushima H, Okazaki K, Takayama Y, Hatakenaka M, Honda H, Izawa T, Nakashima Y, Yamada H, Iwamoto Y (2012) Evaluation of cartilage degradation in arthritis using T1q magnetic resonance imaging mapping. Rheumatol Int 32:2867–2875
Okazaki K, Takayama Y,·Osaki K, Matsuo Y,·Mizuchi H,·Hamai S,·Honda H,·Iwamoto Y. Subclinical cartilage degeneration in young athletes with posterior cruciate ligament injuries detected with T1ρ magnetic resonance imaging mapping. Knee Surg Sports Traumatol Arthrosc 2015; 23:3094–3100.
Nozaki T, Kaneko Y, Yua HJ, Kaneshiro K, Schwarzkopf R, Yoshioka H (2015) Comparison of T1rho imaging between spoiled gradient echo (SPGR) and balanced steady state free precession (b-FFE) sequence of knee cartilage at 3 T MRI. Eur J Radiol 84:1299–1305
Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, Majimdar S (2011) Quantitative MRI using T1 rho and T2 in human osteoarthritis cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging 29:324–334
Goto H, Iwama Y, Fujii M, Aoyama N, Kubo S, Kuroda R, Ohno Y, Sugimura K (2012) A preliminary study of the T1rho values of normal knee cartilage using 3 T-MRI. Eur J Radiol 81:796–803
Li X, Pedoia V, Kumar D, Rivoire J, Wyatt C, Lansdown D, Amano K, Okazaki N, Savic D, Koff MF, Felmlee J, Williams SL, Majumdar S (2015) Cartilage T1 and T2 relaxation times: longitudinal reproducibility and variations using different coils. MR systems and sites Osteoarthritis and Cartilage 23:2214–2223
Bolbos RI, Link TM, Ma CB, Majumdar S, Li X (2009) T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3 T. Osteoarthr Cartil 17:12–18
Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW (2015) Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartil 23:1639–1653
van Tiel V, Kotek G, Reijman M, Bos PK, Bron EE, Klein S, Nasserinejad K, Osch G, Verhaar JAN, Krestin GP, Weinans H, Oel EH (2016) Is T1rho mapping an alternative to delayed gadolinium-enhanced MR imaging of cartilage in the assessment of sulphated glycosaminoglycan content in human osteoarthritic knees? An in vivo validation study. Radiology 2:523–531
Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D (2008) Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1b. Osteoarthr Cartil 16:1560–1571
Settle S, Vickery L, Nemirovskiy O, Vidmar T, Bendele A (2010) Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis. Arthritis & Rheum 62:3006–3015
Takahashi K, Hashimoto S, Nakamura H, Mori A, Sato A, Majima T, Takai S (2015) Medial meniscal posterior root/horn radial tears correlate with cartilage degeneration detected by T1 relaxation mapping. Eur J Radiol 85:1098–1104
Wang L, Vieira RLR, Rybaka LD, Babba JS, Changa G, Krasnokutsky S, Abramson S, Regatte R (2013) Relationship between knee alignment and T1 values of articular cartilage and menisci in patients with knee osteoarthritis. Eur J Radiol 82:1946–1952
Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, Ma CB, Majumdar S (2011) Cartilage in anterior cruciate ligament–reconstructed knees: MR imaging T1 r and T2—initial experience with 1-year follow–up. Radiology 258:505–514
Thuillier DU, Souza RB, Wu S, Luke A, Li X, Feeley BT (2013) T1r imaging demonstrates early changes in the lateral patella in patients with patellofemoral pain and maltracking. Am J Sports Med 41:1813–1818
Anwander H, Melkus G, Rakhra KS, Beaulé PE (2016) T1ρ MRI detects cartilage damage in asymptomatic individuals with a cam deformity. J Orthop Res 6:1004–1009
Deneweth JM, Newman KE, Sylvia SM, McLean SG, Arruda EM (2013) Heterogeneity of Tibial plateau cartilage in response to a physiological compressive strain rate. J Orthop Res 31:370–375
Wong LB, Sah RL (2010) Mechanical asymmetry during articulation of tibial and femoral cartilages: local and overall compressive and shear deformation and properties. J Biomech 43:1689–1695
Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coutts RD, Sa RL (2007) Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthr Cartil 15:1042–1052
Raya JG, Melkus G, Adam-Neumair S et al (2013) Diffusion-tensor imaging of human articular cartilage specimens with early signs of cartilage damage. Radiology 266(3):831–841
Lukas VA, Fishbein KW, Lin PC, Schar E, Schneider E, Neu CP, Spencer RG, Reiter DA (2015) Classification of histologically scored human knee osteochondral plugs by quantitative analysis of magnetic resonance images at 3T. J Orthop Res 33:640–650
Kumar D, Souza RB, Subburaj K, MacLeod TD, Singh J, Calixto NE, Nardo L, Link TM, Li X, Lane NE, Majumdar S (2015) Are there sex differences in knee cartilage composition and walking mechanics in healthy and osteoarthritis populations? Clin Orthop Relat Res 473:2548–2558
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Role of the funding source
The author had no source of funding.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Sasho, T., Katsuragi, J., Yamaguchi, S. et al. Associations of three-dimensional T1 rho MR mapping and three-dimensional T2 mapping with macroscopic and histologic grading as a biomarker for early articular degeneration of knee cartilage. Clin Rheumatol 36, 2109–2119 (2017). https://doi.org/10.1007/s10067-017-3645-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3645-2