Abstract
The evaluation of articular cartilage currently relies primarily on the identification of morphological alterations of the articular cartilage. Unlike anatomic imaging, T2 mapping is sensitive to changes in the chemical composition and structure of the cartilage. Clinical evaluation of T2 mapping of the glenohumeral joint has not been previously reported. The objectives of this study were to evaluate the feasibility of magnetic resonance T2 mapping of the glenohumeral joint in routine clinical imaging, to assess the normal T2 mapping appearance of the glenohumeral joint, and to compare the findings on T2 maps to conventional MR pulse sequences. Magnetic resonance imaging (MRI) examinations of 27 shoulders were performed in a routine clinical setting. All studies included acquisition of T2 mapping using a dedicated software. The T2 maps were analyzed along with the routine MR exam and correlation of cartilage appearance on T2 map and on conventional MR sequences. T2 imaging maps were obtained successfully in all patients. T2 maps and routine MRI correlated in cases of normal cartilage and prolonged T2 values and cartilage defects. In four cases, increased T2 relaxation times in the cartilage and cartilage defects were more apparent on T2 maps. Acquisition of T2 maps at the time of routine MRI scanning is feasible and not time-consuming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Articular cartilage has multiple important functions including frictionless motion of the joint, weight distribution, and shock absorption. Current clinical evaluation of articular cartilage relies primarily on identification of morphological alterations of the articular cartilage [1, 2]. This includes either anatomic magnetic resonance imaging (MRI) with determination of cartilage thickness and detection of cartilage damage or arthroscopy to diagnose cartilage abnormalities [3]. However, conventional MR imaging and arthroscopy are less sensitive in evaluation of cartilage composition [4] and cannot be used to evaluate cartilage with potential and irreversible degeneration or “cartilage at risk” [5, 6]. The importance of such qualitative evaluation of the cartilage is valuable for the assessment of cartilage functional potential, as a non-invasive tool to follow cartilage composition after repair procedures and to monitor the effect of chondroprotective therapy.
Recently, MR parametric mapping techniques, such as cartilage T2 mapping, were developed to exploit the sensitivity of MRI to biophysical properties of the cartilage [7]. Furthermore, it has been shown that this technique can be used to evaluate the biochemical composition and structure of the extracellular cartilage matrix [8]. Unlike anatomic imaging, T2 mapping is sensitive to changes in the chemical composition and structure of the cartilage [5] and can serve as image markers of cartilage degeneration.
T2 mapping of articular cartilage is an imaging technique for the qualitative and quantitative evaluation of the hyaline articular cartilage. Normal articular hyaline cartilage illustrates a predictable spatial variation with depth in T2 relaxation time at MR imaging: an increase in T2 values from the subchondral bone to the articular surface correlating to macroscopic collagen organization and orientation seen in normal articular cartilage [9]. The superficial zone demonstrates very short T2 values which may sometimes be beyond the spatial resolution of images obtained at clinically relevant field strength. Alteration in this orderly transition in T2 values within the cartilage has been shown to correlate to changes in water content, changes in collagen structure, and organization associated with hyaline articular cartilage degradation [9–11]. T2 mapping provides a non-invasive means for the study of cartilage water and interaction of water with the solid components of the extracellular cartilage matrix at a molecular level [5]. So far, clinical evaluation of the feasibility of T2 mapping has been focused on evaluation of articular cartilage of the knee [11–17] and interphalangeal joints of the hand [3]. To our knowledge, clinical application of T2 mapping for the evaluation of glenohumeral joint which can potentially provide a useful tool to identify the earliest stages of cartilage degeneration that precede visible cartilage damage has not been reported.
The primary purpose of this study is to evaluate the feasibility of MRI T2 mapping of glenohumeral joint in routine clinical imaging. Secondly, we wished to assess the normal T2 mapping appearance of the glenohumeral joint. Finally, we aimed to compare the findings on T2 maps to conventional MR pulse sequences and identify the potential signs of cartilage degeneration on T2 maps.
Materials and methods
MRI examinations of shoulder with T2 mapping were acquired from 27 consecutive patients in a routine daily clinical practice during the period of December 2006–February 2007. The patient population consisted of 16 women and 11 men (ages 21–83 years, mean age 46 years). Sixteen arthrographic and 11 non-arthrographic examinations were performed. The type of the examinations was defined based on the clinical query (labral abnormality versus rotator cuff pathology). In five patients, T2 maps of the shoulder were acquired both before and after intraarticular gadolinium injection, bringing the total to 32 MR examinations. Patients were scanned with a 1.5 T MR imaging system (Signa; 16 channel, Excite, GE Healthcare, Milwaukee, WI, USA) by using the standard surface coils (four-channel, receive only, phased array).
MR sequences for non-arthrographic examination included: axial T2* gradient echo (684/22 (repetition time ms/echo time ms)), coronal/oblique T1 (417/10 (repetition time ms/echo time ms)), coronal/oblique T2 (3,483/87 (repetition time ms/echo time ms)), and sagittal/oblique T2 (4,400/82 (repetition time ms/echo time ms)) fast spin echo fat-saturated images.
MR arthrography included: T1 fat-saturated axial (483/10 (repetition time ms/echo time ms)), coronal/oblique (400/10 (repetition time ms/echo time ms)), sagittal/oblique (383/10 (repetition time ms/echo time ms)), and coronal/oblique T2 (3,716/87 (repetition time ms/echo time ms)) fast spin echo fat-saturated images. All studies included acquisition of MR T2 mapping (see below).
T2 mapping was performed using dedicated software (HDx, T2 Map CartiGgram, GE). First, eight coronal-oblique data sets were obtained through the shoulder. An eight-echo multiecho spin echo pulse sequence 1,050/9–70 (repetition time ms/echo time ms) was used to acquire the data with 14 × 14 cm field of view, matrix of 512 × 512, and slice thickness of 3 mm with no intersection gap. The repetition time and range of echo times were chosen based on the default software settings and to allow comparison with T2 mapping results reported previously for the knee [11–16].
The next step, following the acquisition, is calculation of T2 maps. The MR technologist generated a colored T2 map using the default functions and settings of the software. The T2 maps included 16–22 color coronal-oblique images of the glenohumeral joint. The basic parameters of the T2 intensity were default parameters of 25–75 ms. Color scale included the spectrum range from red to blue. Thus, the cartilage with the lowest T2 intensity (lowest concentration of the extracellular water) was marked with colors of the lower part of the scale (red–orange–yellow), and the cartilage with the highest T2 intensity was marked with the colors of the upper part of the scale (green–blue).
The T2 maps were evaluated by a clinical musculoskeletal fellow and one of two musculoskeletal radiologists (2 and 4 years experience in MSK radiology) and correlated with routine MR images. All results were re-evaluated 1–4 days later by all three readers, with agreement by consensus. Cartilage of both the humeral head and glenoid was evaluated. The following parameters were assessed: hyaline cartilage color on T2 map, hyaline cartilage signal on conventional MR sequences, and presence or absence of defects in hyaline cartilage.
For interpretation of the T2 maps, the following criteria of normal hyaline cartilage were used [3–5, 18]: uniform articular cartilage covering the articular surfaces and spatial variation in cartilage T2, with longer values observed near the articular surface. The cartilage was considered abnormal if T2 values of the cartilage increased, colored green–blue in the spectrum and/or T2 pattern without illustration of a normal gradient transition of low-to-high T2 values from deep-to-superficial zones, or if the cartilage had a defect, was thin, or absent. On routine MRI sequences, hyaline cartilage was considered normal if uniform in thickness and intermediate signal intensity. The cartilage was considered abnormal if there was increased signal or if the cartilage had a defect, was thin, or absent.
Results
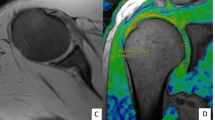
T2 maps were obtained successfully in all patients. Acquisition time to acquire the source images for T2 maps was 5–6 min. Total time including post-processing was approximately 8 min. Comparison of the five joints imaged before and after arthrographic intraarticular injection showed that the joint distention created an interface between the articular surfaces, which allowed better visualization of the cartilage on arthrographic than on non-arthrographic examinations (Fig. 1).
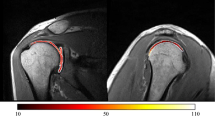
Hyaline cartilage of 12 joints had normal appearance on both T2 map and conventional MR sequences (Tables 1 and 2; Fig. 2). Cartilage of these joints had red–orange color in its deeper layers, orange–yellow color in the more superficial layer on T2 map, and normal signal intensity with no cartilage defects intensity on conventional MR sequences.
A 20-year-old male with normal glenohumeral joint cartilage. a T2 map after intraarticular contrast shows normal color layering along the humeral head and the glenoid. Spatial variation in cartilage T2, with longer values (yellow—white arrow) are observed near the articular surface and shorter values (red—black arrow), in deeper cartilage layers. b Coronal T2 FSE fat-saturated image shows normal cartilage (arrow) in the midportion of the joint
In nine joints, the cartilage had higher than normal T2 values which appeared as green–blue color. In seven of these joints, increased signal in cartilage on conventional MR sequences was readily demonstrated (Fig. 3). In two other joints, an increased signal in cartilage on conventional MR sequences was less apparent than the green–blue color on T2 map in the corresponding segment of the articular surface (Fig. 4). Eight joints demonstrated absence of the colored covering or absence of color on part or the whole articular surface on T2 map. On conventional MR sequences of six of these joints, cartilage defects or cartilage loss was readily seen, and in two other joints, these findings were less apparent. Two joints had a combination of cartilage edema and areas of cartilage loss and were included in the count of both groups (Fig. 5). Due to the sphericity of the humerus, there was a possibility for the prolongation of T2 values due to the magic angle effect over the humeral head. Although there was a possibility that the signal out of the color range or due to elevated noise would appear as a defect on T2 map, the fact that in all the cases this finding on T2 map corresponded to cartilage defect on standard sequences could suggest that probably this was not the case.
A 26-year-old woman with history of SLE and multiple bone infarcts. Non-arthrographic examination shows a cartilage edema in the blue spectrum at superior and midjoint of T2 map (arrowheads) and b increased signal on coronal/oblique T2 FSE (3,483/87) fat-saturated image (arrowheads). Normal red–orange spectrum of the T2 map in the inferior and superior portions of glenohumeral joint is present (arrows)
A 79-year-old man with rotator cuff tear and cartilage loss. Non-arthrographic examination with a cartilage defect in the humeral head, seen as a fragmentation of the colored covering of the lower portion of the humerus on T2 map (arrow). Note area of cartilage edema seen as abnormal green color on T2 map in the central area of the glenoid (arrowheads). b Cartilage abnormalities are less apparent on the corresponding T2 FSE fat-saturated image (arrow)
Discussion
In this work, we evaluated cartilage T2 mapping of the glenohumeral joint. To the best of our knowledge, there is no study in the English literature that describes this new technique in this joint in a clinical setting.
Primarily, the results of this study show that T2 maps can be successfully created and interpreted during routine MR imaging of the shoulder. Secondly, normal T2 maps of hyaline articular cartilage demonstrated red–orange color in its deeper layers and orange–yellow color in more superficial layers correlating with normal cartilage on conventional MR images. Finally, increased signal in the cartilage and cartilage defects on T2 maps and conventional MR sequences correlated as well. In 23.4% of the cases, increased T2 signal in the cartilage and cartilage defects were more apparent on T2 maps.
The limitation of this study is that the cartilage findings at T2 mapping and routine MRI were not confirmed with arthroscopy. While arthroscopy could be used to diagnose cartilage defects, it would be inadequate to evaluate for early or subtle intrinsic cartilage abnormalities as shown on T2 maps. Further studies with cadavers and histologic cartilage examination would be required. Another limitation is that the T2 maps and routine MR images were not reviewed independently. However, this study was planned and completed as a preliminary investigation describing the correlation between T2 maps and conventional MRI.
Cartilage is an important factor in joint disease. Indications for MR imaging of the cartilage include both the diagnosis of cartilage damage and monitor treatment such as cartilage repair procedures and pharmacological therapies [18]. Early detection of cartilage damage, either post-traumatic or degenerative, is indispensable for treatment at an early stage of osteoarthritis [19, 20]. The optimal goal of MR imaging of the cartilage is not only to visualize cartilage morphology and thickness but also to analyze cartilage quality. Both quantitative and qualitative measurements of the cartilage are essential for the follow-up of disease progression and its response to treatment.
In the past, joint space narrowing on weight-bearing radiographs was used as a marker for loss of cartilage. Conventional MRI scan of the cartilage allows quantitative assessment. It provides information about the shape and thickness of the cartilage and visualizes cartilage defects. However, currently used conventional MR cartilage imaging techniques such as proton-density or T2-weighted fast spin echo and 3D spoiled gradient echo or fast low-angle shot sequences with or without fat suppression [18] are somewhat insensitive to intra-substance alterations of cartilage composition [4, 6]. This is, however, an important early marker for degenerative joint disease and suggests that qualitative evaluation of the cartilage is necessary. New MR imaging parametric mapping techniques were suggested: 3D T1 rho-relaxation for the assessment of proteoglycan loss from articular cartilage [21] and delayed gadolinium-enhanced MR imaging of cartilage for in vivo evaluation of glycosaminoglycan in articular cartilage [22]. These methods and T2 cartilage mapping [14] demonstrated sensitivity to the biochemical and structural changes in the extracellular cartilage matrix and thus have the potential to serve as image markers of cartilage degeneration.
T2 is sensitive to slow molecular motions of water protons and anisotropy of the tissue matrix [23–25]. The limited mobility of cartilage water within a highly anisotropic matrix produces relatively short T2 values in the cartilage. Results of prior studies showed that normal articular cartilage demonstrates an increase in T2 values from the deep zone, where the T2 value is shortest to the transitional zone [4]. Cartilage T2 mapping uses intrinsic cartilage water as a probe to study the structural integrity of the extracellular matrix. Changes of the spatial distribution of the T2 values reflect areas of increased and decreased water content and may be used to diagnose cartilage degeneration even before decrease of cartilage can be appreciated. Healthy subjects have lower mean cartilage T2 values than patients with mild and severe osteoarthritis [11]. Asymptomatic increase in T2 associated with aging is different in appearance than the focally increased T2 observed in damaged articular cartilage [16].
Cartilage T2 mapping has valuable potential practical applications. It can be used to diagnose “cartilage at risk” and cartilage with irreversible damage [5]. This may be extremely helpful in the assessment of functional potential, especially in young patients and sportsmen. Furthermore, it can be used as a non-invasive tool to study cartilage composition of repair tissue post-cartilage repair procedures [4] or to monitor the effect of chondroprotective therapy. Cartilage T2 mapping of glenohumeral joint may also have clinical research applications in the study of arthritis and may provide novel information that improves understanding of the pathophysiology of generalized osteoarthritis.
In conclusion, T2 maps of articular cartilage of the glenohumeral joint correlate with the findings at conventional MRI. In addition, the results of this study demonstrate the feasibility of acquiring cartilage T2 maps of the shoulder in routine clinical settings. Incorporation of T2 maps with clinical MR imaging and cadaveric studies are needed to determine the role of T2 maps in the early diagnosis of degenerative joint disease.
References
McCauley TR, Recht MP, Disler DG (2001) Clinical imaging of articular cartilage in the knee. Semin Musculoskelet Radiol 5:293–304
Recht M, White LM, Winalski C et al (2003) MR imaging of cartilage repair procedures. Skeletal Radiol 32:185–200
Lazovic-Stojkovic J, Mosher TJ, Smith HE, Yang QX, Dardzinski BJ, Smith MB (2004) Interphalangeal joint cartilage: high-spatial-resolution in vivo MR T2 mapping—a feasibility study. Radiology 233:292–296
White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R (2006) Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology 241:407–414
Mosher TJ, Dardzinski BJ (2004) Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol 8:355–368
Zaim S, Lynch JA, Li J, Genant HK, Peterfy CG (2001) MRI of early cartilage degeneration following meniscal surgery: a three-year longitudinal study. Proc Int Soc Mag Reson 9:76
Gray ML, Burstein D, Xia Y (2001) Biochemical (and functional) imaging of articular cartilage. Semin Musculoskelet Radiol 5:329–343
Gold G, Beaulieu C (2001) Future of MR imaging of articular cartilage. Semin Musculoskelet Radiol 5:313–327
Glaser C (2005) New techniques for cartilage imaging: T2 relaxation time and diffusion weighted MR imaging. Radiol Clin North Am 43:641–653
Spandonis Y, Heese FP, Hall LD (2004) High resolution MRI relaxation measurements of water in the articular cartilage of the meniscectomized rat knee at 4.7 T. Magn Reson Imaging 22:943–951
Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S (2004) T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology 232:592–598
Maier CF, Tan SG, Hariharan H, Potter HG (2003) T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging 17:358–364
Smith HE, Mosher TJ, Dardzinski BJ et al (2001) Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging 14:50–55
Dardzinski BJ, Mosher TJ, Li S, Van Slyke MA, Smith MB (1997) Spatial variation of T2 in human articular cartilage. Radiology 205:546–550
Dardzinski BJ, Laor T, Schmidthorst VJ, Klosterman L, Graham TB (2002) Mapping T2 relaxation time in the pediatric knee: feasibility with a clinical 1.5-T MR imaging system. Radiology 225:233–239
Mosher TJ, Dardzinski BJ, Smith MB (2000) Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2—preliminary findings at 3 T. Radiology 214:259–266
Frank LR, Wong EC, Luh WM, Ahn JM, Resnick D (1999) Articular cartilage in the knee: mapping of the physiologic parameters at MR imaging with a local gradient coil—preliminary results. Radiology 210:241–246
Link TM, Stahl R, Woertler K (2007) Cartilage imaging: motivation, techniques, current and future significance. Eur Radiol 17:1135–1146
Disler DG, Recht MP, McCauley TR (2000) MR imaging of articular cartilage. Skeletal Radiol 29:367–377
Guermazi A, Zaim S, Taouli B, Miaux Y, Peterfy ChG, Genant HK (2003) MR findings in knee osteoarthritis. Eur Radiol 13:1370–1386
Duvvuri U, Kudchodkar S, Reddy R, Leigh JS (2002) T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis Cartilage 10:838–844
Bashir A, Gray ML, Boutin RD, Burstein D (1997) Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd-(DTPA)(2-)-enhanced MR imaging. Radiology 205:551–558
Harrison R, Bronskill MJ, Henkelman RM (1995) Magnetization transfer and T2 relaxation components in tissue. Magn Reson Med 33:490–496
Packer KJ (1977) The dynamics of water in heterogeneous systems. Philos Trans R Soc Lond B Biol Sci 278:59–87
Henkelman RM, Stanisz GJ, Kim JK, Bronskill MJ (1994) Anisotropy of NMR properties of tissues. Magn Reson Med 32:592–601
Acknowledgments
Authors graciously thank MRI technologists of St. Paul’s Hospital and Monika Ferrier, BA for their important and enthusiastic help.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maizlin, Z.V., Clement, J.J., Patola, W.B. et al. T2 Mapping of Articular Cartilage of Glenohumeral Joint with Routine MRI Correlation—Initial Experience. HSS Jrnl 5, 61–66 (2009). https://doi.org/10.1007/s11420-008-9106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11420-008-9106-3